- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Arabidopsis-Green Peach Aphid Interaction: Rearing the Insect, No-choice and Fecundity Assays, and Electrical Penetration Graph Technique to Study Insect Feeding Behavior
Published: Vol 8, Iss 15, Aug 5, 2018 DOI: 10.21769/BioProtoc.2950 Views: 10421
Reviewed by: Renate WeizbauerZhao ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Insect Feeding Assays with Spodoptera exigua on Arabidopsis thaliana
Yanrong You [...] Chuanyou Li
Mar 5, 2020 5310 Views

Quantification of Methylglyoxal Levels in Cowpea Leaves in Response to Cowpea Aphid Infestation
Jacob R. MacWilliams [...] Isgouhi Kaloshian
Oct 20, 2020 3497 Views

Image-Based Lignin Detection in Nematode-Induced Feeding Sites in Arabidopsis Roots
Muhammad Amjad Ali and Krzysztof Wieczorek
May 5, 2025 1802 Views
Abstract
Aphids constitute a large group of Hemipterans that use their slender stylets to tap into the sieve elements of plants from which they consume copious amounts of phloem sap, thus depriving the plant of photoassimilates. Some aphids also transmit viral diseases of plants. Myzus persicae Sülzer, commonly known as the green peach aphid (GPA), which is a polyphagous insect with a host range that covers 50 plant families, is considered amongst the top 3 insect pest of plants. The interaction between Arabidopsis thaliana and the GPA is utilized as a model pathosystem to study plant-aphid interaction. Here we describe the protocol used in our laboratories for rearing the GPA, and no-choice and fecundity bioassays to study GPA performance on Arabidopsis. In addition, we describe the procedure for the electrical penetration graph (EPG) technique to monitor feeding behavior of the GPA on Arabidopsis.
Keywords: Myzus persicaeBackground
Aphids are important pests of plants that utilize their mouthparts, which are modified into stylets, to remove phloem sap from the sieve elements. As part of their feeding process, aphids deposit saliva into the plant tissue. While some salivary components elicit plant defenses, others manipulate host physiology to benefit the insect, including suppressing plant defenses (Nalam et al., 2018). Plants utilize a variety of defenses to control aphid infestation. These include antibiosis, which adversely impacts aphid growth, development and fecundity, and antixenosis, which affects insect behavior, including feeding behavior. These defenses are exerted at various steps, including at the cell surface, during the intercellular penetration of leaf tissue by the insect stylet, when the stylet tip is inside plant cells, and when the stylet tip is in the sieve elements (Nalam et al., 2018). The green peach aphid (GPA), Myzus persicae Sülzer, is an important pest of plants in numerous families, including the Brassicaceae, Solanaceae, Cucurbitaceae, Rosaceae, Asteraceae, Malvaceae, Amaranthaceae (Blackman and Eastop, 2000). In addition, the GPA transmits several viral diseases (Kennedy et al., 1963; Matthews, 1991). During the last decade, the interaction between Arabidopsis thaliana, which belongs to the Brassicaceae family, and the GPA has been increasingly utilized to study plant-aphid interaction (Louis et al., 2012; Louis and Shah, 2013). This pathosystem has facilitated understanding of the physiological and molecular processes that determine the outcome of plant-aphid interaction, including plant defense mechanisms and their impact on insect population growth, fecundity and behavior (Louis et al. 2012; Louis and Shah, 2013).
The direct current (DC)-electrical penetration graph (EPG) system, which measures the electromotive force (EMF) signal and fluctuations in electrical resistance resulting from aphid stylet penetrations, provides a sensitive method to monitor aphid feeding behavior on plants (Tjallingii, 1985; Salvador-Recatalà and Tjallingii, 2015). When the aphid stylet is inserted intercellularly, the voltage is positive and when inserted intracellularly, the voltage is negative (Tjallingii, 2006). The different EPG waveform patterns are indicative of the different activities in which the insect is engaged. Moreover, the duration of each type of waveform provides a quantitative measure of the effect of plant genotype and/or treatment on insect feeding behavior, including the time spent by the insect feeding from the sieve elements. However, despite the success of the Arabidopsis-GPA pathosystem, the protocols utilized to study aphid population growth, fecundity and feeding behavior have not been described in detail. Here, we detail the protocols for rearing a Brassicaceae-adapted colony of the GPA, no-choice assays for monitoring GPA population growth, fecundity assays to monitor insect reproductive rate, and the EPG analysis to monitor GPA feeding behavior on Arabidopsis.
Part I: Rearing the green peach aphid
The green peach aphid (Myzus persicae) colony is maintained on a mix of radish (Raphanus sativus ‘Early Scarlet Globe’) and mustard (Brassica juncea ‘Florida Broadleaf’) plants, which like Arabidopsis belong to the Brassicaceae family. On Brassicaceae, GPA reproduces asexually by releasing live apterous (wingless) nymphs.
Materials and Reagents
- T.O. Plastics Standard Flats 1020 tray with bottom holes (Hummert International, model: STE-1020-OPEN - WITH HOLES, catalog number: 11-3000-1 )
- T.O. Plastics Standard Flats 1020 tray without holes (Hummert International, model: STE-1020-NH - NO HOLES, catalog number: 11-3050-1 )
- Square injection molded plastic pots (4.5” [11.43 cm] width x 3.75” [9.53 cm] height) with holes at the bottom (International Greenhouse, catalog number: CN-SQK )
- Twist ties
- Biohazard autoclave bags (Fisher Scientific, catalog number: 01-830D )
- NalgeneTM polypropylene heavy duty sterilizing tray (Thermo Fisher Scientific, catalog number: 6900-0020 )
- Soil Mix (Sunshine® Mix #8, Sun Gro Horticulture, model: Fafard®-2 )
- Radish seeds (Radish Early Scarlet Globe) (Main Street Seed & Supply, catalog number: 13307-13 )
- Mustard seeds (Florida Mustard Broad Leaf) (Main Street Seed & Supply, catalog number: 12501-13 )
- Green peach aphid colony (Specimen number 194 deposited with Kansas State University Museum of Entomological and Prairie Arthropod Research)
Equipment
- Plant growth chamber (Percival Scientific, model: AR-66L2 )
Note: Programmed for a 14/10 h day (80-100 μE m-2 sec-1)/night photoperiod at 22 °C. - Autoclave
Procedure
- Place soil in an autoclave bag. Break up any lumps and add tap water. Knead the soil-water mix, till the soil is evenly moist. Close the bag with a plastic tie. With a scissor make four small incisions in the plastic bag to facilitate venting after the bag is removed from the autoclave at the end of Step 2.
- Place the bag containing soil in an autoclavable plastic tray and autoclave for 1 h at 121 °C/15 psi on a liquid cycle. After the autoclaving is completed, pull the tray with soil out of the autoclave and let the soil cool down to room temperature. It is best to let the soil cool down overnight.
- To prepare pots for planting radish and mustard, take a flat plastic tray with holes and place it inside a flat plastic tray without holes. Place eight, 4.5” (11.43 cm) pots in the tray with holes. Fill these pots loosely with the wet autoclaved soil, making sure to fill the pots to the top.
- Sprinkle 30-40 seeds each of radish and mustard on the top of the soil in each pot (see Figure 1).

Figure 1. Radish and mustard seeds in a 4.5” (11.43 cm) pot
- Spray the seeds generously with water.
Note: All the seeds should be nicely sprayed with water. If seeds are left dry, they will not germinate. - Place the trays with the pots containing the fresh radish and mustard seeds in the growth chamber that contains GPA-colonized mix of radish and mustard plants (Figure 2). The tray with fresh seeds is placed adjacent to the tray containing GPA colonized mustard and radish plants. The growth chamber should be preferably located in a contained area where plants for other use are not cultivated.

Figure 2. Radish and mustard plants with GPA (indicated by black arrows) - The radish and mustard seed mix should begin germinating within 2-3 days. The aphids will begin moving to the fresh plants in a few days.
- Repeat this procedure every week to maintain a healthy GPA colony.
- Unwanted plants and soil are emptied into autoclave bags and autoclaved for 1 h at 121 °C/15 psi on a liquid cycle before disposing of.
Steps to prevent insect escaping out of contained facility: If feasible, have the insect colony in a room that is physically separated from the room in which plants are normally cultivated. To limit spread of the insect out of the insect room, avoid going from the insect room to a room where plants are cultivated. If feasible, avoid wearing shirts/lab coats with long sleeves, or roll-up the sleeves so that insects do not accidentally crawl on to your shirts and escape out of the insect room. Further, wash hands well before conducting any other work.
Part II: No-choice bioassay
The no-choice bioassay provides a simple assay to compare aphid population growth on plants of different genotypes. It provides a measure of differences between the resistance levels of plants of different genotypes. It can also be used to compare the impact of various treatments (e.g., chemicals) on aphid population growth. The no-choice assay measures the combined effect of antibiosis and antixenosis. Antibiosis is the effect of host defenses on insect growth, development and reproduction, while antixenosis reflects the effect of plant mechanisms on insect behavior. The Electrical penetration graph technique, which is described later, measures the impact of host genotype or treatments on insect feeding behavior.
Materials and Reagents
- T.O. Plastics Standard Flats 1020 tray with bottom holes (Hummert International, model: STE-1020-OPEN - WITH HOLES, catalog number: 11-3000-1 )
- T.O. Plastics Standard Flats 1020 tray without holes (Hummert International, model: STE-1020-NH - NO HOLES, catalog number: 11-3050-1 )
- Pots for rearing green peach aphid: Square injection molded plastic pots (4.5” [11.43 cm] width x 3.75” [9.53 cm] height) with holes at the bottom (International Greenhouse, catalog number: CN-SQK )
- Pots for cultivating Arabidopsis: Square injection molded plastic pots (3.5” [8.9 cm] width x 37/8” [9.84 cm] height) with holes at the bottom (International Greenhouse, catalog number: CN-SQK )
- Soil Mix (Sunshine® Mix #8, Sun Gro Horticulture, model: Fafard®-2 )
- Clear vinyl propagation dome to fit 1020 flats (Hummert International, catalog number: 11-3360-1 )
- Twist ties
- Biohazard autoclave bags (Fisher Scientific, catalog number: 01-830D )
- NalgeneTM polypropylene heavy duty sterilizing tray (Thermo Fisher Scientific, catalog number: 6900-0020 )
- Camel hair paint brush (size 2 or smaller) (Fisher Scientific, General Data, catalog number: 15-183-35 )
- Tooth picks
- Labeling tape 0.5” (1.27 cm) width (SP Scienceware - Bel-Art Products - H-B Instrument, catalog number: F13481-0050 )
- Fine-tip permanent alcohol/waterproof lab marker (VWR, catalog number: 52877-310 )
- Green peach aphid colony (Specimen #194 deposited with Kansas State University Museum of Entomological and Prairie Arthropod Research)
- Arabidopsis thaliana seeds accession Columbia (However, other accessions should also be fine)
- Radish seeds (Radish Early Scarlet Globe) (Main Street Seed & Supply, catalog number: 13307-13 )
- Mustard seeds (Florida mustard broadleaf) (Main Street Seed & Supply, catalog number: 12501-13 )
- Peters 20:20:20 General Purpose fertilizer (Hummert International, catalog number: 07-5400-1 )
Equipment
- Two plant growth chambers (Percival Scientific, model: AR-66L2 )
Note: The growth chambers are programmed for a 14/10 h day (80-100 μE m-2 sec-1)/night photoperiod at 22 °C. One is required for cultivating Arabidopsis, and the second chamber is required for the no-choice assay with aphids. - Autoclave
- Cold room or refrigerator (4-10 °C)
Procedure
- Propagating Arabidopsis for experiments with the GPA
- Autoclave the soil and prepare the 3.5” (8.9 cm) pots with soil as described above in Part I Procedure. At least five pots are required for each Arabidopsis genotype. Stick a 2-3 cm length of labeling tape on each pot and with a fine-tip waterproof marker, write down the plant genotype.
- Place the soil-filled pots in the tray with holes. Each tray can take a maximum of 18, 3.5” (8.9 cm) pots. A minimum of five pots are required for each Arabidopsis genotype.
- Dissolve 0.1 g of Peter’s 20:20:20 fertilizer in one liter of tap water.
- Fill the tray without holes with the fertilizer solution to a depth of 1-1.25” (2.54-3.81 cm).
- Place the tray (with holes) containing the soil-filled pots in the tray containing the fertilizer solution. The fertilizer solution will rise up through the soil by capillary action. When the top of the soil appears moist (approximately 10-15 min), lift the top tray containing the pots and place it at a slight angle so that it sits on the top edges of the lower tray. Allow the excess fertilizer solution to water drain out into the lower tray.
- Soil is now ready for sowing seeds. At least 10 Arabidopsis plants (two per pot) are required for each genotype. Place two Arabidopsis seeds of the same genotype diagonally across from each other, 1-1.25 cm from the edge of the pot. The seeds can be picked up, one at a time, by touching the moistened tip of a toothpick to the seed. Seeds will adhere to the tip of the toothpick. Seeds can now be placed on soil by touching the seed, which is adhered to the tip of the toothpick, to the moistened soil surface.
Note: Do not push the seed into the soil and have only seeds of the same genotype in each pot. - Cover the tray with the clear vinyl propagation dome.
- Put the tray in a cold room or refrigerator for 48 h (light is not required during this period). This will allow the seeds to imbibe water and increase the chance of seeds germinating uniformly.
- At the end of the cold treatment, move the trays and pots covered with the clear vinyl propagation dome into the growth chamber. The seeds should germinate, and a pair of green cotyledons should emerge within 2-3 days.
- Once the first pair of true leaves has appeared (usually 8-9 days after placing the pots in the growth chamber), remove the transparent plastic dome. Leave the plants uncovered in the growth chamber.
- Fertilize plants every two weeks, by placing the pots contained in a tray with holes in a tray containing the fertilizer solution (0.1 g Peter’s 20:20:20 fertilizer per liter of water).
- The No-choice bioassay setup and analysis
- When the plants are 24-26 days old (see Figure 3), take them to the insect room.

Figure 3. Arabidopsis plants for no-choice bioassay - Use a camel hair paint brush to gently pick up adult apterous (wingless) aphids (~1 mm size), one at a time, from the radish/mustard plants, and place them at the center of each Arabidopsis plant. Repeat the process till 20 aphids have been placed on each Arabidopsis plant.
- Place the infested plants in the growth chamber. Space the pots to ensure that the pots and plants in adjacent pots are not touching each other.
- Fouty-eight hours later, count the total number of aphids (nymphs + adults) on each plant. Most of the aphids will be on the abaxial side of the leaves (see Figure 4).

Figure 4. GPA on the abaxial side of Arabidopsis leaves - When comparing insect population size on two genotypes (see Figure 5), Student’s t-test can be used to determine if the mean of insect population size on the two genotypes is significantly different (P < 0.05).
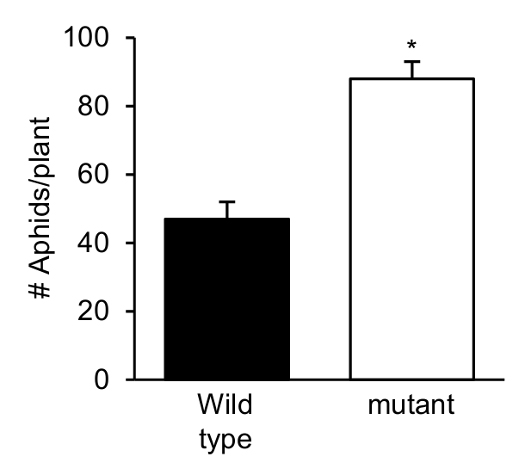
Figure 5. Representative no-choice experiment data. No choice experiment was conducted with the wild type and a mutant genotype of Arabidopsis that exhibits lowered resistance to the GPA. Aphid counts (adult + nymphs) were taken 2 days-post-infestation. Shown is the average aphid counts of aphids on each plant (n = 10). Error bars represent standard error. An asterisk indicates that values between the two genotypes are significantly different (P < 0.05; t-test). - At the end of the experiment, the plants and the soil are emptied into autoclave bags and autoclaved for 1 h at 121 °C/15 psi on a liquid cycle before disposing of.
Part III: Fecundity assay
The fecundity assay is used to determine the reproductive capacity of the GPA on an individual genotype or to compare the reproductive capacity of the GPA between plants of different genotypes, or in plants treated with a chemical compared to control plants.
Materials and Reagents
- Supplies for propagating GPA and cultivating Arabidopsis and radish/mustard plants as described above in Part I and Part II, respectively
- Camel hair paint brush (size 2 or lower) (Fisher Scientific, General Data, catalog number: 15-183-35 )
- 14 day old Arabidopsis plants; two per pot
- Petri dish 100 mm wide x 15 mm deep (Fisher Scientific, catalog number: FB0875713 )
- Radish seeds (Radish Early Scarlet Globe) (Main Street Seed & Supply, catalog number: 13307-13 )
- Mustard seeds (Florida mustard broad leaf) (Main Street Seed & Supply, catalog number: 12501-13 )
Equipment
- Two plant growth chambers (Percival Scientific, model: AR-66L2 )
Note: The growth chambers are programmed for a 14/10 h day (80-100 μE m-2 sec-1)/night photoperiod at 22 °C. One is required for cultivating Arabidopsis, and the second chamber is required for the fecundity assay with aphids. - Autoclave
- Cold room or refrigerator (4-10 °C)
Procedure
- Prepare soil and plant Arabidopsis and radish/mustard seeds as described above in Part I and Part II Materials and Reagents.
- A day before initiating experiment with Arabidopsis, with a camel hair paint brush place several apterous adult (~1-1.5 mm) insects on healthy radish/mustard plants.
- Next day, with a camel hair paint brush, collect the newly emerged nymphs in a Petri dish.
- Use the paint brush to release two 1-day-old nymphs on each Arabidopsis plant. Each nymph is released on a separate leaf. At least 10 Arabidopsis plants are required for each genotype.
- Place the infested plants in the growth chamber. Most of these nymphs will grow in size and start reproducing in approximately 6-8 days. The fecundity assay will determine the number of nymphs produced by these (mother) aphids.
- From Day 4 onwards, check each plant every 2 days for newly emerged nymphs. Count the number of nymphs and discard them, leaving only the mother aphids on the plant.
- Continue counting the number of newly emerged nymphs for a period of 17-18 days post release of the mother aphid on the Arabidopsis plant.
- Determine the total number of nymphs that were recovered from each plant over the duration of the experiment. Fecundity, which is represented as the average number of nymphs released per day, per aphid, is calculated with the following equation:
Fecundity = N ÷ 2(D)
where, N is the total number of newly emerged nymphs recovered from each plant over the duration of the experiment and ‘D’ is the total number of days in the experiment. The number 2 indicates the number of insects that were released on each Arabidopsis plant at the start of the experiment. - When comparing GPA fecundity on two genotypes (Figure 6), Student’s t-test can be used to determine if the fecundity value on the two genotypes is significantly different (P < 0.05) from each other.

Figure 6. Aphid fecundity assay. Shown is the average number of nymphs released per mother aphid per day over an 18 day period on Arabidopsis wild-type and mutant genotypes (n = 10). Error bars represent standard error. An asterisk, indicates that values between the two genotypes are significantly different (P < 0.05; t-test). - At the end of the experiment, the plants and the soil are emptied into autoclave bags and autoclaved for 1 h at 121 °C/15 psi on a liquid cycle before disposing of.
Part IV: Electrical penetration graph to study the green peach aphid feeding behavior
The Electrical penetration graph (EPG) measures the time spent by the aphid on various feeding activities. It can be used to determine the effect of plant genotype and or environmental factors (e.g., chemical treatment) on aphid feeding behavior. A cartoon of the EPG set-up is shown in Figure 7. In EPG, an uninsulated electrode, the plant electrode, is placed in the soil in which a plant is growing, thus electrifying the plant with a very low-voltage, low amperage current. An insect electrode, which at one end contains an extremely fine and flexible gold-wire is glued to the dorsum (back) of an aphid. The other end of the gold wire is connected to a copper wire, which in turn is connected to a brass nail that is hooked up to an amplifier. When the aphid is walking on the surface of the plant tethered to gold wire of the insect electrode, the 'switch' is open in this electrical circuit. However, as the stylet contacts the conductive tissues of the electrified plant, a potential drop occurs and unique waveform patterns reflecting different activities are produced, including (i) non-probing phase (baseline), (ii) pathway phase when stylet is inserted into leaf, but not in the phloem, (iii) time to first probe, (iv) sieve element phase (SEP) when the insect is feeding from sieve elements, (v) time to first SEP, and (vi) xylem phase. Below we describe the steps involved in preparation of the insect probe, the attachment of the insect electrode to the aphid, data acquisition and analysis.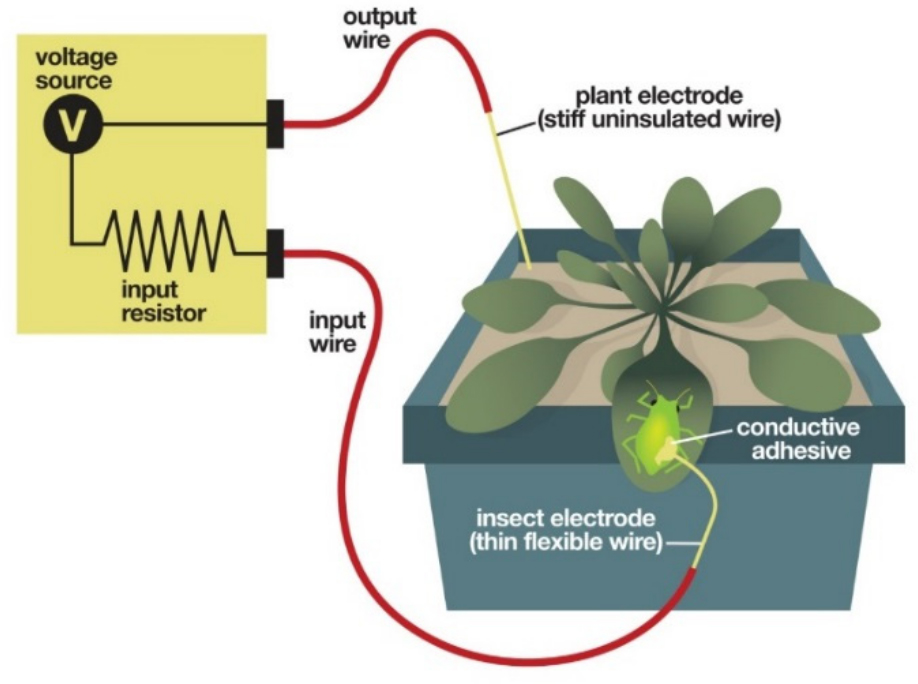
Figure 7. Illustration of an EPG setup. A thin gold wire (insect electrode), which permits unhindered movement of aphid, is connected to the EPG monitor. An output wire (stiff copper wire), which is part of the plant electrode, is inserted into the soil of the pot, in which the plant is rooted. The other end of the plant electrode is connected to the EPG monitor. The plant is electrified with a low-voltage, low amperage current. Once the aphid starts feeding on the plant, the aphid stylet comes into contact with the electrified plant, the circuit will be closed and current will flow through the insect and into the monitor, thus producing different waveforms. Illustration by Nick Sloff, taken with permission from Louis et al. (2012) The Arabidopsis Book e0159. doi: 10.1199/tab.0159, www.arabidopsisbook.org).
Materials and Reagents
- Supplies for propagating GPA and cultivating Arabidopsis and radish/mustard plants as described above in Part I and Part II, respectively
- Thin gold wire Ø 18 μm (thickness range 10-20 μm; available at https://www.epgsystems.eu/epg/products)
- Brass connector pins/nails, Ø 1.2 mm (comes with Giga-8d EPG System; http://www.epgsystems.eu/)
- Copper wire Ø 0.2 mm (AliExpress, model: YT1303 )
- Adjustable swivel clamps to hold the EPG probes (EPG Systems, comes with Giga-8d EPG System; http://www.epgsystems.eu/)
- Lead soldering roll (can be purchased from a local hardware store)
- Petri dish 100 mm wide x 15 mm deep (Fisher Scientific, catalog number: FB0875713 )
- Camel hair paint brush (size 2 or lower) (Fisher Scientific, General Data, catalog number: 15-183-35 )
- Dissecting needle (Thermo Fisher Scientific, catalog number: 19010 )
- Paper pins or T pins
- Kimwipes® (KCWW, Kimberly-Clark, catalog number: 34120 )
- Andwin Scientific Miracloth pore size 22-25 μm (Fisher Scientific, catalog number: NC9147303)
Manufacturer: Andwin Scientific, catalog number: 475855 . - Styrofoam/Polystyrene box
- Radish/mustard plants for propagating GPA
- 21-28 days old Arabidopsis plants; one per pot
- Conductive silver paint-Colloidal Silver (Ted Pella, catalog numbers: 16031 , 16034 )
Note: Alternatively, prepare your own silver glue as described at https://www.epgsystems.eu/downloads-install-files-manuals/file/24-add-ons-and-hints
Equipment
- Tweezers
- GIGA-8 direct current amplifier (http://www.epgsystems.eu/)
- EPG probes (http://www.epgsystems.eu/)
- Plant electrode (http://www.epgsystems.eu/)
- Grounding cable and test cable (see GIGA manual; http://www.epgsystems.eu/)
- Stereo dissecting microscope
- A computer to record and store the real-time monitoring of EPG waveforms
- Faraday cage to prevent external noises*
- Vacuum-operated plate for wiring aphids*
- Soldering iron (can be purchased from a local hardware store) to attach copper wire to brass nails
- Fume hood for soldering
Note: *Construction of the Faraday cage and the vacuum suction device have been explained in detailed by W.F. Tjallingii in the Giga 8d Manual (https://www.epgsystems.eu/downloads-install-files-manuals/file/24-add-ons-and-hints).
Software
- Stylet+ (http://www.epgsystems.eu/)
- JKI Macro for Excel (https://www.epgsystems.eu/downloads-install-files-manuals/category/4-epg-data-processing)
- Excel Workbook for automatic parameter calculation (Sarria et al., 2009)
- Ebert 1.0 for parameter calculation (http://www.crec.ifas.ufl.edu/extension/epg/sas.shtml)
- Microsoft Excel®
- Analysis of Variance (ANOVA) (SAS Institute Inc, SAS v5.1)
- Minitab® 18.1 for non-parametric Analysis (Kruskal-Wallis Test and Mann-Whitney U test)
Procedure
The following protocol is provided for studies on Arabidopsis thaliana and the green peach aphid (GPA), Myzus persicae.
- Preparation of the insect electrode
The insect electrode (Figure 8) consists of a brass connector pin (Ø 1.2 mm) to which a copper wire (2-4 cm long; Ø 0.2 mm) is attached by soldering the wire to the head of the brass connector pin (Video 1; Part A). A gold wire (2-4 cm long; Ø 18 μm) is attached to the opposite end of the copper wire using silver glue or colloidal silver paint (Video 1; Part B).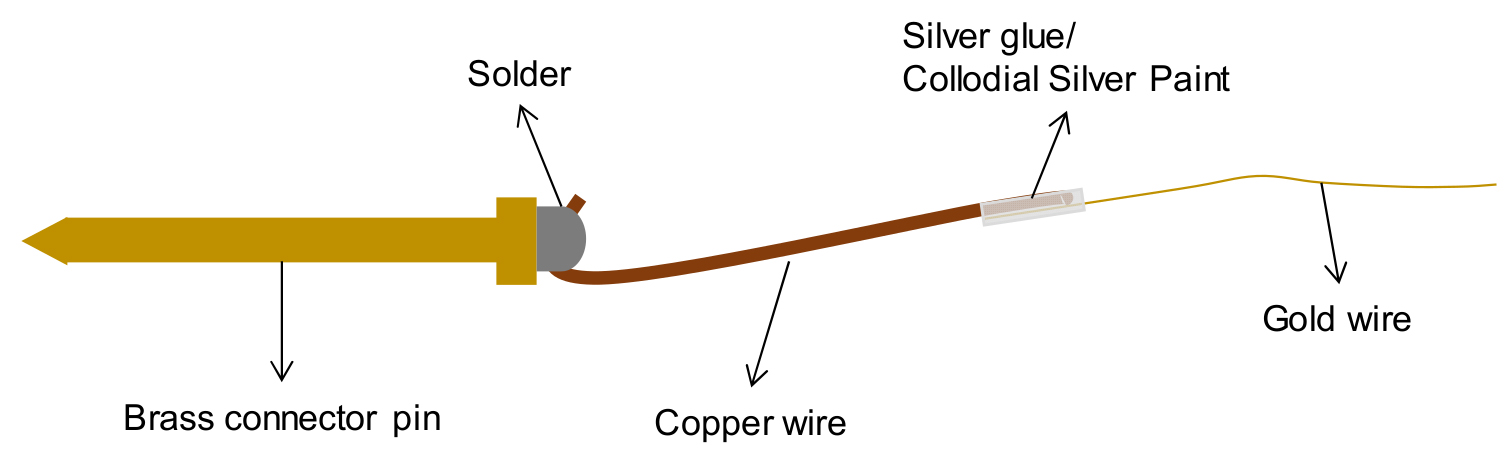
Figure 8. The various components of an insect electrodeVideo 1. Preparation of insect electrode- Using the soldering bolt, melt some soldering wire at its tip onto a hard, heat resistant surface.
- Apply soldering fluid to the head of the brass connector pin and dip the brass connector pin into the melted soldering fluid.
- Immediately bring the brass connector pin with the soldered fluid on the head into contact with the one end of a 2-4 cm piece of precut copper wire (Video 1; Part A).
- Hold the copper wire in place until the soldering fluid cools and solidifies on the brass connector pin-head creating a strong bond with the copper wire.
Note: The above Steps A1-A4, should be performed under the fume hood. - Dip the free end of the copper wire into a tube containing silver glue or colloidal silver paint in order to create a fine sheath of glue/colloidal paint on the free end.
Note: In order to ensure a uniform distribution of silver particles and glue, thoroughly shake the sliver glue/colloidal paint before use. - Touch the glue/paint end of the copper wire to the end of a 2-4 cm piece of precut gold wire and gently lift the gold wire. Alternatively, make a few turns of the gold wire on the copper wire and apply the sliver glue/paint over it (Video 1; Part B).
- Using a dissection needle, gently manipulate the gold wire to distribute the glue/paint to overlap the copper wire and about 0.5 cm of one end of the gold wire (Video 1; Part B).
- Allow the glue/paint to dry completely before applying a second coat of the glue/paint using the tip of the dissection needle to create a strong bond between the copper wire and the gold wire. This process is also done to ensure that there are no glue/paint-free parts of contact between the copper wire and the gold wire.
- After the glue/paint is dry, the insect electrode is ready and can be stored in a styrofoam/polystyrene box.
Note: The insect electrode can be prepared using either a stereo dissection microscope or under sufficient illumination on a laboratory bench.
- Using the soldering bolt, melt some soldering wire at its tip onto a hard, heat resistant surface.
- Preparation of aphids for wiring
Collect enough apterous adult aphids (at least 10-15 for preparing eight insect electrode-wired insects) from the aphid colony and transfer them to a Petri dish (100 mm wide x 15 mm deep).
Prior to the wiring, aphids are starved for one hour to ensure feeding upon the start of the EPG experiment.
Note: A Giga-8 system can at a time, simultaneously monitor the feeding behavior of a maximum of 8 aphids. Hence, to obtain readings from 15-20 aphids, the experiments will have to be conducted on multiple days. - Connecting aphids to the insect electrode
The insect electrode (Figure 8) described above is connected to the aphid with a silver glue/colloidal paint (Figures 9A and 9B). For the following procedure, the use of a stereo dissecting microscope will greatly ease the process. A vacuum suction device can be utilized to hold the aphid in place while attaching the insect electrode to the dorsum of the aphid. Alternatively, the aphid can be placed on a KimWipe® or MiraCloth® in order to reduce the mobility of the aphid.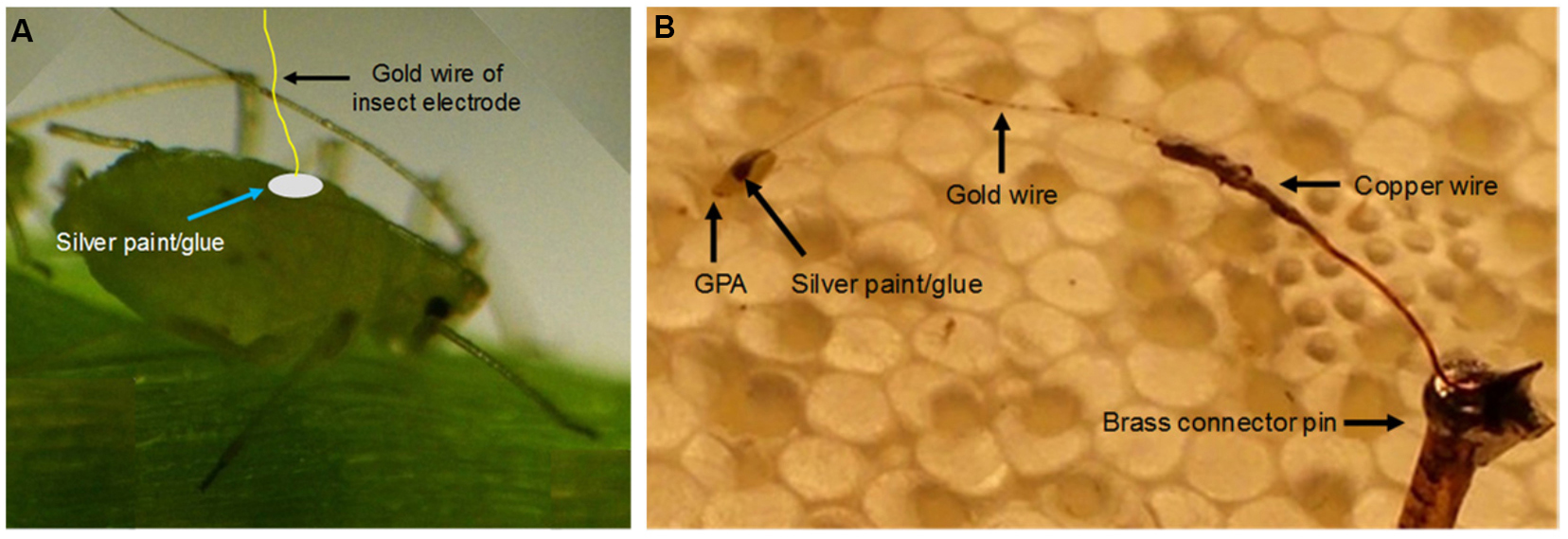
Figure 9. A wired green peach aphid. A. Cartoon depicting the gold wire component of the insect electrode attached to the dorsum of a GPA with sliver paint/glue. B. An image of a GPA connected to the insect electrode via the gold wire and sliver paint/glue. - Ensure that a smooth emulsion of the silver glue/colloidal paint is available by shaking the tube thoroughly.
- With a camel hair paint brush lift an aphid from the Petri dish where the apterous adult aphids are being starved.
- Using a pin/dissecting needle, apply a small drop of glue/colloidal silver paint on the dorsum of an adult aphid (Figures 9A and 9B). Allow the glue/colloidal silver paint to dry for several minutes. Ensure that the glue/colloidal silver is not applied to the head, antennae or legs of the insect.
- Once the first drop of glue/colloidal sliver paint is dry, apply a second drop of glue/colloidal sliver paint to the same spot on the back of the aphid.
- Immediately, insert the free end of the gold wire of an insect electrode into the second drop of glue/colloidal silver paint (Video 2). Ensure that the other end of the gold wire points upwards from the insect and does not hinder the movement of the aphid’s legs or antennae.Video 2. Connecting an aphid to the insect electrode. Connecting the aphid to the gold wire end of the insect electrode with silver glue/colloidal silver paint.
- Hold the insect electrode in place until the glue/colloidal paint is dry. This process will take couple of minutes, during which minimal movement of the aphid and the insect electrode is recommended.
Note: If the glue/colloidal silver paint smears over the antennae or the legs of the aphid or if the gold wire of the insect electrode does not attach, discard the aphid and repeat the process with a fresh aphid. Avoid adding a third droplet of glue/colloidal paint on the aphid back. - At this point, turn off the suction device (if using one) or lift the aphid away from the stage of the stereo dissecting microscope. The camel hair paint brush can be used to assist in lifting the aphid.
- The insect electrode with attached aphid can then be stored until use, by inserting the brass connector pin end into a styrofoam/polystyerene block (Video 3).Video 3. A GPA tethered to an insect electrode
- Repeat Steps C1-C8 until all the aphids needed for the EPG experiment are prepared and ready.
- Plant access
- Plants for EPG should be well watered the day before the experiment. Place the Arabidopsis plants in the Faraday cage on a non-conductive surface (Figures 10A and 10B). Examples of non-conductive surfaces include Petri-dishes, cardboard from shipping containers or pieces of styrofoam cut to the appropriate size to be placed under the pots.
- If using all 8 channels of the Giga-8, arrange eight Arabidopsis plants in the Faraday cage in a manner that the no part of the leaves or pots touch each other.
- Insert the plant electrode into the soil of each pot as close to the base of the pot as possible causing minimal damage to the plant roots.
Note: The plant electrode (http://www.epgsystems.eu/) consists of a copper wire (10-12 cm long; Ø 0.6 mm) connected to a cable and connector pin for connection to the Giga-8 system. - Insert the brass pin of the insect electrode with a wired aphid into the input connector of the EPG probe. The proper position of the insect should be such that the legs should be in the walking position on the plant surface.
- Lift the EPG probe (Figure 10C) so that the insect is not in contact with the plant surface and then proceed to connect the remaining prepared insect electrodes with wired aphids to the remaining channels.
- If required, use the swivel clamps to adjust the height of the EPG probes (Figure 10C).
- Once all the insect electrodes are connected and the aphids are hanging a few centimeters above the selected leaves/plants, start data acquisition using the Stylet+ software (Detailed methodology of the using the software can be found in the Stylet+ manual at https://www.epgsystems.eu/downloads-install-files-manuals/category/7-hard-software).
- Lower the insects one at a time on to the plant surface and ensure that the aphid is able to place its legs on the plants surface and that there is sufficient slack to allow the aphid to move around to find an appropriate place for stylet penetration (Figure 10D).

Figure 10. The EPG setup. A. A Faraday cage containing the Giga-8 setup; B. A close up of the Giga-8 setup monitoring the activity of five GPA on Arabidopsis; C-D. A close-up view of GPA attached by the aphid electrode to the Giga-8 system.
Note: Some users might want to increase the length of the gold wire in the insect electrode to allow for sufficient room for the aphid to move. However, a longer gold wire can result in entanglements on plant structures resulting in noise during the EPG recording. - The amplifier settings may be adjusted once the aphids have started probing (details can be found in Giga 8d Manual: https://www.epgsystems.eu/downloads-install-files-manuals/category/7-hard-software).
- Plants for EPG should be well watered the day before the experiment. Place the Arabidopsis plants in the Faraday cage on a non-conductive surface (Figures 10A and 10B). Examples of non-conductive surfaces include Petri-dishes, cardboard from shipping containers or pieces of styrofoam cut to the appropriate size to be placed under the pots.
- Important considerations
To obtain data with minimal variance between samples, the following considerations should be taken into account.- Optimal EPG Recording time: For GPA feeding on Arabidopsis, the optimal recording time is 8 h. Longer recording times are possible. Pilot experiments are necessary to accurately determine the duration of EPG recordings.
- Age of plants: Ensure that the age of the plants is as close as possible. In order to achieve this, follow staggered planting so that plants for the experiments are of uniform age.
- Site of aphid feeding: If possible and your EPG set-up allows for it, place the aphids connected to the insect electrode on the same-aged leaf of each plant i.e., avoid placing aphids on leaves of different ages.
- Time of day: Aphids exhibit circadian rhythm like any other living organism and aphid feeding behavior may vary with time of day. Avoid setting up and running EPG experiments during the night. Endeavor to run all of your experiments at the same time of the day.
- Number of replications: The appropriate number of replicates should be such that they compensate for behavior variability among individual aphids. Twenty successful replicates are ideal, but a minimum of 15 is essential for all experiments.
- Optimal EPG Recording time: For GPA feeding on Arabidopsis, the optimal recording time is 8 h. Longer recording times are possible. Pilot experiments are necessary to accurately determine the duration of EPG recordings.
- Analysis of EPG waveforms
The Stylet+ Analysis (Ana) is used for EPG waveform analysis. Feeding by the GPA on Arabidopsis plants reveals the presence of nine distinct waveforms: A, B, C (Pathway phase), pd (Potential drops), E1 and E2 (Phloem phase or Sieve element phase, SEP), G (Xylem phase), F (Derailed stylet phase) and NP (non-probing) (Figure 11). Further details on the characteristics of the waveforms can be found at: https://www.epgsystems.eu/downloads-install-files-manuals/category/4-epg-data-processing.- Descriptions of Waveforms
- During the EPG experiment, periods of inactivity when no stylet penetration (Figure 11) occurs and the aphid stylet is retracted and not located in any host tissue can be observed. This period is referred to as non-probing phase (NP).
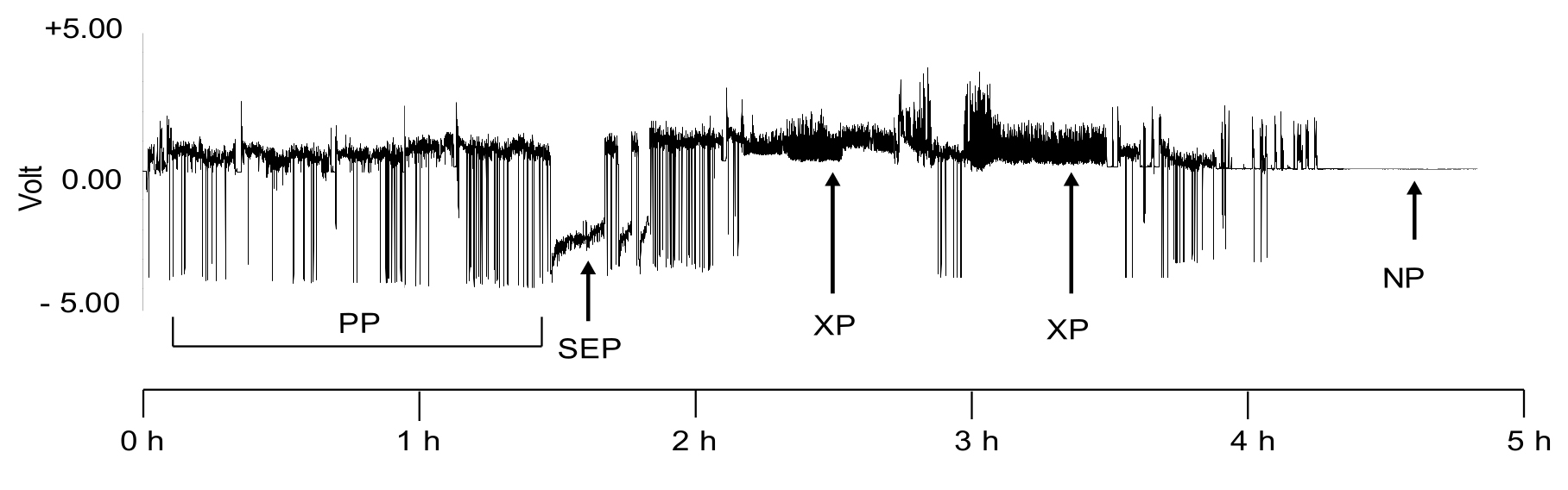
Figure 11. A representative EPG waveform pattern of GPA feeding on Arabidopsis wild-type accession Columbia plant over a 5 h period. The Phloem or sieve element phase (SEP), Xylem Phase (XP); Pathway Phase (PP), and Non-probing phase (NP) are identified. - The waveforms A, B and C (Figure 12A) represent the location of the stylet in the epidermis and mesophyll tissues. The A waveform indicates the presence of the aphid stylet in the epidermis, the B waveform indicates the presence of the stylet in the epidermis or mesophyll and the C waveform represents the presence of the stylets in any tissue. The three waveforms overlap and are hard to tease apart. Therefore, they are lumped together as ‘pathway phase’ or ‘stylet pathway’ and are labeled as waveform C in EPG analysis (Figure 12A). Pathway phase (PP), or stylet pathway phase, is referred to as the phase of stylet penetration that is not the phloem or xylem phase and encompasses a variety of stylet penetration behaviors including intercellular stylet advancement and withdrawal, and brief intracellular punctures by the stylet tips. It is during this probing phase that the insect attempts to locate its primary ingestion site (i.e., the sieve element), and makes decisions regarding host acceptance or rejection.
- Potential drops or pd represent the punctures of plant cells by the stylet (Figure 12A). The pd waveform can be further sub-divided into II-1, II-2 and II-3 (Figure 12B). These characterizations are important if the experiment involves studying the inoculation of persistent viruses by the aphids. These waveforms are not required to be labeled if not required by the research question.
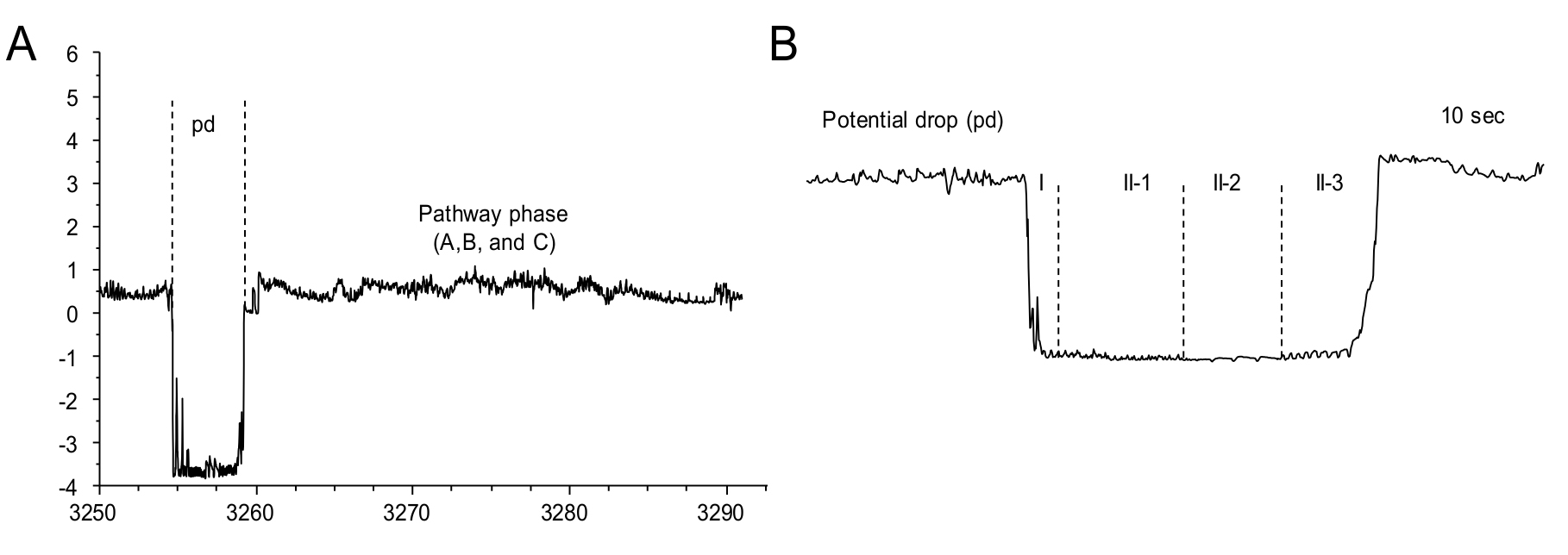
Figure 12. Aphid waveforms of the pathway phase including a potential drop. A. The pathway phase consists of A, B and C waveforms lumped into one category as C. B. A potential drop (pd) showing II with 3 sub-phases (II-1, II-2 and II-3). - SEP occurs when the stylet tips are in a phloem sieve element, which is the site of phloem sap ingestion by aphids. The SEP consists of E1 (Figures 13A and 13B) and E2 (Figures 13A and 13C). During E1, the aphid stylet is located in the sieve elements and the aphid is actively salivating, presumably in an effort to suppress host responses. During E2, the aphid stylet is located within the sieve element and the aphid is ingesting phloem sap.
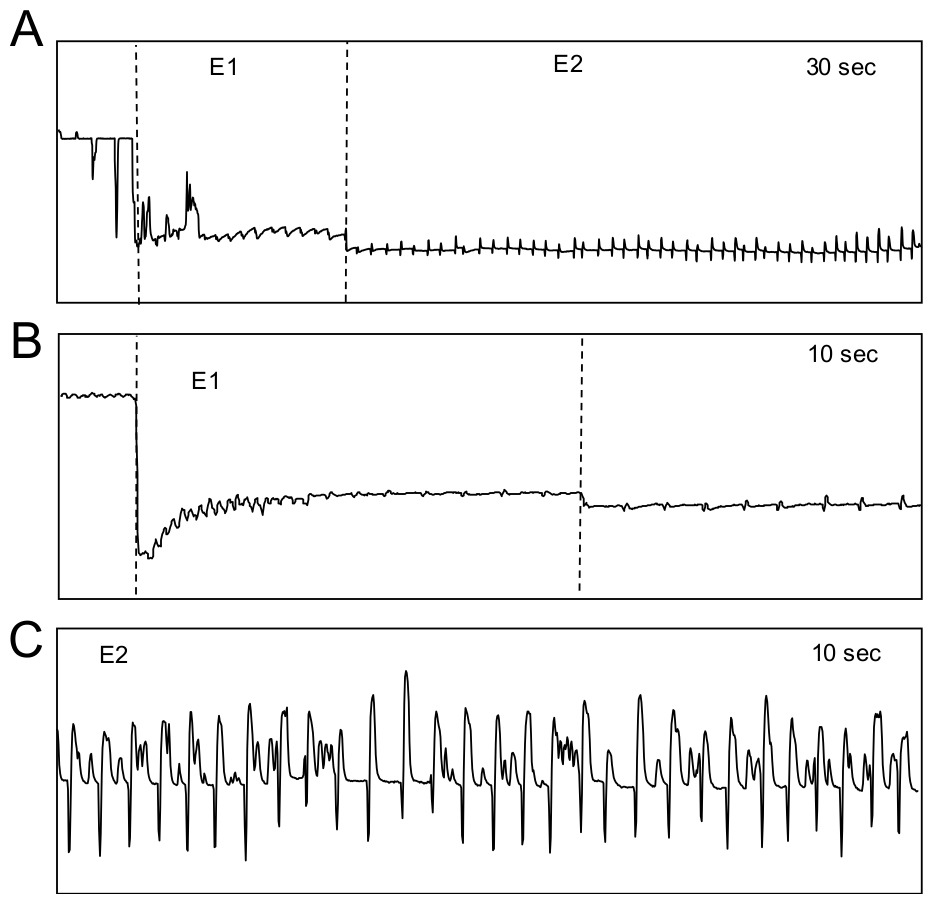
Figure 13. Aphid waveforms of the Sieve element phase (SEP). A. SEP phase showing E1 and E2; B-C. Expanded view of E1 and E2, respectively. - During the G waveform (Figure 14), the aphid stylet is located in the xylem and the aphid is ingesting xylem sap.
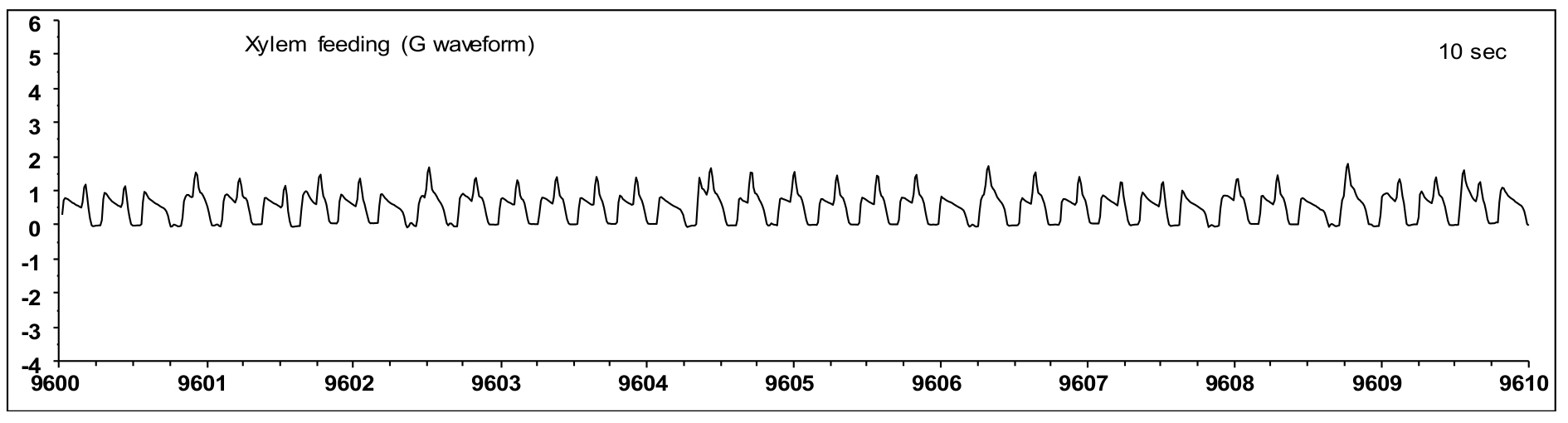
Figure 14. Aphid waveforms of the xylem feeding/ingestion phase (G) - The waveform F refers to derailed stylets (Data not shown), meaning the aphid stylet is encountering penetration difficulties. During this phase, the aphid stylet is located in the host tissue but due to difficulties in stylet mechanics, the aphid is not involved in feeding behaviors.
- During the EPG experiment, periods of inactivity when no stylet penetration (Figure 11) occurs and the aphid stylet is retracted and not located in any host tissue can be observed. This period is referred to as non-probing phase (NP).
- Waveform analysis
- The identification of various waveforms are performed visually and labeling the waveforms is performed using the Stylet+ software (detailed methodology to use the software can be found at: https://www.epgsystems.eu/downloads-install-files-manuals/category/7-hard-software).
- Stylet+ allows the user to create analysis grids and store the analysis data in “.ana” files that are required for downstream data processing.
- If waveform analysis reveals that an aphid spent > 70% of the recording time in Np+F+G activities, discard that aphid.
- The identification of various waveforms are performed visually and labeling the waveforms is performed using the Stylet+ software (detailed methodology to use the software can be found at: https://www.epgsystems.eu/downloads-install-files-manuals/category/7-hard-software).
- Descriptions of Waveforms
- Calculation of EPG events
The length of EPG events and the complexity of the parameters used to explore insect feeding behavior make the analysis of EPG data a time-consuming process. The ability to automatically calculate a large number of EPG parameters greatly reduces the time involved, increases efficiency and provides an accurate view of aphid insect probing and feeding behavior. The availability of several published programs: Backus 1.0 (Backus et al., 2007), Sarria Workbook version 4.4.3 (Sarria et al., 2009), EPG-Calc 6.1 (Giordanengo, 2014), Excel macro JKL 2.0 (www.epgsystems.eu) and Ebert 1.0 (Ebert et al., 2015) are valuable for accelerating the speed and accuracy of processing the large amount of data generated during EPG experiments. The choice of the program that researchers choose for data analysis depends on the experimental setup, insect-plant system and accessibility to the software tools. Ebert et al. (2015) provide a comprehensive review of three of the above-listed programs (Backus 1.0, Sarria Workbook version 4.4.3 and Ebert 1.0).
Data analysis
The data obtained from EPG recordings is considered non-parametric data since they do not conform to the assumptions of one-way ANOVA and must therefore be analyzed using appropriate statistical tests or the data can be subjected to transformation to meet the assumptions of ANOVA. Two methods of analysis are appropriate:
- Data analysis without transformation: The different parameters obtained from EPG analysis worksheets listed in G are first compared using the Kruskal-Wallis test, which is a distribution-free test. Parameters that show significant differences (α = 0.05) between treatments (if more than two) can be further evaluated using the Mann-Whitney U (MWU) test. MWU is used to compare differences between two treatments and therefore, repeated MWU tests have to be carried out between all treatments to determine significant differences (α = 0.05).
For example, if your EPG experiment consists of three treatments: T1, T2 & T3, and the Kruskal-Wallis test indicates that there are significant differences between the three treatments for parameter X (P ≤ 0.05), then to identify which treatments are significantly different from each other, the MWU tests are carried out T1 vs. T2, T1 vs. T3 and T2 vs. T3. - Data analysis with Rank transformation: In order to use ANOVA to analyze the different parameters obtained from the EPG recordings, the data can be rank transformed. Rank transformation means that the values of the activity of every treatment for each parameter are arranged in rank order. In order to carry out this transformation, the data for each parameter for all treatments is arranged in a sequential manner using the RANK.AVG function in MS Excel. This function returns the rank of a number in a list of numbers and if more than one value has the same rank, it returns the average rank. The rank-transformed data for each parameter can then be analyzed using ANOVA (either is SAS or Minitab) to determine differences between treatments. Parameters that show a P-value of ≤ 0.05 can be subjected to a post-hoc test such as Tukey’s Honest Significant Difference test to determine the difference between means of the different treatments.
Note: Ebert 1.0 is a SAS program that provides both data compilation and statistical analysis of EPG variables. Data analysis is performed using a mixed model analysis of variance (Ebert et al., 2015).
Notes
- Arabidopsis plants should not be overwatered. Overwatering increases chances of algal and fungal growth in soil. It is best to sub-irrigate only when the soil surface shows the first sign of drying. This will also facilitate healthy growth of Arabidopsis.
- Maintain a back-up of the GPA colony in a separate growth chamber to minimize the chance of losing the colony due to equipment malfunction. Periodically, clean up the growth chamber to remove dead insects and debris. Further, wipe the interior walls and shelving clean with a wet cloth followed by 70% ethanol to get rid of aphid honeydew that might have collected in the chamber.
- Under our experimental conditions, we have found that in order to obtain 20 successful replicates for data analysis of aphid feeding behavior, for each treatment at least twice that number of aphids have to wired and connected to the Giga 8d and a plant.
- Wiring the insect is not difficult, but requires patience and practice. In order to connect the adult aphid to the insect electrode, any variation of the procedure can be used. For instance, Video 2 shows an alternate method of connecting an aphid to the insect electrode. The important point is to ensure that the glue and the gold wire do not hinder aphid movement.
- Although it can be done manually, the accuracy and efficiency of calculating the EPG events can be greatly enhanced by using the various available programs Backus 1.0 (Backus et al., 2007), Sarria Workbook version 4.4.3 (Sarria et al., 2009), EPG-Calc 6.1 (Giordanengo, 2014), Excel macro JKL 2.0 (www.epgsystems.eu) and Ebert 1.0 (Ebert et al., 2015).
- Although the EPG protocol described here is for uninfested aphids, it can also be utilized to study the effect of viral infections on GPA feeding behavior.
Acknowledgments
We would like to thank Travis Isaacs and Sarah Moh for Video 1 and Video 2. Work in the Nalam laboratory was supported by faculty startup funds provided by Indiana University-Purdue University Fort Wayne. Work in the Louis laboratory was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Act (Accession # 1007272) through the USDA National Institute of Food and Agriculture. Work in the Shah laboratory was supported at varied times by grants from the National Science Foundation and the US Department of Agriculture. All the authors declare no conflict of interest.
References
- Backus, E. A., Cline, A. R., Ellerseick, M. R. and Serrano, M. S. (2007). Lygus hesperus, (hemiptera: Miridae) feeding on cotton: new methods and parameters for analysis of nonsequential electrical penetration graph data. Ann Entomol Soc Am 100(2): 296-310.
- Blackman, R. L., and Eastop, V. F. (2000). Aphids on the world’s crops: an identification and information guide. Chichester: John Wiley and Sons.
- Ebert, T. A., Backus, E. A., Cid, M., Fereres, A. and Rogers, M. E. (2015). A new SAS program for behavioral analysis of electrical penetration graph data. Comput Electron Agr 116(C): 80-87.
- Giordanengo, P. (2014). Epg-Calc: a PHP-based script to calculate electrical penetration graph (EPG) parameters. Arthropod-Plant Inte 8(2): 163-169.
- Kennedy, J. S., Day, M. F., and Eastop, V. F. (1963). A conspectus of aphids as vectors of plant viruses. London: Commonwealth Institute of Entomology pp: 114.
- Louis, J. and Shah, J. (2013). Arabidopsis thaliana-Myzus persicae interaction: shaping the understanding of plant defense against phloem-feeding aphids. Front in Plant Sci 4: 213.
- Louis, J., Singh, V. and Shah, J. (2012). Arabidopsis thaliana-aphid interaction. Arabidopsis Book 10: e0159.
- Matthews, R. E. F. (1991). Relationships between plant viruses and invertebrates. Plant Virology (3rd edition). In: Matthews, R. E. F. (Ed.) San Diego, CA: Academic Press pp: 520-561.
- Nalam, V., Louis, J. and Shah, J. (2018). Plant defense against aphids, the pest extraordinaire. Plant Sci
- Salvador-Recatalà, V. and Tjallingii, W. F. (2015). A new application of the electrical penetration graph (EPG) for acquiring and measuring electrical signals in phloem sieve elements. J Vis Exp (101): e52826.
- Sarria, E., Cid, M., Garzo, E. and Fereres, A. (2009). Excel workbook for automatic parameter calculation of EPG data. Comput Electron Agr 67(1-2): 35-42.
- Tjallingii, W. F. (1985). Electrical nature of recorded signals during stylet penetration by aphids. Entomol Exp Appl 38(2): 177-186.
- Tjallingii, W. F. (2006). Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57(4): 739-745.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Nalam, V., Louis, J., Patel, M. and Shah, J. (2018). Arabidopsis-Green Peach Aphid Interaction: Rearing the Insect, No-choice and Fecundity Assays, and Electrical Penetration Graph Technique to Study Insect Feeding Behavior. Bio-protocol 8(15): e2950. DOI: 10.21769/BioProtoc.2950.
Category
Plant Science > Plant immunity > Plant-insect interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












