- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transient Expression Assay in NahG Arabidopsis Plants Using Agrobacterium tumefaciens
Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2894 Views: 10056
Reviewed by: Zhibing LaiAraceli Castillo GarrigaShunping Yan

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Near-Infrared Autofluorescence Imaging of Nuclei in Living Plant Roots
Akira Yoshinari and Masayoshi Nakamura
Apr 20, 2025 2122 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1688 Views
Abstract
Agrobacterium-mediated transient expression has greatly contributed to research in molecular plant biology but has low efficiency and inconsistency in Arabidopsis thaliana (Arabidopsis). Here, we describe a simple, efficient and fast protocol to make transient gene expression in NahG Arabidopsis plants using Agrobacterium tumefaciens. This protocol has been successfully used to assess protein sub-cellular localization and accumulation, enzyme activity, and protein-protein interaction. In addition, this assay overcomes the use of Nicotiana benthamiana plants as a surrogate system for transient gene expression assays. Finally, the use of this protocol does not require complex inoculation methods or specific growth conditions, and can be used with different Agrobacterium strains with similar results.
Keywords: NahG ArabidopsisBackground
Agrobacterium tumefaciens (hereafter referred to as Agrobacterium)-mediated transient transformation assays have greatly contributed to research in molecular plant biology. These methods have many advantages over the laborious and time-consuming stable transformation approaches including, among others, a higher efficiency, simplicity, and fast, consistent results when the transient transformation assays are carried out in Nicotiana benthamiana. On the other hand, these assays are inefficient and lack robustness when carried out in the model plant Arabidopsis thaliana (hereafter referred to as Arabidopsis), forcing Arabidopsis researchers to use N. benthamiana as a heterologous system, which entails obvious limitations and might generate misleading results.
Many efforts have been made in the past to increase the efficacy of Agrobacterium-mediated transient transformation in Arabidopsis (reviewed in Krenek et al., 2015). Recently, we described a protocol to perform transient gene expression using NahG Arabidopsis plants, overcoming previous limitations (Rosas-Díaz et al., 2017). Using Arabidopsis NahG plants, which contain low levels of salicylic acid (SA) due to the expression of an SA hydroxylase from the bacterium Pseudomonas putida (Lawton et al., 1995), we have been able to obtain high accumulation of marker proteins such as GUS and GFP, and carry out sub-cellular localization and protein-protein interaction experiments. Remarkably, this protocol for transient expression can be used with, at least, three widely used Agrobacterium strains, LBA4404, GV3101 and C58C1. In summary, this protocol shows that expression of the NahG transgene greatly enhances the efficiency of Agrobacterium-mediated transformation in rosette leaves in Arabidopsis, enabling the routine use of this technique in the model plant.
Materials and Reagents
- Materials
- Soil mix or substrate such as Compo Sana® Universal Ligera (COMPO, TSUSTPROF25L)
- Plant pots such as Desch vol 11 (Desch Plantpak, catalog number: 1055278 )
- Seed tray–40 cavities
- Plant trays
- Cling film
- Syringes 1 ml or 2 ml
- Tissue paper
- Petri dishes
- Falcon tubes
- Eppendorf tubes
- Pipette tips
- Sterile toothpicks
- Syringe filter 0.22 µm
- Soil mix or substrate such as Compo Sana® Universal Ligera (COMPO, TSUSTPROF25L)
- Biological materials
- Arabidopsis thaliana NahG seeds (Lawton et al., 1995)
- Agrobacterium tumefaciens strain (LBA4404, GV3101 or C58C1) carrying a binary vector with the gene of interest
- Arabidopsis thaliana NahG seeds (Lawton et al., 1995)
- Reagents
- Sterile deionized water
- Glycerol (CARLO ERBA Reagents, catalog number: 453752 )
- NaCl (AppliChem, catalog number: 121659.1210 )
- Tryptone (Biolife, catalog number: 412290 )
- Yeast Extract (AppliChem, catalog number: 403687.1210 )
- Bacteriological agar (MICROKIT, catalog number: BCB006+ )
- MES (2-(N-morpholino) ethanesulfonic acid) (Sigma-Aldrich, catalog number: M2933 )
- MgCl2 (AppliChem, catalog number: 131396.1210 )
- DMSO (Sigma-Aldrich, catalog number: M81802 )
- Acetosyringone (Sigma-Aldrich, catalog number: D134406 )
- Rifampicin (Duchefa Biochemie, catalog number: R0146.0005 )
- Tetracycline (Sigma-Aldrich, catalog number: T3383 )
- Gentamycin (Sigma-Aldrich, catalog number: G3632 )
- Spectinomycin (Duchefa Biochemie, catalog number: S0188.0005 )
- Kanamycin (Sigma-Aldrich, catalog number: K4378 )
- LB medium (see Recipes)
- 1 M MES (see Recipes)
- 1 M MgCl2 (see Recipes)
- 0.1 M Acetosyringone (see Recipes)
- Infiltration solution (see Recipes)
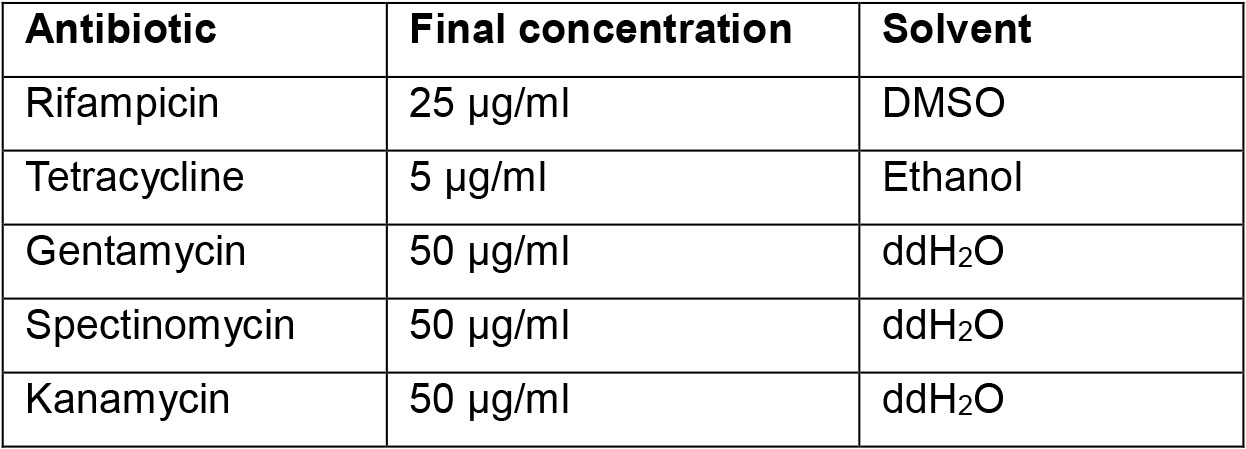
- Antibiotics solution (depending on construct and Agrobacterium strain, see Recipes)
- Sterile deionized water
Equipment
- Sterile Erlenmeyer flasks
- Plant growth chamber capable of sustaining 21 °C under short-day conditions (8 h light/16 h dark) with 140-150 µmol m-2 sec-1 light intensity (Radiber SA, catalog number: AGP-1400 )
- Spectrophotometer capable of OD600 measurements such as Shimadzu UV-1601 (Shimadzu, catalog number: 206-67001-34 )
- Automatic P1000, P200 and P20 micropipettes
- Incubator set at 28 °C such as Incubator D-6450 Hanau (Heraeus Instruments, model: D-6450 )
- Incubator shaker capable of sustaining 28 °C and 180 rpm such as New BrunswickTM I26 (Eppendorf, New Brunswick scientific, model: I26, catalog number: M1324-0000 )
- Centrifuge for 50 ml tubes such as Rotofix 32A (Hettich, catalog number: 1206-01 )
- Autoclave
Procedure
- Plant growth conditions
Note: The preparation of Arabidopsis NahG plants is a key step in obtaining a satisfactory transient gene expression/protein expression. In this protocol, ~4 weeks old Arabidopsis NahG plants are used for Agrobacterium infiltration.- Sow seeds in water-soaked soil mix in a plant pot. Cover the pot with cling film and place it in a growth chamber with 8 h light/16 h dark cycle at 21 °C.
- Grow until the seedlings have two true leaves (around 7-10 days).
- Carefully transplant seedlings to the final destination in seed trays with 40 cavities.
- Grow plants for approximately 3 more weeks inside the growth chamber with 8 h light/16 h dark cycle at 21 °C.
- Plants are ready for infiltration when they have a robust rosette of 10-12 leaves and have not started flowering.
- Sow seeds in water-soaked soil mix in a plant pot. Cover the pot with cling film and place it in a growth chamber with 8 h light/16 h dark cycle at 21 °C.
- Agrobacterium cultures
Note: This protocol can be used with, at least, three different commonly used strains of Agrobacterium: LBA4404, GV3101 and C58C1.- Using a glycerol stock and a sterile toothpick, streak the Agrobacterium clone(s) to be used in LB solid plates supplemented with the appropriate antibiotics. Place the plates inside a 28 °C incubator for 48 h to obtain fresh and single colonies.
- The day before starting the infiltration, start liquid Agrobacterium cultures in LB liquid medium using the fresh colonies on the plates. Pick Agrobacterium biomass from a single colony, using a sterile toothpick, place it inside a sterile Erlenmeyer flask with 20 ml LB liquid media supplemented with the appropriate antibiotics, and culture them at 28 °C and 180 rpm overnight.
- Using a glycerol stock and a sterile toothpick, streak the Agrobacterium clone(s) to be used in LB solid plates supplemented with the appropriate antibiotics. Place the plates inside a 28 °C incubator for 48 h to obtain fresh and single colonies.
- Infiltration
- Agrobacterium preparation
- Pour saturated cultures into 50 ml Falcon tubes. Spin down cells at 4,000 x g for 10 min.
- Discard LB medium supernatant by decanting. Eliminate as much supernatant as possible.
- Resuspend with vortex the cell pellets using 1 volume of freshly prepared infiltration buffer.
- After resuspension, leave cultures for 2-4 h in darkness at room temperature.
- Prepare a 1/20 dilution of the saturated culture, measure OD600 and calculate necessary volume to have a final OD600 of 0.05. Dilute using infiltration buffer.
- Pour saturated cultures into 50 ml Falcon tubes. Spin down cells at 4,000 x g for 10 min.
- Infiltration procedure
- Fill a 1 or 2 ml needleless syringe with the resuspended culture at a final OD600 of 0.05. Perform the infiltration by pressing the syringe (without needle) on the abaxial side of the leaf while exerting counter-pressure with a fingertip on the adaxial side. Observe how the liquid spreads within the leaf if the infiltration is successful. Infiltrate whole leaves (ca. 100 µl of bacterial suspension/leave).
- Dry the excess of culture from the leaf surface using tissue paper (Figure 1, Video 1).
- Two to four days after infiltration, observe fluorescence of infiltrated proteins or harvest infiltrated leaves to do a protein extraction.

Figure 1. Experimental procedure of transient expression in NahG Arabidopsis thaliana plants using Agrobacterium tumefaciens. A. Eppendorf tube with Agrobacterium carrying the desired construct, 1 ml syringe, 4-week-old NahG Arabidopsis plants, and piece of paper. B. Plant infiltration (see details in the procedure section).Video 1. Experimental procedure of transient expression in NahG Arabidopsis thaliana plants using Agrobacterium tumefaciens
- Fill a 1 or 2 ml needleless syringe with the resuspended culture at a final OD600 of 0.05. Perform the infiltration by pressing the syringe (without needle) on the abaxial side of the leaf while exerting counter-pressure with a fingertip on the adaxial side. Observe how the liquid spreads within the leaf if the infiltration is successful. Infiltrate whole leaves (ca. 100 µl of bacterial suspension/leave).
- Agrobacterium preparation
Notes
- Some leaves might suffer necrosis after infiltration with the bacterial suspension.
- Growing NahG Arabidopsis under long day condition (16 h light/8 h dark cycle), might not affect the transient expression if the plants have not started flowering. However, plant leaves are bigger and easier to infiltrate when plants are grown in short day conditions.
- It is also possible to adjust the OD600 first and then incubate the cultures for 2-4 h in darkness.
- Infiltrate no more than 4-5 leaves per plant. Survival rate of the plants diminishes considerably when more leaves are infiltrated.
- Using this protocol the level of protein accumulation is optimal at 3 days after infiltration.
- Practically any leaf of the rosette can be infiltrated with positive results but the eldest leaves tend to show a lower signal and the youngest leaves tend to show a stronger response to the bacterial inoculation, with the concomitant necrosis.
- Drying the excess of culture is not determinant for the success of the infiltration but highly recommendable due to leaves sticking together when wet, causing higher levels of necrosis.
Recipes
- LB medium (1 L)
NaCl 5 g
Tryptone 10 g
Yeast extract 5 g
(Only for solid medium) Bacteriological agar 16 g - 1 M MES (100 ml)
17.5 g MES
Complete with sterile deionized water
Adjust the pH to 5.6
Sterilize by filtration
Store at room temperature - 1 M MgCl2 (100 ml)
20.3 g MgCl2
Complete with sterile deionized water
Sterilize by autoclaving
Store at room temperature - 0.1 M Acetosyringone (10 ml)
0.196 g acetosyringone
10 ml DMSO
Prepare 1 ml aliquots and store them at -20 °C - Infiltration solution (100 ml)
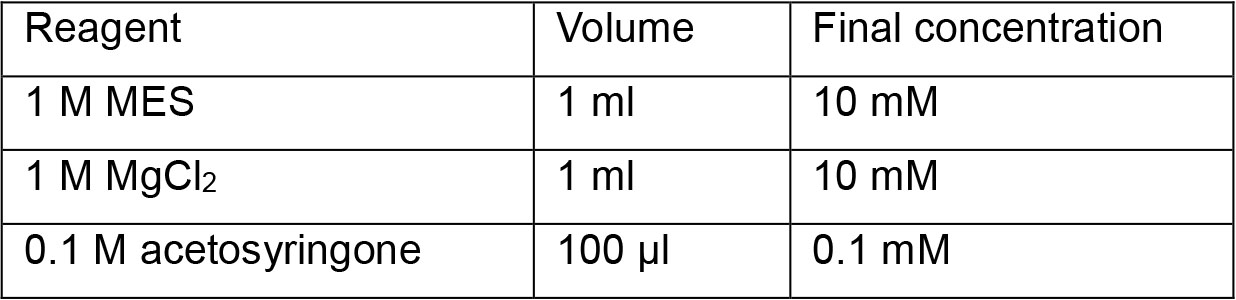
Top up with sterile deionized water
Note: Prepare infiltration solution just before use. - Antibiotics solution
Acknowledgments
The authors thank Huang Tan for his technical help during the imaging. This work was supported by the Spanish Ministerio de Ciencia y Tecnología (AGL2016-75819-C2) and the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences. This protocol is derived from a previous publication (Rosas-Díaz et al., 2017).
Competing interests
The authors declare that they have no conflict of interest.
References
- Krenek, P., Samajova, O., Luptovciak, I., Doskocilova, A., Komis, G. and Samaj, J. (2015). Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol Adv 33(6 Pt 2): 1024-1042.
- Lawton, K., Weymann, K., Friedrich, L., Vernooij, B., Uknes, S. and Ryals, J. (1995). Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant Microbe Interact 8(6): 863-870.
- Rosas-Díaz, T., Cana-Quijada, P., Amorim-Silva, V., Botella, M. A., Lozano-Duran, R. and Bejarano, E. R. (2017). Arabidopsis NahG plants as a suitable and efficient system for transient expression using Agrobacterium tumefaciens. Mol Plant 10(2): 353-356.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cana-Quijada, P., Bejarano, E. R., Lozano-Durán, R. and Rosas-Díaz, T. (2018). Transient Expression Assay in NahG Arabidopsis Plants Using Agrobacterium tumefaciens. Bio-protocol 8(12): e2894. DOI: 10.21769/BioProtoc.2894.
Category
Plant Science > Plant physiology > Biotic stress
Cell Biology > Cell imaging > Confocal microscopy
Systems Biology > Interactome > Protein-protein interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link



.jpg)









