- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Embryonic Intravitreous Injection in Mouse
Published: Vol 8, Iss 14, Jul 20, 2018 DOI: 10.21769/BioProtoc.2929 Views: 6457
Reviewed by: Miao HeEhsan KheradpezhouhAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visual-stimuli Four-arm Maze test to Assess Cognition and Vision in Mice
Jean-Philippe Vit [...] Maya Koronyo-Hamaoui
Nov 20, 2021 5282 Views

Intravitreal NHS-Biotin Injection and Immunohistochemistry to Label and Image Protein Transport in the Mouse Optic Nerve
Caroline R. McKeown [...] Hollis T. Cline
Aug 20, 2025 2697 Views

Mouse Vestibulo-Ocular Reflex Testing for Otolith Organs and Horizontal Semicircular Canal
Tong Zhao [...] Fangyi Chen
Nov 20, 2025 1199 Views
Abstract
Axons of retinal ganglion cells (RGCs) relay visual information from the retina to lateral geniculate nucleus (LGN) and superior colliculus (SC), which are two major image-forming visual nuclei. Wiring of these retinal projections completes before vision begins. However, there are few studies on retinal axons at embryonic stage due to technical difficulty. We developed a method of embryonic intravitreous injection of dyes in mice to visualize retinal projections to LGN and SC. This study opens up the possibility of understanding early visual circuit wiring in mice embryos.
Keywords: EmbryoBackground
Retinal axons begin to project to LGN and SC as early as embryonic day 14.5 (E14.5) in mice. To investigate axon projections in embryos, dyes need to be injected intravitreally while keeping the embryos alive for at least 10 h prior to injection surgery. Previous studies showed embryos can be cultured in vitro using mouse serum with oxygen. However, issues such as nutrition diversity, oxygen saturation and temperature regulation impinge the physiological condition of the embryo. In our research, we conducted intravitreous injection through uterine wall and eyelids in embryos and kept them in uterine after injection. Retinal axon projections to LGN and SC were nicely labeled from E15.5 to E18.5.
Materials and Reagents
- 50 ml centrifuge tube (Corning, catalog number: 430828 )
- Cotton ball (Winner Medical Group, catalog number: 50401050 )
- Sterile gauze (Winner Medical Group, catalog number: 016935 )
- Suture needle (Ningbo Medical Needle Co., LTD, 7/0)
- Microscope slides (Fisher Scientific, catalog number: 12-550-15 )
- Microscope cover glass (Fisher Scientific, catalog number: 12-544-18 )
- Dropper (Shanghai Baiqian Biotechnology, catalog number: J00082 )
- Mouse (Shanghai SLAC, strain: C57BL/6J)
- Redistilled water (ddH2O)
- 75% alcohol (Sinopharm Chemical Reagent, catalog number: 80176960 )
- Mineral oil (Sigma-Aldrich, catalog number: M8410 )
- Cholera toxin B (CTB), Alexa FluorTM 555 conjugate (Thermo Fisher Scientific, InvitrogenTM, catalog number: C22843 )
- Isoflurane (RWD Life Science, catalog number: R510-22 )
- Iodophor (Shanghai Likang Disinfectant Hi-Tech, catalog number: 310100 )
- Lidocaine (MP Biomedicals, catalog number: 190111 )
- Sucrose (AMRESCO, catalog number: M117 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 16005 )
- Optimal cutting temperature compound (O.C.T) (Sakura, Tissue-Tek®, catalog number: 4583 )
- Diamidino-phenyl-indole (DAPI) (Sigma-Aldrich, catalog number: D9542 )
- AQUA-MountTM Mounting medium (Thermo Fisher Scientific, catalog number: 13800 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Sodium phosphate dibasic dihydrate (Na2HPO4·2H2O) (Sigma-Aldrich, Fluka, catalog number: 71645 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886 )
- Ampicillin (INALCO, catalog number: 1758-9314 )
- Trichloroacetaldehyde hydrate (Sinopharm Chemical Reagent, catalog number: 30037517 )
- Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: S5011 )
- Tris (Sangon Biotech, catalog number: A100826 )
- Tris hydrochloride (Tris-HCl) (AMRESCO, catalog number: T0234 )
- Triton X-100 (AMRESCO, catalog number: 0694 )
- DAPI (Sigma-Aldrich, catalog number: D9542 )
- 0.1 M Phosphate buffer saline solution(PBS) (see Recipes)
- Ampicillin solution (see Recipes)
- 0.9% Sodium chloride solution (see Recipes)
- 1% lidocaine solution (see Recipes)
- 10% Chloral hydrate solution (see Recipes)
- 0.2 M Phosphate buffer (PB) (see Recipes)
- 0.05 M Tris buffered saline (TBS) (see Recipes)
- 0.5%/0.05% Triton solution (see Recipes)
- CTB solution (see Recipes)
- 4% paraformaldehyde solution (see Recipes)
- 30% sucrose solution (see Recipes)
- DAPI solution (see Recipes)
Equipment
- Glass Capillaries for Nanoliter 2010 (referred to as pipette in this manuscript) (World Precision Instruments, catalog number: 504949 )
- Scissors (RWD Life Science, catalog number: S12003-09 )
- Tweezers (VETUS, catalog number: ST-10 )
- Ophthalmic scissors (World Precision Instruments, catalog number: 14003-G )
- Water bath (Jinghong Experimental Equipment, model: XMID-8222 )
- Nanoject II Auto-Nanoliter Injector (Drummond Scientific, model: Nanoject II , catalog number: 6584)
- DC temperature controller (FHC, model: 40-90-8D )
- Anesthesia machine (RWD Life Science, catalog number: R610 )
- Flaming/brown micropipette puller (Sutter Instrument, model: P-97 )
- Shaver (Codos, catalog number: KP-3000 )
- Fluorescence microscope (Nikon Instruments, model: Eclipse Ni-U )
- -80 °C freezer (Thermo Fisher Scientific, catalog number: ULT 1386-3-V42 )
- Freezing microtome (Leica Biosystems, model: Leica CM1950 )
Software
- ImageJ (NIH, USA)
- Matlab (Mathworks Inc, USA)
Procedure
- Put one male mouse and two female mice into a home cage at 20:00. Detect the presence of Vaginal plug at 8:00 AM next day. If the female mouse has a vaginal plug, the embryonic stage is denoted by 0.5 days (E0.5). Otherwise, divide male and female mice into two home cages and put them into one home cage again at 20:00.
- Sterilize all metal surgical instruments for 30 min at a station of high temperature (121 °C) and high pressure (0.12 MPa). Disinfect the other nonmetal apparatuses with 75% ethanol.
- Fill PBS and ampicillin solution into two centrifuge tubes respectively. Keep the tubes in a water bath at 45 °C.
- Pull a Pipette using a micropipette puller. Break the Pipette tip with scissors to make it open. Fill the Pipette with mineral oil and then fit it in Nanoject II. CTB-555 is inhaled into pipette using Nanoject II.
Note: The diameter of the pipette tip is about 50 μm. - Anesthetize the pregnant mouse with 1.5% isoflurane in oxygen in a chamber until its respiratory frequency decreases to 60 times per min and then transfer it onto a heat pad at 32 °C controlled by DC temperature controller.
- Place the head of the pregnant mouse into a face mask to make sure that mouse is anesthetized by 0.5-1% isoflurane in oxygen continuously until respiratory frequency maintains between 80-100 times per min.
- Shave the mouse abdomen and disinfect successively by using a cotton ball with 75% ethanol and iodophor.
- Cover the mouse abdomen with a sterile gauze which is cut a 2-cm long slit to expose the position of the abdominal incision.
- Slit the abdominal skin and peritoneum carefully at the middle of abdomen from outside to inside.
Note: Incision at the precise middle of abdomen can avoid capillary vessels. Otherwise, bleeding induced by damaged capillary vessels is easier to cause wound infection. - Pull Embryos out carefully with tweezers and place them on dry sterile gauze over the abdomen.
Note: The tension should be slight to avoid tearing uterine wall. Be careful not to damage vessels and placenta. - Cut a slit (~1 mm) on the uterine wall over the eyeball of the embryo a slit (~1 mm) using ophthalmic scissors (Video 1).
Note: If there are vessels on the uterine wall over the eyeball of the embryo, the embryo is rotated by hand softly to avoid vessels.Video 1. The posture of intravitreous injection - One hand holds embryo. Another hand holds Nanoject II. Pipette pass through the slit of uterine wall, eye slide, sclera and then plug it in vitreous chamber. Inject 1 μl CTB-555 into the vitreous chamber of one eye at the speed of 69 nl per time, 1-2 sec interval (Video 1).
Notes:- One millimeter long pipette tip is inserted subretinally. After injection, the pipette stays in the eye for 5 sec.
- A second person adds warm PBS using a dropper to the surface of uterine wall to keep it moist throughout the whole process.
- Resting the arms of the experimentalist on the table helps to prevent disturbance during the injection process.
- One millimeter long pipette tip is inserted subretinally. After injection, the pipette stays in the eye for 5 sec.
- Inject eyes of other embryos successively with the similar volume and speed.
- Replace the embryos into abdomen carefully with tweezers.
- Fill the abdomen cavity of the pregnant mouse with 1 ml ampicillin solution to prevent infection. Absorb the extra solution with gauze over the abdomen.
- Suture the peritoneum and abdominal skin from inside to outside.
- Apply 1% lidocaine solution on the wound to relieve the pain.
- Anesthetize the pregnant mouse continually with 0.5-1% isoflurane for 10 h.
Note: The pregnant mouse is anesthetized to make the surgical procedure smooth. - After 10 h, anesthetize pregnant mouse with 10% chloral hydrate solution at the dose of 0.06 ml per 10 g weight.
Note: The pregnant mouse still has a heartbeat after chloral hydrate solution injection to avoid fetal hypoxia. - Cut the abdomen open again to expose embryos. Made a slit on the uterine wall using scissors. Cut the umbilical cord to take out embryo.
- Fix the embryo on a board and implement heart perfusion using 4% PFA solution.
Note: The process of transcardial perfusion of embryos is similar to that of adults. It is best to perform transcardial perfusion under an atomic microscope to reveal the structure of the heart. - Take brains of embryos out and fix in 4% PFA for 12-18 h.
Note: Retinas of embryos are taken out and fixed in 4% PFA for 2 h. Retinas are mounted on slides and observed with the fluorescence microscope. It is necessary to make sure that majority of retinal ganglion cells are labeled by CTB-555. - Dehydrate brains in 30% sucrose.
- Embed brains in O.C.T after they sink in 30% sucrose solution, and then rapidly transfer into a -80 °C freezer. Half an hour later, take the brains into freezing microtome to rewarm for 20 min.
- Cut brains into coronal slices of 20 μm thick. Wash the brain slices 5 times (5 min per time) using TBS solution to remove O.C.T away.
Note: All brain slices of LGN and SC section are collected. - Stain brain slices with DAPI (see Recipe 12) for 7 min, and then wash 5 times with TBS solution (how long each time).
- Mount brain slices on microscope slides. Apply mounting medium on slices until slices are dry in the air without light. Finally, coverslips are mounted on slices.
- Take pictures of brain slices under a fluorescence microscope (Figure 1). The results of embryonic intravitreous injection can also be found online at: https://academic.oup.com/cercor/article/28/4/1168/3064954.
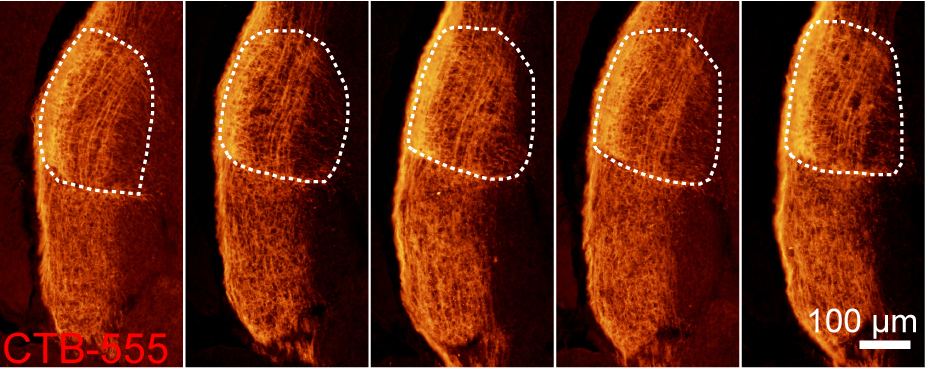
Figure 1. Images of LGN labeled with CTB-555 after embryonic intravitreous injection at E18.5. The dorsal LGN is outlined with white dashed lines.
Data analysis
Analysis of retinal projection was conducted as previously described (from our lab) (Diao et al., 2018). Briefly, the background of all images was subtracted in ImageJ (NIH, USA). Quantifications of fluorescently labeled areas, such as the fraction of contralateral projections and sizes of LGN and SC, were performed using Matlab (Mathworks Inc, USA). For 20 μm brain slices, a threshold of 30% (fluorescence intensity of pixels above 30% of the maximum intensity) is applied in the calculation of fraction of contralateral projections. CTB-555 and DAPI fluorescent signals helped outlining the territory of LGN and SC.
Note: The birth-dating of embryos could be differed by ± 0.5 days. It is necessary to use sufficient number of pregnant females to obtain sufficient embryos for analysis.
Recipes
- 0.1 M Phosphate buffer saline solution (PBS)
0.02 M KH2PO4, 0.04 M Na2HPO4·2H2O and 0.1567 M NaCl in ddH2O - Ampicillin solution
0.1 mg/ml ampicillin in 0.1 M PBS - 0.9% Sodium chloride solution
0.9% NaCl in ddH2O - 1% lidocaine solution
1% lidocaine in 0.9% NaCl solution
Note: Add a little of hydrochloric acid into the solution to help dissolve. - 10% Chloral hydrate solution
10% Chloral hydrate in 0.9% NaCl solution - 0.2 M Phosphate buffer (PB)
0.04 M NaH2PO4 and 0.2 M Na2HPO4·2H2O in ddH2O - 0.05 M Tris buffered saline (TBS)
0.0123 M Tris, 0.0377 M Tris-HCl, 0.1538 M NaCl in ddH2O - 0.5%/0.05% Triton solution
0.5%/0.05% Triton X-100 in TBS - CTB solution
500 μg CTB solid powder is dissolved by 10 μl dimethyl sulphoxide (DMSO) and then add 230 μl 0.9% sodium chloride solution - 4% paraformaldehyde solution
4% PFA in PB - 30% sucrose solution
30% sucrose in PB - DAPI solution
1 mg/ml DAPI in ddH2O for storage
1:3,000 diluted in TBS for working concentration
Acknowledgments
The authors declare no competing interests. This protocol was adapted from Diao et al. (2018). We thank the following funding agencies for supporting this work: the NSF of China (31421091 and 31422025), the Young 1000 Plan and Ministry of Science and Technology of the People’s Republic of China (2015AA020512).
References
- Diao, Y., Cui, L., Chen, Y., Burbridge, T. J., Han, W., Wirth, B., Sestan, N., Crair, M. C. and Zhang, J. (2018). Reciprocal connections between cortex and thalamus contribute to retinal axon targeting to dorsal lateral geniculate nucleus. Cereb Cortex 28(4): 1168-1182.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cui, L., Diao, Y. and Zhang, J. (2018). Embryonic Intravitreous Injection in Mouse. Bio-protocol 8(14): e2929. DOI: 10.21769/BioProtoc.2929.
Category
Neuroscience > Neuroanatomy and circuitry > Optic nerve
Neuroscience > Sensory and motor systems > Visual system
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link