- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluating Working Memory on a T-maze in Male Rats
Published: Vol 8, Iss 14, Jul 20, 2018 DOI: 10.21769/BioProtoc.2930 Views: 10270
Reviewed by: Edgar Soria-GomezArnau Busquets-GarciaShauna Parkes

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

The Traveling Salesman Problem (TSP): A Spatial Navigation Task for Rats
R. E. Blaser
Jun 5, 2018 5709 Views

Classic Labyrinth Test for Neurobehavioral Evaluation in Wistar Rats
Salim Gasmi
Sep 20, 2018 6876 Views

Consummatory Successive Negative Contrast in Rats
Ana María Jiménez-García [...] Ignacio Morón
Apr 5, 2019 5051 Views
Abstract
Working memory is short-term memory, so temporal improvement does not reflect the consolidation of a memory trace, rather the functionality of the underlying neuronal circuits and molecular signaling cascades. The administration of drugs–either one-time or through daily injection–can elucidate the underlying mechanisms. The T-maze is especially suitable for studying dopamine-dependent working memory, since it is less stressful than other tests, for example, water maze-based paradigms (Bezu et al., 2016 and 2017). Here, we present a training protocol for evaluating the underlying mechanisms that lead to the development of spatial working memory in rats. Our approach uses a T-maze, and it can be used to get high temporal resolution.
Keywords: LearningBackground
Spatial working memory is a short-time process where spatial information is encoded (Dudchenko, 2004) to influence subsequent behavior. Until now, only a few behavioral paradigms have been developed to test spatial working memory (Wenk, 2001). One of the most commonly used paradigms is the T-maze, which consists of a start arm and two arms arranged in a T-shape. In this paradigm, rats intrinsically tend to switch arm visits during consecutive trials, which suggests the rats remember the first arm that was visited, which is called “spontaneous alternation” (Lalonde, 2002). This tendency can be reinforced by baiting the arms with food when animals are mildly food deprived. Usually, protocols aim to train animals to reach a certain criterion of correct choices before pharmacological treatment starts or to accumulate the results over (Crawley and Goodwin, 1980; Deacon and Rawlins, 2006). We are interested in the role of the dopaminergic system in spatial learning and memory. For this reason, we treat animals with dopamine transporter (DAT) inhibitors, which block the reuptake of dopamine into the synapse and results in an increased concentration of extracellular dopamine. Further, the role of different dopamine receptors, like D1- and D2-like receptors, was elucidated through experiments that used receptor-specific agonists and antagonists. In this protocol, we use the T-maze for studying working memory, because this task is particularly sensitive to changes in the dopaminergic system compared with other working memory tests like water maze-based tasks (Bezu et al., 2017). Our overall goal is to synthesize of new compounds with high specificity to the target molecules and low side effects (Aher et al., 2016; Saroja et al., 2016; Sase et al., 2016; Hussein et al., 2017; Kristofova et al., 2018). Thus, we analyze a variety of brain structures involved in working memory processing, like the hippocampus, septum, basal forebrain, and prefrontal cortex (Chudasama and Robbins, 2004; Gruber et al., 2006) for regulation and modification of molecular signaling molecules and receptor complexes involved in memory processes (Baddeley, 1992). Rats were trained over a three day period, and we did not notice differences between pharmacologically treated rats and control rats that received additional training (up to six days) (Bezu et al., 2017).
Materials and Reagents
- Custom made T-maze, made of Acrylic glass GS black (Bilek + Schüll, Vienna, Austria)
- Male Sprague-Dawley rats (12-13 weeks old)
Note: They were bred and maintained in the Core Unit of Biomedical Research, Division of Laboratory Animal Science and Genetics, Medical University of Vienna. - Pharmacological agents
Note: They were applied in the experimental room since taking the animals to a different location causes novelty stress, that may potentiate the brief stress of intraperitoneal injection that we conducted. - Standard maintenance food (ssniff, R/M-H, Soest, Germany)
- Food reward: e.g., food pellets were provided (Dustless Precision Pellets®, Rodent purified diet, 45 mg; Bio-Serv, catalog number: F0021 )
- Incidin® Extra N (Ecolab, catalog number: 30 125 30 , PZN 002 357 95)
- 1% incidin (see Recipes)
Equipment
- Three lamps (LED chip, 20 W) for indirect illumination (mounted on a stand placed 1.3-1.5 m above the floor directed to the ceiling); Illumination within arms: 40-50 lux
- Recording camera (video camera, ABUS AUGUST BREMICKER SÖHNE KG, model: EyseoEcoLine, catalog number: TV7003 )
- T-maze
For the apparatus see Figure 1. Reward food pellets were placed outside the T-maze scattered over the table to mask olfactory cues during training. Visual cues (equipment, walls and doors, Figure 1B) were identifiable around the maze. Additional cues like paper printouts of black and white figures were placed on room walls two meters above the floor (Sánchez-Santed et al., 1997). The maze was cleaned with 1% Incidin after the training of each animal to remove olfactory cues. Indirect illumination (from floor to ceiling) provides equal light intensities (40-50 lux) in each arm. Trials were monitored with a camera (mounted on the ceiling directly above the maze- and videos stored on a PC. A freely available video capture program (Debut Video Capture) was used to store the videos on a computer. Paper printouts of figures (210 mm x 279 mm) placed at room walls and equipment served as visual cues.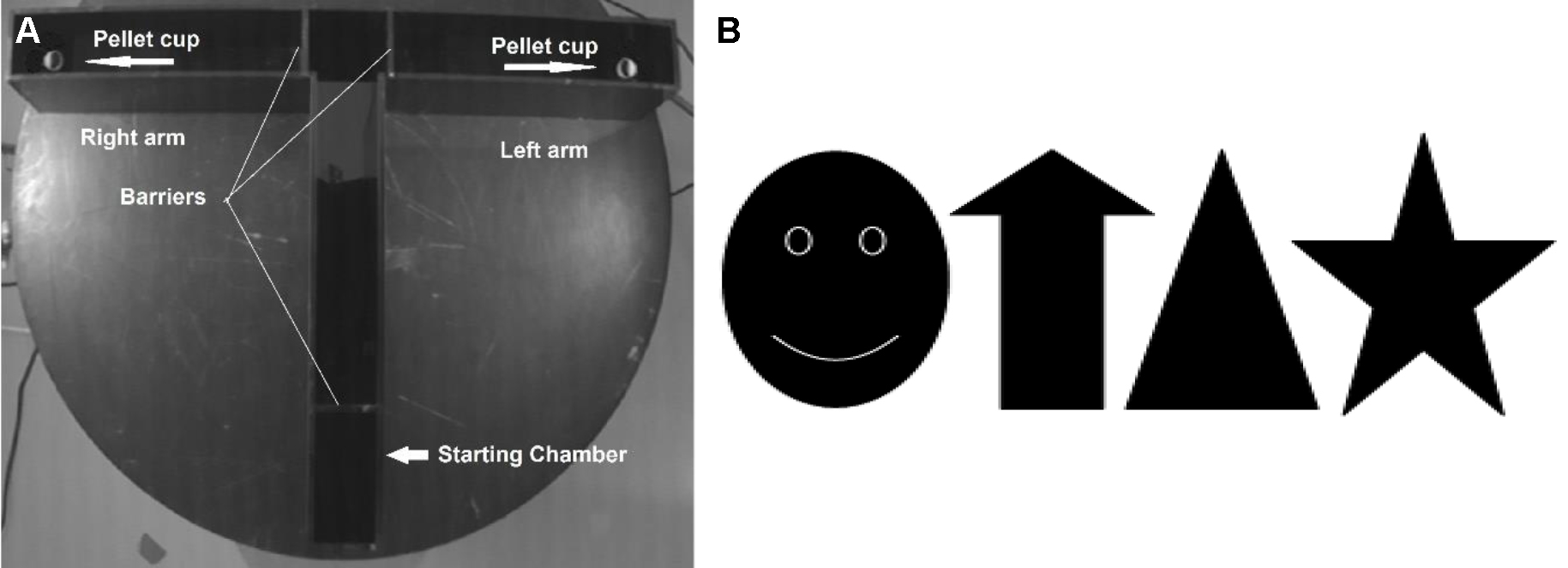
Figure 1. T-maze placed on a desk (A) and printouts of external cues placed on experimental room walls (B). Two-goal arms (50 cm long, 10 cm in width, with walls of 25 cm height) and the starting arm (70 cm) could be separated by a guillotine door (A). The maze was placed on a table with a height of 80 cm. The start arm was equipped with a starting box (20 cm in length) separated from the maze by a guillotine door. At the end of each goal arm, reward food pellets were provided in a small plastic cup (30 mm in diameter and 12 mm in height) to mask the food pellet.
Procedure
Note: All experiments are conducted according to the guidelines of the Ethics committee, Medical University of Vienna approved by the Federal Ministry of Education, Science and Culture, Austria. Code number: BMWFW-66.009/0206-WF/V/3b/2015.
- Housing
Rats were housed individually in standard Makrolon cages (type 4, 59 cm x 38 cm and 20 cm in height), the grounds of which were covered with commercial bedding material (autoclaved wood chips). Standard food (ssniff, R/M-H, Soest, Germany) and tap water were given ad libitum until the beginning of handling. The animals were adapted to the testing room place, with the same conditions as in the colony room (22 ± 2 °C; humidity: 55 ± 5%; 12 h artificial light/12 h dark cycle: light on at 7 AM). Rats were adapted to the experimental rooms for at least 24 h before starting the experiment and remained in the experimental room, throughout the entire duration of the experiment. - Animal handling
- All experimental procedures start at 9 AM in the light phase (overview in Figure 2).
- Pick the rats up by the body (not the tail), and let them set on the experimenter's arm. If the rat becomes agitated, then it should be released and returned to its home cage. Handling is essential to reduce stress effects during experiments, which can severely affect the behavioral outcome of the tests.
- Handle each rat for 15 min each day for three consecutive days before habituation. Record the body weight of the animals from the first day of handling throughout the experiment. The animals are mildly food deprived (6 g of standard food per rat/day) during this period to decrease the body weight to 85% of initial free feeding and maintain this throughout the experiment while tap water is given ad libitum. Give 15 reward food pellets in the home cage during the handling days prior to training in order to familiarize the rat with the pellets.
- The experimenter stays in the room behind a wall barrier observing the rats on the computer screen connected with the video camera.
- All experimental procedures start at 9 AM in the light phase (overview in Figure 2).
- Habituation
Habituate the rats to the T-maze on days 4 and 5. During habituation the rats are allowed to freely explore the maze for 15 min. Food reward pellets are available during habituation. At day 4, scatter 20 pellets over the maze in a short distance to each other in order to let the animals learn that food is available in the maze and to motivate the rat to move forward. At day 5, pellets are located only in the cups at the end of the two-goal arms. - Training
- Day 6 is the first day of training. Place the animals in the box of the starting arm and release after 10 sec (Figure 3A).
- During the forced trial, place a reward pellet at the end of one randomly selected open arm, block the opposite arm with the guillotine door (Figure 3B).
- In the following trial, open both arms but only bait the opposite arm of that arm baited during the forced trial (Figure 3C).
- After completion of each trial (Figures 3D-3E) pick up the rats and transfer them to their home cage until the next trial starts (intertrial interval of 5 min).
- Place a food reward pellet in the food cup of the arm not previously rewarded.
- Record arm entries when the entire animal (including the tail tip) has moved into one arm.
- After selection of the unbaited arm, apply a self-correction procedure by keeping the baited arm baited until it is visited, allowing the rat to shift their choice. The food reward remains in its location until it is found and eaten.
- Ten trials (including the forced trial) are performed.
- At days 7 and 8, repeat Steps D1 to D7 (Figure 2).
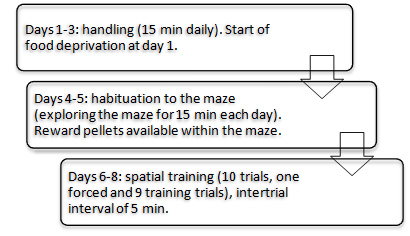
Figure 2. Scheme of the experimental protocol
Figure 3. Example of a training sequence starting with the forced trial (A-C) followed by a training trial (D-F). A. The rat is waiting for 10 sec in the starting chamber; B. Forced trail; C. The rat is eating the pellet; D-F. The rat in the second trial should always choose the opposite arm of its previous visit, otherwise a memory error is recorded.
Notes:
- The T-maze is cleaned with 1% Incidin between trials to remove any olfactory cues.
- It is very useful to measure the time the animal needs to leave the start box until entering an arm (latency) in order to control for possible sensory or motoric side effects of treatment.
- If the rat enters the unbaited arm, record a working memory error and place the rat back at the start position without a food reward. Record the number of correct entries into baited arms, number of reentries into unbaited arms (Video 1).
- Indirect illumination provides similar light intensities in the three arms (40-50 lux). Higher illumination differences between arms cause a bias of the rat responses to the darker arm since rats prefer dark areas for safety reasons.Video 1. T-maze protocol
Data analysis
WME (working memory error) = visit of an unbaited arm (each repeated visit is considered as a working memory error)
Correct choice = visit of a baited arm
WMI (working memory index) = number of correct choices/number of total trials (Table 1, Figure 4)
Latency= time to enter one goal arm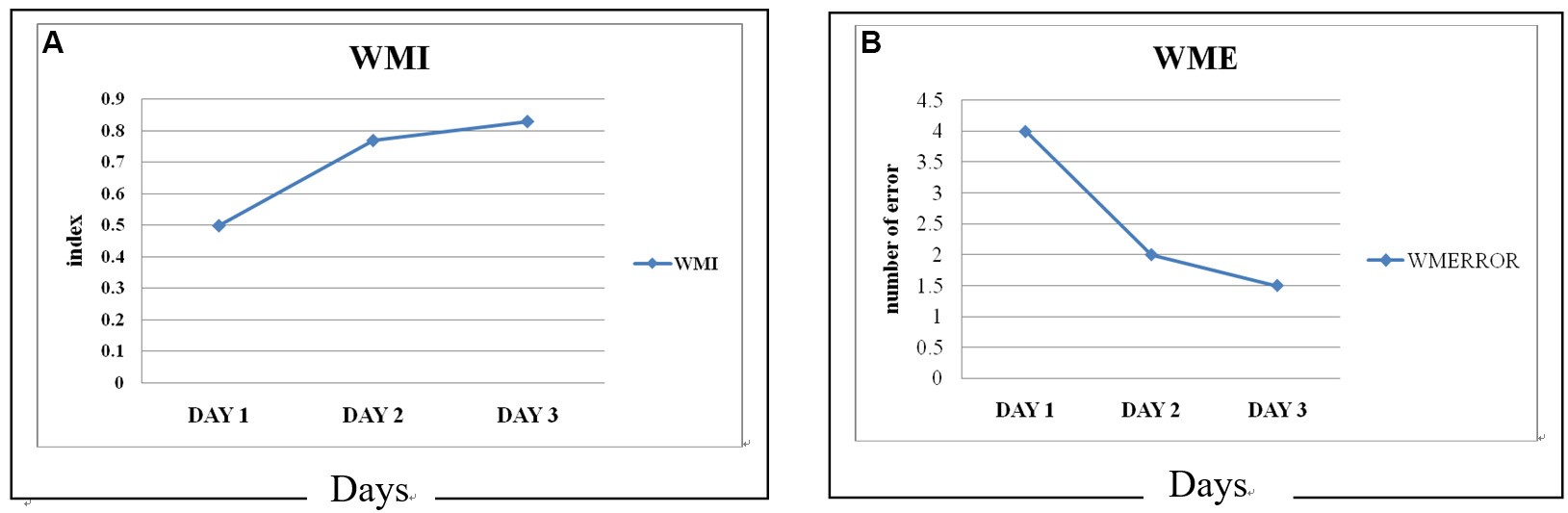
Figure 4. Working memory indices (A) and numbers of working memory errors (WME, B) of three animals
Table 1. Example of data analysis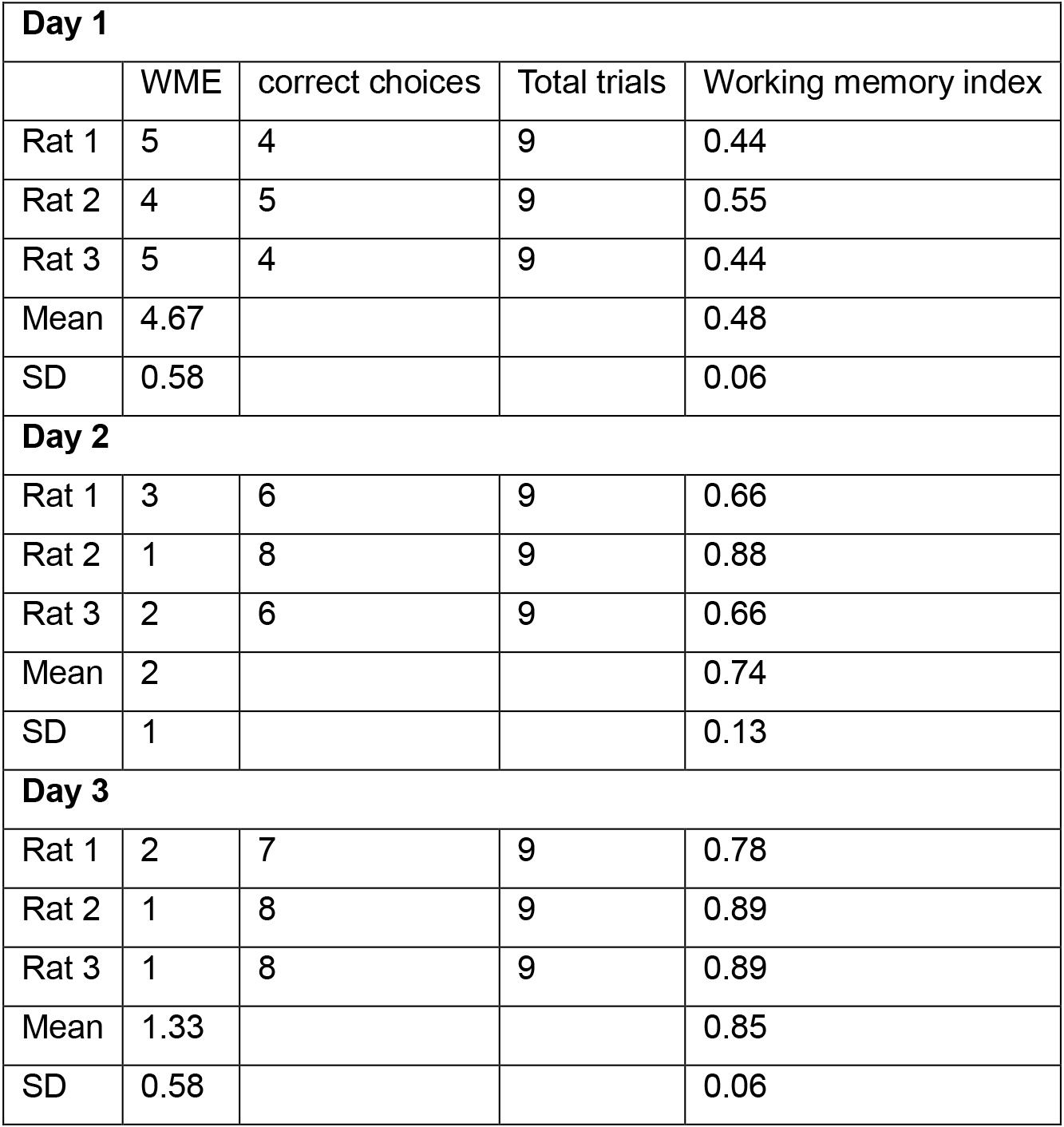
Notes
If the animals do not move or move very slowly:
- Check the home cage for uneaten pellets. If the pellets are not eaten, the rat may not recognize the pellets as familiar food and thereby they will not be motivated to chase for it.
- Insufficient body weight loss may cause low motivation to search for food in the maze.
- Insufficient habituation.
- Excessive fear may be exhibited by individual rats indicated by staying frozen in one place or by defecation and urination. The rat will also squeal when being picked up.
Recipes
- 1% incidin
Incidin diluted in distilled water (1%)
Note: It is used for cleaning the maze between trials.
Acknowledgments
This protocol was adapted from Bezu et al. (2016 and 2017). The authors have no conflicts of interest or competing interests to declare.
References
- Aher, Y. D., Subramaniyan, S., Shanmugasundaram, B., Sase, A., Saroja, S. R., Holy, M., Hoger, H., Beryozkina, T., Sitte, H. H., Leban, J. J. and Lubec, G. (2016). A novel heterocyclic compound CE-104 enhances spatial working memory in the radial arm maze in rats and modulates the dopaminergic system. Front Behav Neurosci 10: 20.
- Baddeley, A. (1992).Working memory. Science 255: 556-559.
- Bezu, M., Malikovic, J., Kristofova, M., Engidawork, E., Hoger, H., Lubec, G. and Korz, V. (2017). Spatial working memory in male rats: Pre-experience and task dependent roles of dopamine D1- and D2-Like receptors. Front Behav Neurosci 11: 196.
- Bezu, M., Shanmugasundaram, B., Lubec, G. and Korz, V. (2016). Repeated application of Modafinil and Levodopa reveals a drug-independent precise timing of spatial working memory modulation. Behav Brain Res 312: 9-13.
- Chudasama, Y. and Robbins, T. W. (2004). Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology 29(9): 1628-1636.
- Crawley, J. and Goodwin, F. K. (1980). Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13(2): 167-170.
- Deacon, R. M. and Rawlins, J. N. (2006). T-maze alternation in the rodent. Nat Protoc 1(1): 7-12.
- Dudchenko, P. A. (2004). An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28(7): 699-709.
- Gruber, A. J., Dayan, P., Gutkin, B. S. and Solla, S. A. (2006). Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci 20(2): 153-166.
- Hussein, A. M., Aher, Y. D., Kalaba, P., Aher, N. Y., Dragacevic, V., Radoman, B., Ilic, M., Leban, J., Beryozkina, T., Ahmed, A., Urban, E., Langer, T. and Lubec, G. (2017). A novel heterocyclic compound improves working memory in the radial arm maze and modulates the dopamine receptor D1R in frontal cortex of the Sprague-Dawley rat. Behav Brain Res 332: 308-315.
- Kristofova, M., Aher, Y. D., Ilic, M., Radoman, B., Kalaba, P., Dragacevic, V., Aher, N. Y., Leban, J., Korz, V., Zanon, L., Neuhaus, W., Wieder, M., Langer, T., Urban, E., Sitte, H. H., Hoeger, H., Lubec, G. and Aradska, J. (2018). A daily single dose of a novel modafinil analogue CE-123 improves memory acquisition and memory retrieval. Behav Brain Res 343: 83-94.
- Lalonde, R. (2002). The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26(1): 91-104.
- Sánchez-Santed, F., de Bruin, J. P., Heinsbroek, R. P. and Verwer, R. W. (1997). Spatial delayed alternation of rats in a T-maze: effects of neurotoxic lesions of the medial prefrontal cortex and of T-maze rotations. Behav Brain Res 84(1-2): 73-79.
- Saroja, S. R., Aher, Y. D., Kalaba, P., Aher, N. Y., Zehl, M., Korz, V., Subramaniyan, S., Miklosi, A. G., Zanon, L., Neuhaus, W., Hoger, H., Langer, T., Urban, E., Leban, J. and Lubec, G. (2016). A novel heterocyclic compound targeting the dopamine transporter improves performance in the radial arm maze and modulates dopamine receptors D1-D3. Behav Brain Res 312: 127-137.
- Sase, A., Aher, Y. D., Saroja, S. R., Ganesan, M. K., Sase, S., Holy, M., Hoger, H., Bakulev, V., Ecker, G. F., Langer, T., Sitte, H. H., Leban, J. and Lubec, G. (2016). A heterocyclic compound CE-103 inhibits dopamine reuptake and modulates dopamine transporter and dopamine D1-D3 containing receptor complexes. Neuropharmacology 102: 186-196.
- Wenk, G. L. (2001). Assessment of spatial memory using the T maze. Curr Protoc Neurosci Chapter 8: Unit 8 5B.
- Day 6 is the first day of training. Place the animals in the box of the starting arm and release after 10 sec (Figure 3A).
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hussein, A. M., Bezu, M. and Korz, V. (2018). Evaluating Working Memory on a T-maze in Male Rats. Bio-protocol 8(14): e2930. DOI: 10.21769/BioProtoc.2930.
Category
Neuroscience > Behavioral neuroscience > Animal model > Rat
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









