- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Long-term in vitro Culture of Cryptosporidium parvum
Published: Vol 8, Iss 15, Aug 5, 2018 DOI: 10.21769/BioProtoc.2947 Views: 9337
Reviewed by: Adler R. DillmanMichael ArrowoodSmita Nair

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sex-specific Separation of Plasmodium falciparum Gametocyte Populations
Melanie C. Ridgway [...] Alexander G. Maier
Jun 5, 2021 3890 Views

Plasmodium cynomolgi Berok Growth Inhibition Assay by Thiol-reactive Probe Based Flow Cytometric Measurement
Jessica Jie Ying Ong [...] Jin-Hee Han
Sep 5, 2021 3452 Views

Babesia duncani in Culture and in Mouse (ICIM) Model for the Advancement of Babesia Biology, Pathogenesis and Therapy
Vandana Kumari [...] Choukri Ben Mamoun
Nov 20, 2022 1929 Views
Abstract
Continuous in vitro growth of Cryptosporidium parvum has proved difficult and conventional in vitro culture techniques result in short-term (2-5 days) growth of the parasite resulting in thin-walled oocysts that fail to propagate using in vitro cultures, and do not produce an active infection using immunosuppressed or immunodeficient mouse models (Arrowood, 2002). Here we describe the use of hollow fiber bioreactors (HFB) that simulate in vivo conditions by providing oxygen and nutrients to host intestinal cells from the basal surface and permit the establishment of a low redox, high nutrient environment on the apical surface. When inoculated with 105 C. parvum (Iowa isolate) oocysts the bioreactor produced 108 oocysts per ml (20 ml extra-capillary volume) after 14 days, and was maintained for over 2 years. In vivo infectivity studies using a TCR-α-immune deficient mouse model showed that oocysts produced from the bioreactor at 6, 12 and 18 months were indistinguishable from the parent Iowa isolate used to initiate the culture. HFB produced oocysts had similar percent excystation profiles to the parent Iowa isolate.
Keywords: ParasitologyBackground
Cryptosporidium parvum is an intracellular obligate parasite of the intestinal tract of man and other mammals resulting in an acute diarrhea. The disease is self-limiting in immunocompetent individuals, however, in immunocompromised adults and young children, the disease can be life-threatening (Kotloff, 2017). It is amongst the top three diagnosed enteric diseases of children in economically low resource countries (Kotloff et al., 2013; Sow et al., 2016), and is estimated to account for 9% of child deaths globally (Bhutta and Black, 2013; Checkley et al., 2015). In countries with a high burden of pediatric diarrheal disease, it has been shown that there is a correspondingly high incidence of malnutrition, stunting, and impaired cognitive functions (Lang and MA-LED Network Investigators, 2015). Despite the significant health risks and global distribution of this disease, there is no consistently effective therapy for the most-at-risk population. Recent advances in manipulating the parasite genome (Vinayak et al., 2015) and chemotherapeutic profiling (Arnold et al., 2017; Love et al., 2017; Manjunatha et al., 2017) are an encouraging sign that this bleak situation will change. Culture of the parasite has concentrated on developing a 3D culture system using adult murine colon cells (Baydoun et al., 2017) or novel bioengineered human intestinal cells (DeCicco RePass et al., 2017) which has produced novel insights into parasite invasion and significantly extended the length of culture time compared to the 2D culture method. However, these techniques are limited in terms of parasite numbers obtained. The 3D models overcome the major obstacle associated with conventional 2D culture methods where host cells receive nutrients and oxygen from the apical surface (except for those systems that use porous membrane inserts like the Costar Transwell system); this is contrary to the in vivo situation where the enterocytes receive nutrients and oxygen from the basal surface and the apical surface faces the lumen of the gut. However, current intestinal implant models fail to provide the low oxygen environment present inside the gut lumen which restricts the long-term growth of the parasite. The use of hollow fiber technology allows the creation of the biphasic environment present in the gut and overcomes many problems associated with the long-term culture of C. parvum (Morada et al., 2016). This protocol describes the establishment of hollow fiber bioreactors that can be used to simulate in vivo conditions by providing oxygen and nutrients to the basal surface of host intestinal cells that are attached to the outside of the hollow fibers (Figure 1). The environment inside the reactor is adjusted to mimic the lumen of the gut hence the apical surface of the intestinal cells are established in a low redox, high nutrient environment, that favors high growth rates and long-term maintenance of C. parvum. The use of this method provides 108-109 oocysts which can be used for molecular and biochemical studies and has the advantage of avoiding the use of harsh chemicals such as K-dichromate, which is used as a long-term storage medium at (4 °C) and has the advantage of sanitizing the oocysts; and chlorine currently used as both a sanitizer and to enhance excystation of oocysts obtained from animal sources (Arrowood, 2008).
Figure 1. Hollow Fiber Bioreactor. A. Schematic representation of the HFB; B. HFB setup.
Materials and Reagents
- BrandTechTM BRANDTM reagent reservoirs (BrandTech Scientific, catalog number: 703459 )
- Aluminum foil 18 in x 500 ft (Sigma-Aldrich, catalog number: Z185159-1EA )
- BD syringe with various tips, 60 ml (BD, catalog number: 309653 )
- BD Diagnostic Systems SYR only Luer-LokTM 10 ml 200PK RX (Thermo Fisher Scientific, catalog number: B302995)
Manufacturer: BD, catalog number: 302995 . - BD Disposable syringes with Luer-LokTM Tips, 3 ml (BD, catalog number: 309657 )
- FisherbrandTM sterile alcohol prep pads (Fisher Scientific, catalog number: 22-363-750 )
- BD VacutainerTM general use syringe needles (BD, catalog number: 305180 )
- FisherbrandTM borosilicate glass disposable serological pipets with regular tip, standard length, 5 ml (Fisher Scientific, catalog number: 13-678-27E )
- FisherbrandTM borosilicate glass disposable serological pipets with regular tip, standard length, 10 ml (Fisher Scientific, catalog number: 13-678-27F )
- FisherbrandTM borosilicate glass disposable serological pipets with regular tip, short length, 25 ml (Fisher Scientific, catalog number: 13-678-36D )
- EMD MilliporeTM MillexTM-GP sterile syringe filters with PES membrane (Fisher Scientific, catalog number: SLGP033RS)
Manufacturer: Merck, catalog number: SLGP033RS . - CorningTM polyethylene terephthalate (PET) centrifuge tubes, 15 ml (Corning, catalog number: 430055 )
- CELLSTARTM Greiner Bio-OneTM TC treated cell culture flasks, 250 ml (Greiner Bio One International, catalog number: 658175 )
- FisherbrandTM premium microcentrifuge tubes, 1.5 ml (Fisher Scientific, catalog number: 05-408-129 )
- FisherbrandTM low-retention pipet tips–filtered, 10 μl (Fisher Scientific, catalog number: 02-717-158 )
- FisherbrandTM low-retention pipet tips–filtered, 20 μl (Fisher Scientific, catalog number: 02-717-161 )
- FisherbrandTM low-retention pipet tips–filtered, 200 μl (Fisher Scientific, catalog number: 02-717-165 )
- QuibitTM assay tubes (Thermo Fisher Scientific, InvitrogenTM, catalog number: Q32856 )
- MicroAmpTM optical 96-well reaction plate with barcode (Thermo Fisher Scientific, Applied BiosystemsTM, catalog number: 4306737 )
- MicroAmpTM optical adhesive film (Thermo Fisher Scientific, Applied BiosystemsTM, catalog number: 4311971 )
- Filters, bottle top 1 L (Thermo Fisher Scientific, catalog number: 597-4520 )
- DynabeadsTM MPCTM-S magnetic particle concentrator (Thermo Fisher Scientific, catalog number: A13346 )
- DynabeadsTM MPCTM-6 magnetic particle concentrator (Thermo Fisher Scientific, catalog number: 12002D )
- DynabeadsTM L10 tubes (Thermo Fisher Scientific, catalog number: 74003 )
- VWR® 8-425 screw thread vials (VWR, Avantor, catalog number: 66020-950 )
- Cap screw top black, with PTFE/silicone pre-slit septa, 10 mm, packed in a clean environment (PerkinElmer, catalog number: N9306052 )
- C. parvum 18S-rRNA primers: Cp18S-995F: 5’-TAGAGATTGGAGGTTCCT-3’ and Cp18S-1206R: 5’-CTCCACCAACTAAGAACGCC-3’ (Thermo Fisher Scientific, Waltham, MA, USA)
- Human 18S-rRNA primers: Hs18S-F1373: 5’-CCGATAACGAACGAGACACTCTGG-3’ and Hs18S-R1561: 5’-TAGGGTAGGCACACGCTGAGCC-3’ (Thermo Fisher Scientific, Waltham, MA, USA)
- Quant-iTTM Qubit RNA BR assay kit (Thermo Fisher Scientific, InvitrogenTM, catalog number: Q10211 )
- RNeasy mini kit, 250 (QIAGEN, catalog number: 74106 )
- RNase-free DNase set, 50 (QIAGEN, catalog number: 79254 )
- iScriptTM RT-qPCR sample preparation reagent (Bio-Rad Laboratories, catalog number: 1708898 )
- Luna® Universal one step RT-qPCR kit protocol (New England BioLabs, catalog number: E3005 )
- Sterile phosphate buffered saline (Thermo Fisher Scientific, GibcoTM, catalog number: 10010049 )
- Minimum Essential Medium (MEM) with L-glutamine and phenol red, without HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 11095-114 )
- Horse Serum, New Zealand origin (Thermo Fisher Scientific, GibcoTM, catalog number: 16050122 )
- UltraPureTM DNase/RNase-free distilled water (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10977015 )
- Ethanol, 70% (Sigma-Aldrich, catalog number: 1009744000)
Manufacturer: Merck, catalog number: 100974 . - Roche DiagnosticsTM glucose test strips for Accutrend® Plus meters (Fisher Scientific, catalog number: 22-045-871)
Manufacturer: Roche Diagnostics, catalog number: 11447475160 - Crypt-a-GloTM reagent only kit (Waterborne, catalog number: A400FLR-20 )
- Sporo-GloTM (Waterborne, catalog number: A600FLR-20X )
- Trypsin-EDTA (0.25%), phenol red (Thermo Fisher-Scientific, GibcoTM, catalog number: 25200056 )
- L-glutamine (Sigma-Aldrich, catalog number: G3126 )
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- D-glucose (Sigma-Aldrich, catalog number: G8270 )
- Ascorbic acid (Sigma-Aldrich, catalog number: A0278 )
- P-Aminobenzoic acid (Sigma-Aldrich, catalog number: A9878 )
- Calcium pantothenate (Sigma-Aldrich, catalog number: C8731 )
- Folic acid (Sigma-Aldrich, catalog number: F7876 )
- Heparin (Sigma-Aldrich, catalog number: H3393 )
- Antibiotic/antimycotic 100x (Thermo Fisher Scientific, catalog number: 15240062 )
- Taurodeoxycholate (Sigma-Aldrich, catalog number: T0557 )
- Thioglycolic acid (MP Biomedicals, catalog number: 102933 )
- Mannitol (Sigma-Aldrich, catalog number: M4125 )
- Glutathione (Sigma-Aldrich, catalog number: G6013 )
- Taurine (Sigma-Aldrich, catalog number: T8691 )
- Betaine hydrochloride (Sigma-Aldrich, catalog number: B3501 )
- Cysteine (Sigma-Aldrich, catalog number: C7352 )
- Oleic acid (Sigma-Aldrich, catalog number: O1257-10MG )
- Cholesterol (Sigma-Aldrich, catalog number: C4951-30MG )
- DynabeadsTM anti-Cryptosporidium (Thermo Fisher Scientific, catalog number: 73011 )
- Nitrogen Medical Grade 99.998% purity (TW Smith, Brooklyn, NY)
- Carbon dioxide, Bone Dry, 99.998% (TW Smith, Brooklyn, NY)
- ECS medium mix (see Recipes)
- ICS medium mix (see Recipes)
Equipment
- 1 L Bottles, 33 mm cap (Fisher Scientific, catalog number: 0642114)
Manufacturer: DWK Life Sciences, catalog number: 61111T1000 . - 125 ml Bottles, 33 mm cap (DWK Life Sciences, catalog number: 219755 )
- Roche DiagnosticsTM glucose controls for Accutrend® Plus meters (Fisher Scientific, catalog number: 22-045-733)
Manufacturer: Roche Diagnostics, catalog number: 05213231160 . - HausserTM LevyTM hemacytometer chamber set (Hausser Scientific, catalog number: 3520 )
- FiberCellTM Systems Duet Pump (FiberCell Systems, catalog number: P3202 )
- Hollow Fiber Medium Cartridge (FiberCell Systems, catalog number: C2011 )
- UVP UV2 Sterilizing PCR Workstation (VWR, Avantor, Bridgeport, NJ 08014, USA)
- Class II, Type A, Biohazard Cabinet, model number 10276 (Envirco Cedar Grove, NJ 07009, USA)
- Beckman Coulter Microfuge® 16 centrifuge (Beckman Coulter, model: Microfuge® 16 )
- EppendorfTM centrifuge 5810R (Eppendorf, model: 5810 R )
- Quant Studio 6 Flex 44 (Applied Biosystems, Life Technology, Thermo Fisher Scientific, Waltham, MA, USA)
- Thermo Scientific Revco Ultima CO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA)
- DynabeadsTM MX mixer base (Thermo Fisher Scientific, catalog number: 15902 )
- QubitTM 3.0 fluorometer (Thermo Fisher Scientific, catalog number: Q33216 )
- NikonTM Optiphot fluorescence microscope (Nikon, Westbury, NY)
- Fisher Scientific Accumet® AR10 pH benchtop meter (Fisher Scientific, catalog number: 13-636-AB150 )
- Fisher Scientific dry bath incubator (Fisher Scientific, catalog number: 11-718-2 )
- Autoclave (LBR Scientific, Rutherford, NJ)
Software
- Quant Studio RT-PCR software v1.0 (Applied Biosystems, Life Technology, Thermo Fisher Scientific, Waltham, MA, USA)
- Sigma Plot v2001 (Systat Software, Inc, San Jose, CA, USA)
Procedure
- Cartridge preparation
- Sterilization of Reservoir Cap/Bottle Assembly
- Insert a 33 ml reservoir cap into the 125 ml bottle. Fit screw cap loosely.
- Attach fresh reservoir cap tube (non-sterile), included with the cartridge, to the cap.
- Loosely wrap the Luer fittings on the end of the reservoir cap tubing with foil.
- Place entire assembly into an autoclave bag and seal.
- Autoclave at 121 °C, 15 lb/in2 for 20 min (High Vacuum).
- Insert a 33 ml reservoir cap into the 125 ml bottle. Fit screw cap loosely.
- Connection of the reservoir cap to the Flow Path
- Remove Fibercell module from its packaging. Check the Luer fittings between the hollow fiber module and the flow path to ensure that they are finger tight.
- Remove the foil from one of the Luer fittings on the reservoir cap.
- Choose one of the Luer fittings on the flow path. Spray with 70% ethanol and wipe with an alcohol pad.
- Remove the Luer cap from the Luer fitting and attach the Luer fitting to the 33 ml reservoir cap. Apply ½ turn counter rotation to the tubing prior to attachment to prevent kinking of the tubing.
- Repeat steps to attach the other Luer fitting.
- Check fitting and be sure they are tightly attached.
- Remove Fibercell module from its packaging. Check the Luer fittings between the hollow fiber module and the flow path to ensure that they are finger tight.
- Sterilization of Reservoir Cap/Bottle Assembly
- Pre-culture
When working with adherent cells, the cartridge should be washed with 2-volumes of 125 ml of sterile PBS each for 24 h prior to filling the cartridge with 125 ml of cell culture medium.- Ensure the left and right end port side clamps are open. Aseptically fill 100 ml of sterile PBS into 125 ml bottle attach to cartridge using the 33 mm caps (The flow path and cartridge for medium cartridge holds about 30 ml of media).
- Use thumb and forefinger to manually prime the pump until air is pushed out of the tubing then close the left and right end port side clamps.
- Fill a sterile 60 ml syringe with 30 ml sterile PBS and attach to the left extra-capillary space (ECS) port and attach an empty 60 ml syringe to the right ECS port.
- Open the clamps on the left and right ECS side ports and push the sterile PBS into the ECS. Tilt cartridge upwards and fill medium taking care to remove all air present in the ECS. If the volume is not sufficient, repeat.
- Close the left and right ECS side ports, spray with 70% alcohol and remove the syringes, spray with 70% alcohol and wipe with an alcohol pad prior to attaching clean sterile 3 ml syringes to the ports. Open the left and right side port clamps.
- Connect the Fibercell hollow fiber module to the Fibercell pump unit in a 37 °C 5% CO2 incubator. Set a medium flow rate of 5, leave for 24 h.
- After 24 h, the left and right end port clamps are closed and the PBS reservoir bottle is aseptically changed for a fresh 125 ml bottle containing 100 ml of sterile PBS and returned to the incubator for 24 h.
- Aseptically fill a sterile 125 ml reservoir bottle with 100 ml of MEM media. Leave the reservoir cap loose by ½ turn.
- Fill a sterile 60 ml syringe with 30 ml sterile MEM and attach to the left ECS port and attach an empty 60 ml syringe to the right ECS port.
- Open the clamps on the left and right ECS side ports and push the sterile MEM into the ECS. Tilt cartridge upwards and fill medium taking care to remove all air present in the ECS. If the volume is not sufficient, repeat.
- Close the left and right ECS side ports, spray with 70% alcohol and remove the syringes, spray with 70% alcohol and wipe with an alcohol pad prior to attaching clean sterile 3 ml syringes to the ports. Open the left and right side port clamps.
- Connect the Fibercell hollow fiber module to the Fibercell pump unit in a 37 °C, 5% CO2 incubator. Set a medium flow rate of 5 and leave overnight.
- Repeat Steps B8-B12 using MEM plus 10% horse serum.
- Ensure the left and right end port side clamps are open. Aseptically fill 100 ml of sterile PBS into 125 ml bottle attach to cartridge using the 33 mm caps (The flow path and cartridge for medium cartridge holds about 30 ml of media).
- Inoculating Cells into the ECS (refer to Video 1)Video 1. Procedure for loading either host cells or C. parvum into the HFB
- Close the left and right end port slide clamps.
- HCT-8 monolayers are trypsinized using Trypsin-EDTA (0.25%), phenol red, washed with MEM and resuspended 1 x 106 in a total volume of 5 ml of ICS mix (see Recipe 2), and placed into a trough.
- Spray the left side port Luer connection with 70% ethanol and wipe with an alcohol pad. Remove syringes with an alcohol pad.
- Draw up cells using a 10 ml syringe with a blunt end needle.
- Following same aseptic procedure attach an empty syringe to the right side port Luer fitting.
- If closed, open the side port clamps.
- Gently flush the cell suspension through the ECS back and forth between the 2 syringes 3-5 times. This ensures distribution of the cells.
- Loosen the reservoir cap ½ turn.
- Leave the left end port slide clamp closed. Open the right-hand end port slide clamp. This will allow excess medium to flow into the reservoir bottle.
- Close the right side port slide clamp.
- Gently depress the plunger of the syringe attached to the left side port until all the medium and cells have been forced into the ECS.
- Close the left side port slide clamp and open the right side port slide clamp.
- Gently depress the plunger of the syringe attached to the right side port until all the medium and cells have been forced into the ECS.
- Close the side port slide clamps. Replace the syringes with new sterile syringes.
- Tighten the reservoir cap.
- Open the left end port slide clamp.
- Place the cartridge into the Fibercell pump inside a 37 °C, 5% CO2 incubator and set flow rate to 5. After 3 days, increase the flow rate to 10.
- Addition of C. parvum oocysts
Fresh MEM plus 10% HS is replaced daily. The pH and glucose concentration of the 48 h and fresh medium are monitored in the ECS using an Accumet® pH meter and an Accutrend Plus® glucose meter. When the glucose drops by 50% in 24 h, the ECS is seeded with 1 x 105 C. parvum oocysts that have been treated with 10% Chlorox®, washed 7x each with 30 ml sterile distilled water and suspended in 10 ml of MEM containing the supplements shown in Recipe 1 (ECS mix). The intra-capillary space (ICS) medium is replaced with that shown in Recipe 2 (ICS mix).- Remove the unit to a sterile hood and slide the L and R end port clamps to closed.
- Spray the left and right ECS ports with 70% alcohol using an alcohol pad and remove the syringe from the left ECS port.
- Attach the syringe containing the 10 ml of C. parvum oocysts suspended in the ECS mix to the left ECS port.
- Attach a clean sterile 10 ml syringe to the right ECS port.
- Open the slide clamps to the left and right ECS ports. Push down on the syringe attached to the left port (containing C. parvum oocysts), gently pull up on the right syringe.
- When the left syringe is fully depressed, push down on the right syringe to mix the contents of the ECS.
- Repeat the procedure (Steps 5 and 6) 3-4 times end up with both the left and right syringe approximately 50% full (5 ml in each).
- Close the right ECS port clamp, open the right end port slide clamp. Push down on the syringe attached to left ECS port until the contents have been added to the ECS (Take care not to add air to the cartridge). Slide the left ECS port clamp closed. Open the right ECS slide clamp and push down on the syringe until the contents of the syringe attached to this port have been added (Take care not to add air to the cartridge). Slide the right ECS port clamp closed. When inoculating the cartridge initially the user should experience minimal backpressure, after several months (> 6 months) of continuous culture an increase in backpressure can be experienced presumably due to build-up of the host-cell layer and debris from sloughed cells. When using the 3 ml ECS cartridge after a year of continuous culture, the backpressure is significant and it is not recommended for studies beyond this point. The 20 ml ECS cartridge was maintained for over 2 years without encountering this problem and is the cartridge of choice for long-term studies.
- Slide the left end port clamp to open. Spray the left and right ECS ports with 70% alcohol and use an alcohol pad to remove the syringes and replace with clean, sterile 10 ml syringes.
- Ensure both left and right end port clamps are closed and replace the reservoir bottle with 125 ml of the complete ICS medium mix (Recipe 2). Open both left and right end ports and return the cartridge to the pump. Set the flow rate to 10.
- The pH and glucose in the ICS medium are monitored daily, by removing 5 ml directly from the reservoir bottle. When the glucose concentration falls from 360 mg/dl to approx. 200 mg/dl in 24 h, change the reservoir for a 500 ml bottle of the complete ICS medium mix, containing 460 mg/dl of glucose.
- Continue to monitor the pH and glucose, and when the glucose concentration falls to 50% (200-230 mg/dl) in 24 h, change the reservoir for a 1 L bottle of the complete ICS medium mix, containing 500 mg/dl glucose.
- The pH and glucose are monitored every 48 h for the duration of the culture as an indicator of the integrity of the host cell layer.
- Remove the unit to a sterile hood and slide the L and R end port clamps to closed.
- Analysis of C. parvum growth by changes in Cp18S-rRNA
RNA Preparation:- Centrifuge a 50 μl sample, discard the supernatant and add 100 μl of iScript Buffer (lysis buffer).
- Freeze/thaw 6x by immersing in liquid nitrogen for 1 min followed by rapid thaw at 70 °C for 1.5 min.
- Centrifuge the lysate for 5 min at 16,162 x g (14,800 rpm).
- Carefully transfer the supernatant using a 200 μl pipette (DO NOT TOUCH THE PELLET) to a clean tube.
- Add 1 volume of 70% ethanol to the lysate, and mix well by pipetting. Do not centrifuge.
- Transfer up to 700 μl of the sample, including any precipitate, to an RNeasy Mini spin column placed in a 2 ml collection tube.
- Centrifuge for 15 sec at 8,000 x g (10,412 rpm). Discard flow through.
- Add 350 μl of Buffer RW1 to the column. Centrifuge for 15 sec at 8,000 x g (10,412 rpm).
- Add 10 μl DNase I stock solution to 70 μl buffer RDD. Mix gently by inverting the tube. Centrifuge for 15 sec at 8,000 x g (10,412 rpm).
- Add DNase I incubation mix (80 μl) from Step E9 to the column membrane and place on the benchtop for 15 min
- Add 350 μl buffer RW1 to the column. Centrifuge for 15 sec at 8,000 x g (10,412 rpm). Discard flow through.
- Add 500 μl buffer RPE to the column. Centrifuge for 15 sec at 8,000 x g (10,412 rpm) to wash the membrane. Discard flow through.
- Add 500 μl buffer RPE to the column. Centrifuge for 2 min at 8,000 x g (10,412 rpm). Discard flow through
- Place the column in a new 1.5 ml collection tube. Add 30-50 μl of RNase free water directly to the column membrane. Centrifuge for 1 min at 8,000 x g (10,412 rpm) to elute the RNA. Label tubes with sample description, date, and amount of water added.
- Either quantitate RNA immediately or store samples at -80 °C.
- RNA is quantitated using the broad range Qubit RNA assay kit using a Qubit 3.0 fluorometer according to the manufacturer’s instructions.
- Enumerate C. parvum by quantitating Cp18S-rRNA in HFB samples and compare it to a standard prepared using 106, 107 and 108 C. parvum oocysts. Carry out the procedure using 5 ng of RNA with Luna Universal One-Step® RT-qPCR kit (NEB E3005) employing C. parvum 18S-rRNA primers: (Cp18S-995F: 5’-TAGAGATTGGAGGTTCCT-3’ and Cp18S-1206R: 5’-CTCCACCAACTAAGAACGCC-3’) and compare it to human 18S-rRNA primers: (Hs18S-F1373: 5’-CCGATAACGAACGAGACACTCTGG-3’ and Hs18S-R1561: 5’-TAGGGTAGGCACACGCTGAGCC-3’). Perform the procedure according to the manufacturer’s instructions using a Quant Studio 6 Flex 44.
- The HFB reproducibly produces 5 x 107 to 108 oocysts per ml after 2 weeks of culture, and this is maintained if not more than 50% of the ECS volume is sampled every 7-10 days. Sampling 90% of the cartridge volume at one time resulted in 4-week recovery time to obtain 108 oocysts per ml.
- Stain oocysts with Crypt-o-Glo® according to the manufacturer’s instructions and evaluate using a NikonTM Optiphot fluorescence microscope.
- Stain motile stages with Spor-a-Glo® according to the manufacturer’s instructions and evaluate using a NikonTM Optiphot fluorescence microscope.
- Centrifuge a 50 μl sample, discard the supernatant and add 100 μl of iScript Buffer (lysis buffer).
- Tips to aid reproducibility
- Keep the inlet and outlet lines to the reservoir bottle behind the line used to prime the pump, this prevents kinking of the lines and reduces the chance of contamination when handling the cartridge.
- Use an alcohol pad to wrap around fittings when changing the syringes prevents contamination.
- If the glucose concentration of the reservoir bottle does not drop significantly after 48 h, replenish the host cells by adding 106 HCT-8 cells to the extra-capillary space.
- To achieve the consistent growth of C. parvum, it is important to maintain a low redox balance of the extra-capillary medium.
Note: To achieve this, we prepared the redox mix using sterile distilled water that is boiled and purged with 0.4 μm filtered N2 gas. When cooled to ambient temperature, thiols are dissolved under an N2 atmosphere. - Removal of 50% (10 ml) of the culture is possible by displacing the extra-capillary medium with an equal volume of fresh ECS medium. When displacing the cells depress the syringe gently (10 sec with a 10 ml syringe) so as not to cause turbulence. It is advised that the culture not be disturbed for 10-14 days post-sampling to enable repopulation of the culture.
- Keep the inlet and outlet lines to the reservoir bottle behind the line used to prime the pump, this prevents kinking of the lines and reduces the chance of contamination when handling the cartridge.
Data analysis
- Growth profile of total parasite stages from the HFB
- Samples (0.5 ml) were removed from the HFB and RNA isolated and analyzed by qRT-PCR using specific primers for C. parvum 18S-rRNA (Cp18S-995F: 5’-TAGAGATTGGAGGTTCCT-3’ and Cp18S-1206R: 5’-CTCCACCAACTAAGAACGCC-3’) and compared with HCT-8 18S-rRNA (Hs18S-F1373: 5’-CCGATAACGAACGAGACACTCTGG-3’ and Hs18S-R1561: 5’-TAGGGTAGGCACACGCTGAGCC-3’) to enumerate parasite number as described by Zhang and Zhu (2015). Parasite numbers increased from 5 x 103/ml on day one to 5 x 108/ml by Day 15 (Figure 2). Using Dynabeads to purify oocysts from the HFB sample we have obtained 109 oocysts from 10 ml of culture volume.
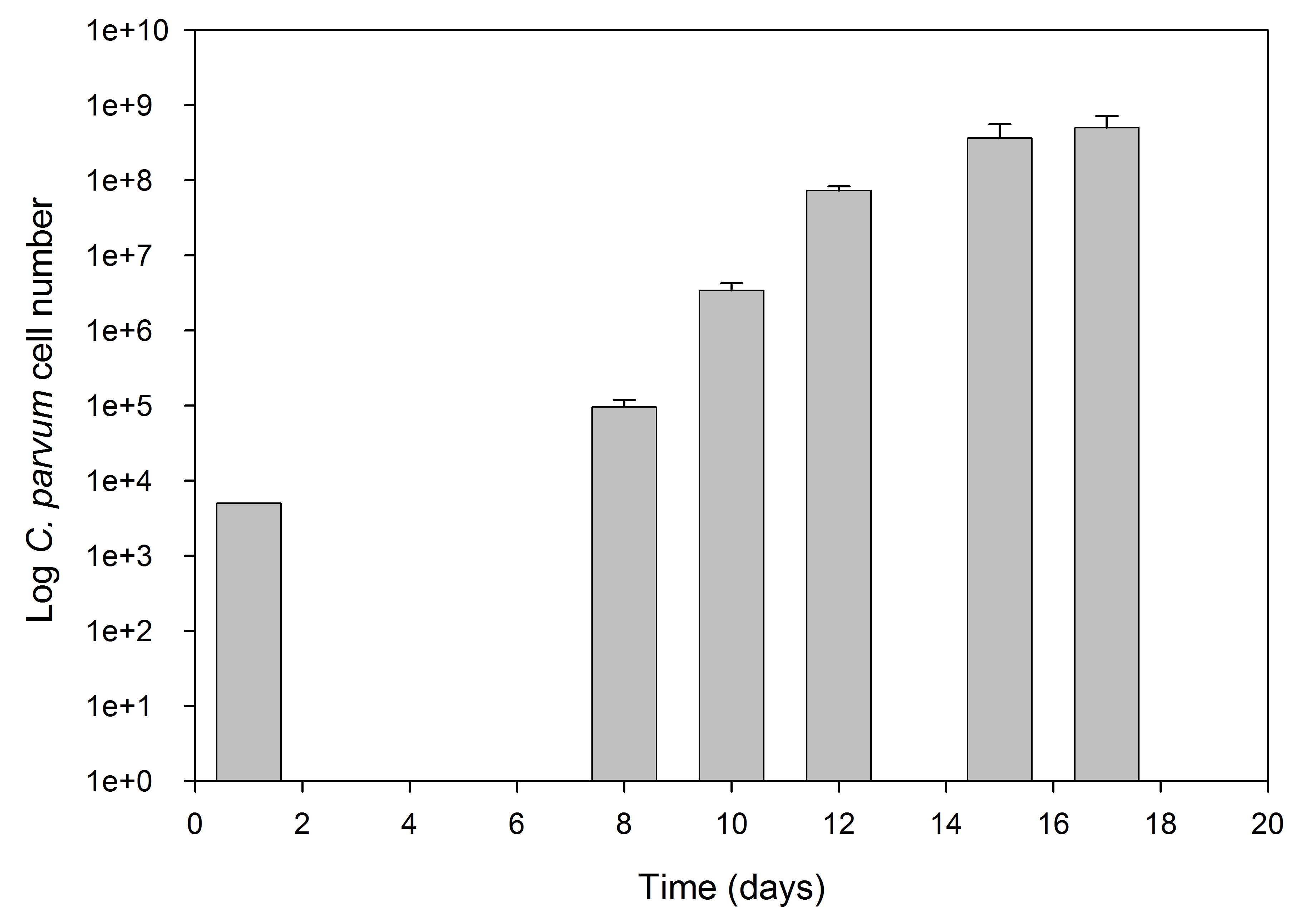
Figure 2. C. parvum growth curve in the HFB - C. parvum numbers are shown as Log of total parasite stages per ml. Parasite numbers were determined by qRT-PCR using primers for the Cp-18SrRNA as described in the procedure and the ΔCT values determined from a standard using 103, 104 and 105 oocysts (Zhang and Zhu, 2015). The culture was inoculated on day 1 with 5 x 103 oocysts/ml (105). Results are presented as triplicate counts ± SEM.
- Oocysts were stained with a monoclonal antibody to the Cryptosporidium oocyst wall protein conjugated to a fluorescent probe (Crypt-a-Glo®) and examined using a fluorescence microscope with an excitation wavelength of 410-485 nm and an emission wavelength of 515 nm (Figure 3A). Motile extracellular stages were stained using a polyclonal antibody conjugated to a red fluorescent probe (Sporo-Glo®) and examined using a fluorescent microscope with an excitation wavelength of 535-550 nm and an emission wavelength of 580 nm (Figure 3B).
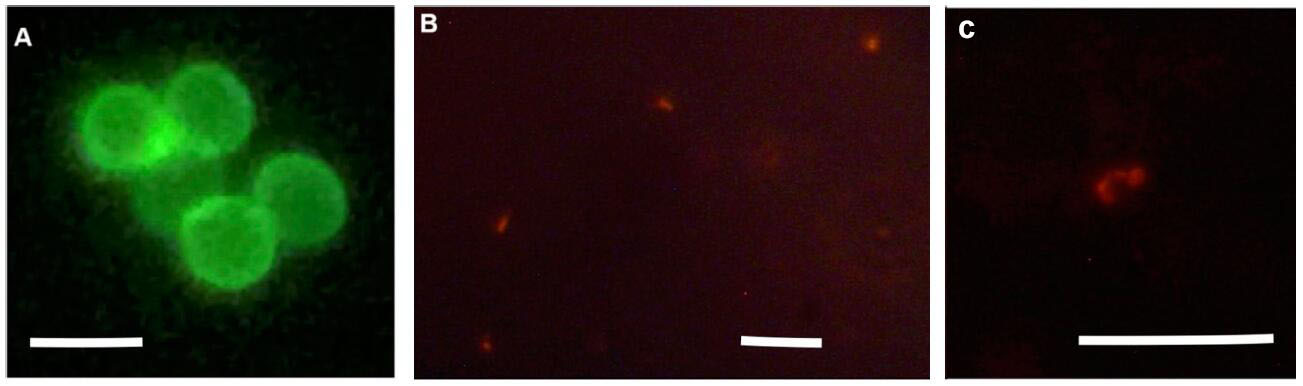
Figure 3. Microscopy of parasites collected from the HFB. A. Oocysts from the HFB stained using Crypt-a-Glo®; B and C. Motile stages from the HFB stained using Sporo-Glo®. Scale bars = 5 μm.
- Samples (0.5 ml) were removed from the HFB and RNA isolated and analyzed by qRT-PCR using specific primers for C. parvum 18S-rRNA (Cp18S-995F: 5’-TAGAGATTGGAGGTTCCT-3’ and Cp18S-1206R: 5’-CTCCACCAACTAAGAACGCC-3’) and compared with HCT-8 18S-rRNA (Hs18S-F1373: 5’-CCGATAACGAACGAGACACTCTGG-3’ and Hs18S-R1561: 5’-TAGGGTAGGCACACGCTGAGCC-3’) to enumerate parasite number as described by Zhang and Zhu (2015). Parasite numbers increased from 5 x 103/ml on day one to 5 x 108/ml by Day 15 (Figure 2). Using Dynabeads to purify oocysts from the HFB sample we have obtained 109 oocysts from 10 ml of culture volume.
- In vivo Infectivity of HFB produced C. parvum
Oocysts collected from the HFB culture were used to infect TCR-α-deficient mice to demonstrate the maintenance of virulence factors. Parasites cultured in 2D culture flasks for 48 h fail to cause an infection in either immunosuppressed or immune-deficient mouse models. Oocysts were collected from the HFB and purified using Dyna beads® according to the manufacturer’s instructions. Groups of three TCR--deficient mice on a C57BL/6 background (Jackson Laboratories, Bar Harbor, ME, USA) were orally dosed with 106 C. parvum oocyts (0.1 ml in PBS) from the HFB, and compared with 106 oocysts of the parent Iowa isolate (positive control) and mice dosed with 0.1 ml PBS (negative control). There is a statistical difference in weight gain of the C. parvum infected mice from 10 days onward compared to negative controls dosed with PBS (Figure 4A), indicative of an active C. parvum infection. Fecal pellets were collected and softened in PBS prior to smearing on microscope slides and staining by the modified cold Kinyoun acid-fast technique and enumerated microscopically at 400x using a confocal microscope (Nikon, Westbury, NY, USA). Oocysts were detected after 7-10 days and shedding increased daily up to 25 days (Figure 4B).
Figure 4. In vivo analysis of C. parvum oocysts from the HFB. A. Mouse model C. parvum infection. Triplicate groups of mice were infected with 0.1 ml by oral gavage containing 106 C. parvum oocysts suspended in PBS collected from the HFB , Six-week old C. parvum Iowa isolate (same source as used to initiate the culture)
, Six-week old C. parvum Iowa isolate (same source as used to initiate the culture)  , and compared to controls gavaged with 0.1 ml PBS
, and compared to controls gavaged with 0.1 ml PBS  . Mice were weighed daily. B. Oocyst shedding by HFB cultured mouse model infection. Mouse feces were softened with 0.1 ml distilled water and smeared onto a glass slide, stained using a modified cold Kinyoun acid-fast technique and oocysts counted microscopically at 400x magnification.
. Mice were weighed daily. B. Oocyst shedding by HFB cultured mouse model infection. Mouse feces were softened with 0.1 ml distilled water and smeared onto a glass slide, stained using a modified cold Kinyoun acid-fast technique and oocysts counted microscopically at 400x magnification. - Excystation of oocysts collected from the HFB
The excystation rate of C. parvum oocysts from the HFB was compared to control Iowa isolate oocysts as an index of oocyst viability. C. parvum oocysts were incubated in PBS, pH 7.4 containing 150 mg of Na taurocholate and 50 mg of trypsin at 37 °C for 30 min and dual stained using Crypt-a-Glo® and Sporo-Glo®. The percent of excysted oocysts was determined by counting oocysts and sporozoites using a hemacytometer using a fluorescent microscope (Nikon Optiphot) at 400x magnification. Crypt-a-Glo® stained oocysts were visualized using an excitation wavelength of 410-485 nm and an emission wavelength of 515 nm; Sporo-Glo® stained sporozoites (spz) were visualized using an excitation wavelength of 535-550 nm and an emission wavelength of 580 nm. The results show that approx. 80% of the oocysts collected from the HFB culture system excyst in 30 min, which is similar to the data obtained with the C. parvum parent Iowa isolate. Oocysts from both the HFB culture and the Iowa isolate stored at 4 °C demonstrated a gradual decrease in percent excystation with time of storage, falling to approx. 40% after 6 months (Figure 5).
Figure 5. Excystation of C. parvum oocysts. Oocysts were excysted in PBS, pH 7.4 containing 150 mg of Na taurocholate and 50 mg of trypsin at 37 °C for 30 min. The percentage of excysted oocysts determined by dual staining with Crypt-a-Glo® and Sporo-Glo® and counting using an Improved Neubauer Hemacytometer. The percent excystation is calculated from the following formula: spz count/4 x oocyst count x 100%. - Changes in glucose concentrations of HFB
The glucose concentration of the intra-capillary and extra-capillary space was measured as an indicator of host cell growth (Figure 6). When the medium glucose concentration falls to 50% of its starting concentration, the new medium must be replenished to prevent loss of host cells. The extra-capillary glucose concentration remains constant at approximately 275 mg/dl (Figure 6, dashed line).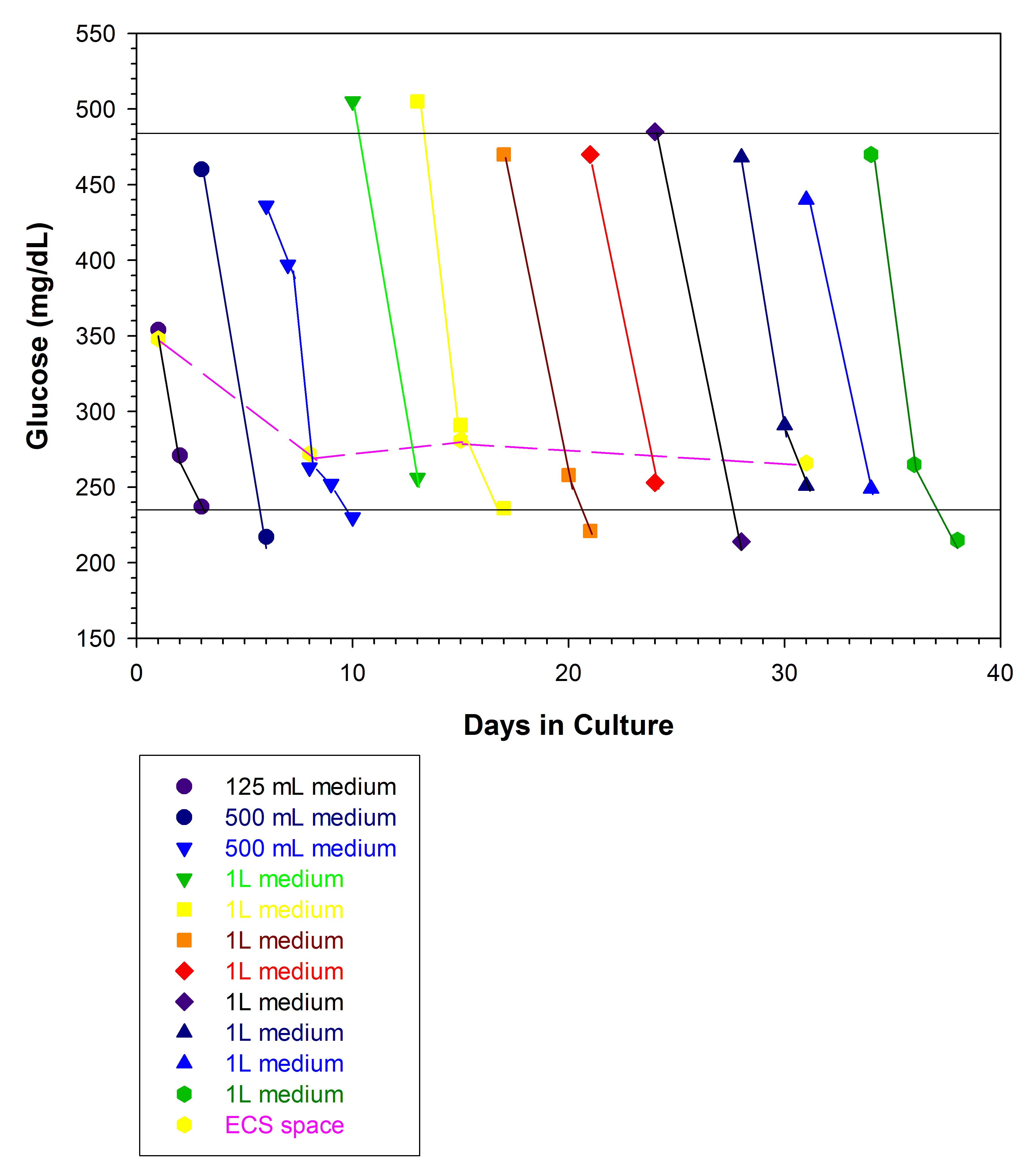
Figure 6. Glucose concentration of intra-capillary space medium. The glucose concentration of the medium providing nutrients to the host cells is monitored daily. The starting concentration is 350 mg/dl in a 125 ml flask which is changed to 425 mg/dl/500 ml flask on day 4. When the glucose concentration drops to 210-225 mg/dl in 48 h, (typically by Day 10) the glucose is increased to 475-500 mg/dl in a 1 L flask. The glucose concentration typically falls to 50% in 48 h, hence the medium is replenished on a Monday, Wednesday, Friday schedule thereafter. The glucose in the extra-capillary space, which contains the parasites, remains constant at 275 mg/dl.
Recipes
- ECS medium mix

Components are added and the medium is filter sterilized
*Prepared using nitrogen degassed water and stored in 1 ml aliquots under a nitrogen gas phase at -20 °C
#Stock kept at 4 °C - ICS medium mix

Components are added and the medium is filter sterilized
Acknowledgments
We would like to thank Dr’s Saul Tzipori and Sangun Lee (Cummings School of Veterinary Medicine, Tufts University, N. Grafton, MA, USA) for helpful advice with the in vivo animal studies. Dr’s Louis Weiss and Leslie Gunther-Cummins (Departments of Pathology and Medicine, and Analytical Imaging Facility, Albert Einstein College of Medicine, Bronx, NY, USA) for electron microscopy. This work was funded by the Bill and Melinda Gates Foundation (NY). The protocol is adapted from Morada et al. (2016). The authors declare no conflicts of interest or competing interests.
References
- Arnold, S. L. M., Choi, R., Hulverson, M. A., Schaefer, D. A., Vinayak, S., Vidadala, R. S. R., McCloskey, M. C., Whitman, G. R., Huang, W., Barrett, L. K., Ojo, K. K., Fan, E., Maly, D. J., Riggs, M. W., Striepen, B. and Van Voorhis, W. C. (2017). Necessity of bumped kinase inhibitor gastrointestinal exposure in treating Cryptosporidium infection. J Infect Dis 216(1): 55-63.
- Arrowood, M. J. (2002). In vitro cultivation of Cryptosporidium species. Clin Microbiol Rev 15(3): 390-400.
- Arrowood, M. J. (2008). In vitro cultivation. In: Cryptosporidium and Cryptosporidosis. Fayer, R. and Xiao, L. (Eds.). CRC Press, Boca Raton: 499-525.
- Baydoun, M., Vanneste, S. B., Creusy, C., Guyot, K., Gantois, N., Chabe, M., Delaire, B., Mouray, A., Baydoun, A., Forzy, G., Chieux, V., Gosset, P., Senez, V., Viscogliosi, E., Follet, J. and Certad, G. (2017). Three-dimensional (3D) culture of adult murine colon as an in vitro model of cryptosporidiosis: Proof of concept. Sci Rep 7(1): 17288.
- Bhutta, Z. A. and Black, R. E. (2013). Global maternal, newborn, and child health--so near and yet so far. N Engl J Med 369(23): 2226-2235.
- Checkley, W., White, A. C., Jr., Jaganath, D., Arrowood, M. J., Chalmers, R. M., Chen, X. M., Fayer, R., Griffiths, J. K., Guerrant, R. L., Hedstrom, L., Huston, C. D., Kotloff, K. L., Kang, G., Mead, J. R., Miller, M., Petri, W. A., Jr., Priest, J. W., Roos, D. S., Striepen, B., Thompson, R. C., Ward, H. D., Van Voorhis, W. A., Xiao, L., Zhu, G. and Houpt, E. R. (2015). A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis 15(1): 85-94.
- DeCicco RePass, M. A., Chen, Y., Lin, Y., Zhou, W., Kaplan, D. L. and Ward, H. D. (2017). Novel bioengineered three-dimensional human intestinal model for long-term infection of Cryptosporidium parvum. Infect Immun 85(3): e00731-16.
- Kotloff, K. L. (2017). The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am 64(4): 799-814.
- Kotloff, K. L., Nataro, J. P., Blackwelder, W. C., Nasrin, D., Farag, T. H., Panchalingam, S., Wu, Y., Sow, S. O., Sur, D., Breiman, R. F., Faruque, A. S., Zaidi, A. K., Saha, D., Alonso, P. L., Tamboura, B., Sanogo, D., Onwuchekwa, U., Manna, B., Ramamurthy, T., Kanungo, S., Ochieng, J. B., Omore, R., Oundo, J. O., Hossain, A., Das, S. K., Ahmed, S., Qureshi, S., Quadri, F., Adegbola, R. A., Antonio, M., Hossain, M. J., Akinsola, A., Mandomando, I., Nhampossa, T., Acacio, S., Biswas, K., O'Reilly, C. E., Mintz, E. D., Berkeley, L. Y., Muhsen, K., Sommerfelt, H., Robins-Browne, R. M. and Levine, M. M. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382(9888): 209-222.
- Lang, D. and MAL-ED Network Investigators. (2015). Opportunities to assess factors contributing to the development of the intestinal microbiota in infants living in developing countries. Microb Ecol Health Dis 26: 28316.
- Love, M. S., Beasley, F. C., Jumani, R. S., Wright, T. M., Chatterjee, A. K., Huston, C. D., Schultz, P. G. and McNamara, C. W. (2017). A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis. PLoS Negl Trop Dis 11(2): e0005373.
- Manjunatha, U. H., Vinayak, S., Zambriski, J. A., Chao, A. T., Sy, T., Noble, C. G., Bonamy, G. M. C., Kondreddi, R. R., Zou, B., Gedeck, P., Brooks, C. F., Herbert, G. T., Sateriale, A., Tandel, J., Noh, S., Lakshminarayana, S. B., Lim, S. H., Goodman, L. B., Bodenreider, C., Feng, G., Zhang, L., Blasco, F., Wagner, J., Leong, F. J., Striepen, B. and Diagana, T. T. (2017). A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 546(7658): 376-380.
- Morada, M., Lee, S., Gunther-Cummins, L., Weiss, L. M., Widmer, G., Tzipori, S. and Yarlett, N. (2016). Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 46(1): 21-29.
- Sow, S. O., Muhsen, K., Nasrin, D., Blackwelder, W. C., Wu, Y., Farag, T. H., Panchalingam, S., Sur, D., Zaidi, A. K., Faruque, A. S., Saha, D., Adegbola, R., Alonso, P. L., Breiman, R. F., Bassat, Q., Tamboura, B., Sanogo, D., Onwuchekwa, U., Manna, B., Ramamurthy, T., Kanungo, S., Ahmed, S., Qureshi, S., Quadri, F., Hossain, A., Das, S. K., Antonio, M., Hossain, M. J., Mandomando, I., Nhampossa, T., Acacio, S., Omore, R., Oundo, J. O., Ochieng, J. B., Mintz, E. D., O'Reilly, C. E., Berkeley, L. Y., Livio, S., Tennant, S. M., Sommerfelt, H., Nataro, J. P., Ziv-Baran, T., Robins-Browne, R. M., Mishcherkin, V., Zhang, J., Liu, J., Houpt, E. R., Kotloff, K. L. and Levine, M. M. (2016). The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 10(5): e0004729.
- Vinayak, S., Pawlowic, M. C., Sateriale, A., Brooks, C. F., Studstill, C. J., Bar-Peled, Y., Cipriano, M. J. and Striepen, B. (2015). Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 523(7561): 477-480.
- Zhang, H. and Zhu, G. (2015). Quantitative RT-PCR assay for high-throughput screening (HTS) of drugs against the growth of Cryptosporidium parvum in vitro. Front Microbiol 6: 991.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yarlett, N. and Morada, M. (2018). Long-term in vitro Culture of Cryptosporidium parvum. Bio-protocol 8(15): e2947. DOI: 10.21769/BioProtoc.2947.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









