- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Hypoxia Reporter Element Assay
Published: Vol 8, Iss 15, Aug 5, 2018 DOI: 10.21769/BioProtoc.2951 Views: 6732
Reviewed by: Jia LiLiku B TezeraPhilipp Wörsdörfer

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Efficient Gene-Editing in Human Pluripotent Stem Cells Through Simplified Assembly of Adeno-Associated Viral (AAV) Donor Templates
Berta Marcó de La Cruz [...] Fredrik H. Sterky
Nov 5, 2024 2876 Views

Temporally and Spatially Controlled Age-Related Prostate Cancer Model in Mice
Sen Liu [...] Qiuyang Zhang
Jan 5, 2025 2691 Views

Leveraging Circular Polymerization and Extension Cloning (CPEC) Method for Construction of CRISPR Screening Libraries
Bengisu Dayanc [...] Serif Senturk
Feb 20, 2025 2775 Views
Abstract
Hypoxia is a condition in which there is a decrease in oxygen supply to the cellular environment. Changes to cellular oxygen levels can lead to transcriptional changes of oxygen-regulated genes. Reporter assays are used to study gene expression alteration and modifications in response to environmental changes. Dual-reporter assays allow the simultaneous measurement of two different genes within a single cell, thus improving experimental accuracy. Within this protocol, we describe the utilization of the LightSwitch Dual Assay System to measure BMX expression in response to hypoxic conditions.
Keywords: LuciferaseBackground
In our recent publication (van Oosterwijk et al., 2018), we sought to examine the regulation of BMX, a nonreceptor tyrosine kinase, in response to sorafenib treatment. BMX, a Tec kinase family member, is known bind to tyrosine-phosphorylated proteins and mediate membrane targeting by binding to phosphatidylinositol 3,4,5-triphosphate (PIP3; Chen et al., 2013). We showed that direct treatment of sorafenib in acute myeloid leukemia (AML) cells lines with or without stromal cell support did not contribute to the upregulation of BMX. Previous studies have shown that BMX expression can be induced by ischemia and that sorafenib has antiangiogenic activity (He et al., 2006; Davis et al., 2011). Therefore, we hypothesized that the antiangiogenic activity of sorafenib causes a hypoxic environment within the bone marrow, thus contributing to a hypoxia-dependent BMX upregulation in AML. Under hypoxic conditions, we were able to show a significant increase in BMX expression in a number of different cell lines. Further analysis of the BMX promoter identified a putative hypoxia-responsive element (HRE; 5’-ACGTG-3’) at -5005. To test whether this HRE was involved in the hypoxia-induced promoter activation of BMX, we developed a hypoxia element reporter assay.
Reporter assays are extensively used throughout the scientific community to study alterations and modifications to gene expression in response to environmental changes. One type of reporter assay that is gaining in acceptance are the dual-reporter assays. This type of reporter assay allows the simultaneous expression and measurement of two different reporters within a single cell and had been widely proven to improve experimental accuracy by reducing extraneous influences. One such dual assay system is the LightSwitch Dual Assay System. This system utilizes the RenSP luciferase reporter gene, a novel luciferase developed by SwitchGear Genomics and the Cypridina luciferase reporter gene. RenSP and Cypridina employ different substrates, thus eliminating cross-reaction between proteins and their substrates. RenSP is used with your favorite gene as the reporter signal, and Cypridina is utilized with a constitutively active promoter as the control signal.
Here, we used the LightSwitch Dual Assay System to evaluate the involvement of the BMX HRE to its upregulation in response to hypoxic conditions.
Materials and Reagents
- Pipette tips
- White 96 well plates (CELLSTAR® Tissue Culture Plates, Greiner Bio One International, catalog number: 655083 )
- HEK293 (293[HEK-293]; ATCC, catalog number: CRL-1573 )
- pLightswitch_Prom_BMX (BMX promoter construct; SwitchGear Genomics, catalog number: S701154 )
- pLightswitch_Prom control vector (SwitchGear Genomics, catalog number: S790005 )
- pTK-Cluc (Cypridina TK control construct; SwitchGear Genomics, catalog number: SN0322S )
- FuGENE® HD (Promega, catalog number: E2311 )
- DMEM, high glucose, pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11995065 )
- QuikChange XL Site-Directed Mutagenesis Kit (Agilent Technologies, catalog number: 200516 )
- Terrific Broth Powder (BD, DifcoTM, catalog number: 243820 )
- Glycerol (Sigma-Aldrich, catalog number: G9012 )
- Poly-D-Lysine (Sigma-Aldrich, catalog number: P6407-5MG )
- Opti-MEM (Thermo Fisher Scientific, GibcoTM, catalog number: 31985070 )
- Fetal Bovine Serum (Thermo Fisher Scientific, GibcoTM, catalog number: 10437028 )
- LightSwitch Dual Assay System (SwitchGear Genomics, catalog number: DA010 )
- Poly-D-Lysine hydrobromide (MP Biomedicals, catalog number: 02150175 )
- Terrific Broth (see Recipes)
- 5x Poly-D-lysine Solution (see Recipes)
- 1x Poly-D-lysine Solution (sterile) (see Recipes)
- Growth media (see Recipes)
Equipment
- Pipettes
- Thermocycler (Eppendorf, model: Mastercycler® pro S , catalog number: 950030020)
- Table-top centrifuge (Eppendorf, model: 5810 R , catalog number: 022625101)
- Swinging bucket rotor (Eppendorf, model: S-4-104 , catalog number: 5820755008)
- High-performance centrifuge (Thermo Fisher Scientific, model: SorvallTM RC 6 Plus , catalog number: 36-101-0816)
- Laminar flow hood (Thermo Fisher Scientific, model: 1300 Series Class II , Type A2, catalog number: 1323TS)
- 37 °C, 5% CO2 water-jacketed incubator (Thermo Fisher Scientific, model: Heracell VIOS 160i , catalog number: 51030285)
- 37 °C non-humidified incubator (Labnet International, model: 311DS , catalog number: I5311-DS)
- Hypoxia chamber (Coy Lab, model: O2 Control InVitro Glove Box with no upgrades)
Standardfeatures include:- Humidified incubation box
- Temperature control
- Control of O2 and CO2 in 0.1% increments
- Humidified incubation box
- Plate luminometer (Molecular Devices, model: SpectraMax i3x , with no upgrades)
Standard features include:- Read modes: Absorbance, Fluorescence, Luminescence
- Wavelength ranges include: Abs: 230-1,000 nm, Fl Ex: 250-830 nm, Fl EM 270-850 nm, Lumi: 300-850 nm
- Read modes: Absorbance, Fluorescence, Luminescence
Software
- GraphPad Prism (GraphPad Software, Version 6)
- QuikChange Primer Design (https://www.genomics.agilent.com/primerDesignProgram.jsp)
Procedure
- Site-directed mutagenesis of pLightswitch_Prom_BMX to mutate the putative HRE within in the BMX promoter
- Primers are designed to mutate 2 base pairs (ACGTG → ATATG) within the HRE of the BMX promoter using the QuikChange Primer Design website (referenced above).
The CG → TA bp change has been described in the literature to effectively disrupt the HRE. - pLightSwitch_Prom_BMX is mutated using the QuikChangeXLSite-Directed Mutagenesis kit per manufacturer’s protocol.
- Extension time: 4.5 min
- Substitute DifcoTM Terrific Broth for NZY+ broth
- Extension time: 4.5 min
- Mutated plasmids are confirmed by sequencing using promoter insert sequencing primers (Forward: 5’-TCCATCAAAACAAAACGAAACAA-3’, Reverse: 5’-AGTCGAGCACGTTCATCTGCTT-3’) listed on the Switchgear Genomics website (http://switchgeargenomics.com/resources/vector-maps).
- Primers are designed to mutate 2 base pairs (ACGTG → ATATG) within the HRE of the BMX promoter using the QuikChange Primer Design website (referenced above).
- Hypoxia reporter assay (LightSwitch Dual Assay System Manual)
Day 1 (Carry out under a Laminar flow hood)- Coat two white 96-well plates with 1x Poly-D-lysine (50 μl/well) for 2 h at room temperature.
- Plate enough wells per plate to carry out in triplicate.
- Example samples:
- Empty vector (pLightSwitch_Prom) + pTK-Cluc
- Wild-type Bmx promoter (pLightSwitch_Prom_BMX) + pTK-Cluc
- HRE mutated BMX promoter (pLightSwitch_Prom_BMXmHRE) + pTK-Cluc
- Empty vector (pLightSwitch_Prom) + pTK-Cluc
- Use one plate per condition (i.e., 2 plates for comparison normoxia to hypoxia).
- Plate enough wells per plate to carry out in triplicate.
- Wash the plates once with sterile H2O (200 μl/well); plate 5 x 103 HEK293 cells per well in 100 μl of growth media and cultured overnight at 37 °C in 5% CO2.
Day 2
Preparation of constructs and reagents- Allow all reagents and plasmids to equilibrate to room temperature.
Note: Plasmids should be purified using a kit or protocol that will remove endotoxin, such as PureYeild Plasmid System from Promega. - Pre-warm Opti-MEM to 37 °C.
Transfection (Carry out under a Laminar flow hood)- Carry out transfections according to protocol details (LightSwitch Dual Assay System; link to protocol found above) for a 96-well plate with minimal changes.
Cypridina TK control construct, pTK-Cluc: 1 ng (One nanogram is used instead of 10 ng due to luciferase saturation)
pLightSwitch_Prom, pLightSwitch_Prom_BMX or pLightSwitch_Prom_BMXmHRE: 30 ng
The ratio of FuGENE® HD to plasmid: 3:1 (0.3 μl FuGENE:100 ng DNA per well) - Briefly, the transfection protocol is:
- Combine reagents as indicated in the following table and mix well.
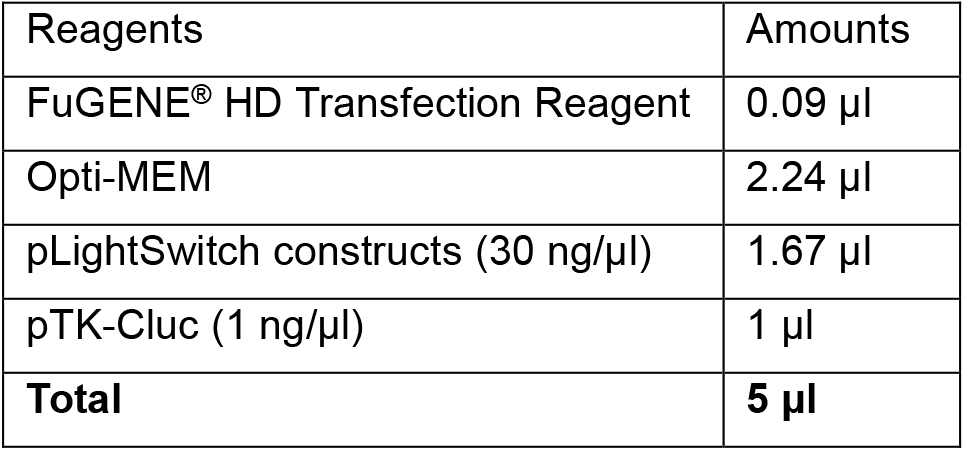
- Let sit at RT for 30 min.
- Gently drop onto seeded wells.
- Gently shake.
- Plates should be incubated at 37 °C for 24 h in normoxia (5% CO2).
- Combine reagents as indicated in the following table and mix well.
Day 3 (Under a Laminar flow hood)- Remove the transfection reagents by changing out the media on both plates.
- Transfer one plate into hypoxia (5% CO2, 94% N2, and 1% O2) at 37 °C and maintain another one in normoxia.
- Incubate both plates for an additional 24 h.
Note: Incubation time can be varied depending on the induction rate of your favorite gene due to hypoxia.
Day 4
Follow the manufacturer's instructions (LightSwitch Dual Assay System) for measuring Renilla (pLightSwitch_Prom vectors) and Cypridina (pTK-Cluc) luciferase activity.- No alterations were made to the protocol detailed by SwitchGear Genomics. Plates were always measured fresh and never frozen.
- For hypoxia conditions (all done within the hypoxia chamber):
- Adding reagents and incubations are all done within the hypoxia chamber.
- Remove the plates from hypoxia and immediately read the luminescence on a plate reader.
- Adding reagents and incubations are all done within the hypoxia chamber.
- For normoxia conditions: Adding reagents and incubation are all done at room temperature on the bench top.
- Coat two white 96-well plates with 1x Poly-D-lysine (50 μl/well) for 2 h at room temperature.
Data analysis
- Renilla (pLightSwitch_Prom) luciferase activity is normalized to Cypridina (pTK-Cluc) luciferase activity and adjusted for background luciferase for each condition.
- Data can then be graphed as relative luciferase activity or as fold change.(see Figure 1)
Note: If fold change is determined, the comparison should be to the wild-type promoter under normoxia conditions.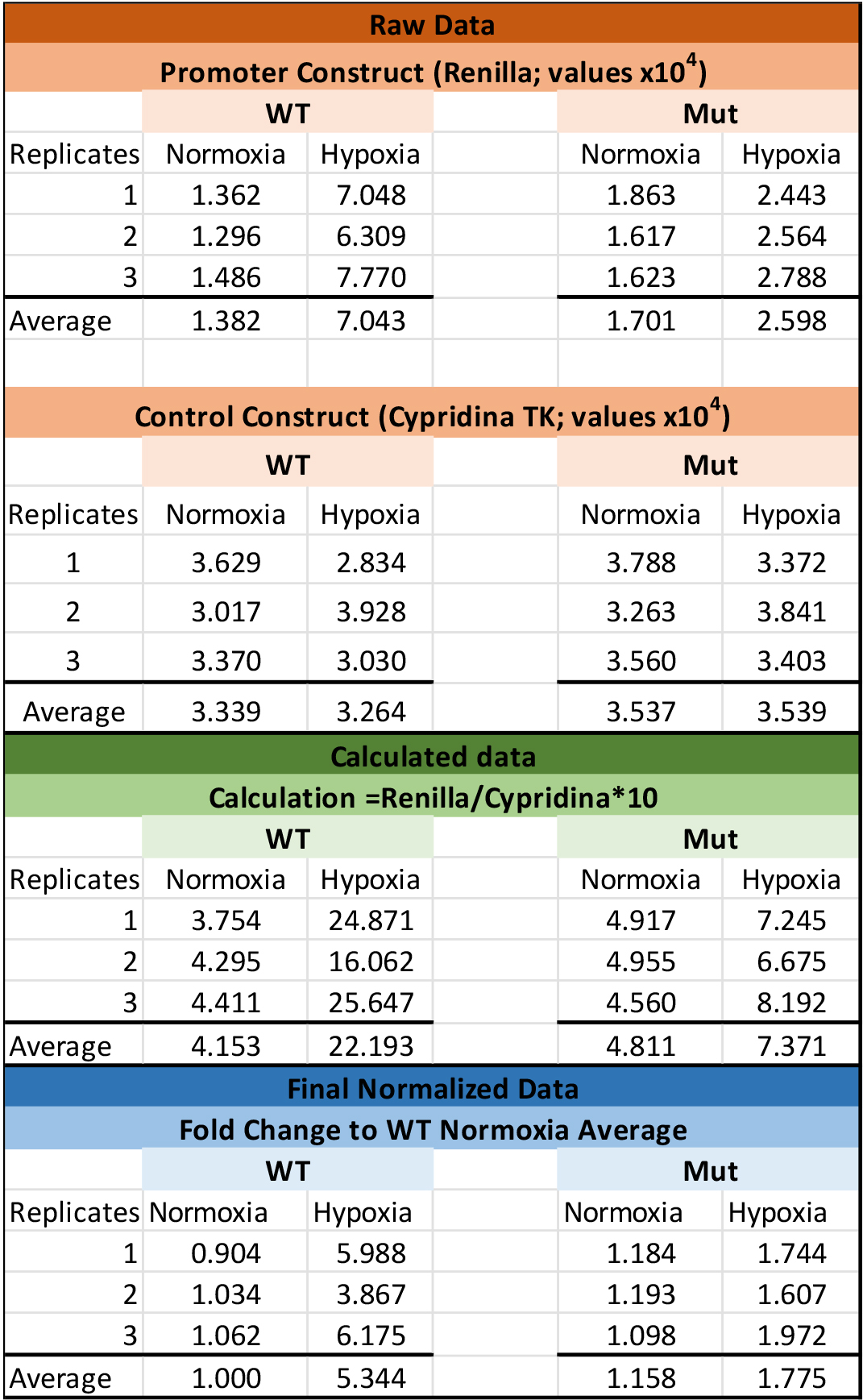
Figure 1. Example data
Notes
While there may be variation in transfection efficiency from experiment to experiment, the LightSwitch Dual Assay system is designed to improve experimental accuracy by reducing extraneous influences. Thus your reproducibility and variability should be minimal after the Renilla luciferase activity has been normalized to the Cypridina luciferase activity.
Recipes
- Terrific Broth
47.6 g Terrific Broth powder
4 ml glycerol
1 L H2O
Autoclaved at 121 °C for 15 min - 5x Poly-D-lysine Solution
Dissolve 5 mg Poly-D-lysine in 1 L ddH2O - 1x Poly-D-lysine Solution (Sterile)
Dilute 200 ml 5x Poly-D-Lysine solution w with 800 ml ddH2O and filter sterilize - Growth media
DMEM
10% FBS
Acknowledgments
We thank Navjotsingh Pabla for assistance with protocol development and helpful comments during manuscript preparation. This study was supported by the American Lebanese Syrian Associated Charities, NIH Cancer Center Support Grant P30 CA021765, and R01 CA138744 (to SDB). This study was also supported by funds from the Ohio State University Comprehensive Cancer Center, Pelotonia foundation, and NIH Cancer Center Support Grant P30 CA016058. Authors declare that there are no conflicts of interest or competing interests.
References
- Chen, S., Jiang, X., Gewinner, C. A., Asara, J. M., Simon, N. I., Cai, C., Cantley, L. C. and Balk, S. P. (2013). Tyrosine kinase BMX phosphorylates phosphotyrosine-primed motif mediating the activation of multiple receptor tyrosine kinases. Sci Signal 6(277): ra40.
- Davis, M. I., Hunt, J. P., Herrgard, S., Ciceri, P., Wodicka, L. M., Pallares, G., Hocker, M., Treiber, D. K. and Zarrinkar, P. P. (2011). Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29(11): 1046-1051.
- He, Y., Luo, Y., Tang, S., Rajantie, I., Salven, P., Heil, M., Zhang, R., Luo, D., Li, X., Chi, H., Yu, J., Carmeliet, P., Schaper, W., Sinusas, A. J., Sessa, W. C., Alitalo, K. and Min, W. (2006). Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest 116(9): 2344-2355.
- van Oosterwijk, J. G., Buelow, D. R., Drenberg, C. D., Vasilyeva, A., Li, L., Shi, L., Wang, Y. D., Finkelstein, D., Shurtleff, S. A., Janke, L. J., Pounds, S., Rubnitz, J. E., Inaba, H., Pabla, N. and Baker, S. D. (2018). Hypoxia-induced upregulation of BMX kinase mediates therapeutic resistance in acute myeloid leukemia. J Clin Invest 128(1): 369-380.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Buelow, D. R. and Baker, S. D. D. (2018). Hypoxia Reporter Element Assay. Bio-protocol 8(15): e2951. DOI: 10.21769/BioProtoc.2951.
Category
Cancer Biology > General technique > Molecular biology technique
Molecular Biology > DNA > Gene expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










