- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Implementation of Blue Light Switchable Bacterial Adhesion for Design of Biofilms
Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2893 Views: 7168
Reviewed by: Elizabeth LibbyTimo A LehtiKumari Sonal Choudhary

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Inexpensive Imaging Platform to Record and Quantitate Bacterial Swarming
Weijie Chen [...] Jay X. Tang
Sep 20, 2021 3636 Views

Purification of the Bacterial Amyloid “Curli” from Salmonella enterica Serovar Typhimurium and Detection of Curli from Infected Host Tissues
Murugesan Sivaranjani [...] Aaron P. White
May 20, 2022 3233 Views

A Guideline for Assessment and Characterization of Bacterial Biofilm Formation in the Presence of Inhibitory Compounds
Bassam A. Elgamoudi and Victoria Korolik
Nov 5, 2023 3104 Views
Abstract
Control of bacterial adhesions to a substrate with high precision in space and time is important to form a well-defined biofilm. Here, we present a method to engineer bacteria such that they adhere specifically to substrates under blue light through the photoswitchable proteins nMag and pMag. This provides exquisite spatiotemporal remote control over these interactions. The engineered bacteria express pMag protein on the surface so that they can adhere to substrates with nMag protein immobilization under blue light, and reversibly detach in the dark. This process can be repeatedly turned on and off. In addition, the bacterial adhesion property can be adjusted by expressing different pMag proteins on the bacterial surface and altering light intensity. This protocol provides light switchable, reversible and tunable control of bacteria adhesion with high spatial and temporal resolution, which enables us to pattern bacteria on substrates with great flexibility.
Keywords: Bacterial adhesionBackground
Controlling the biofilm formation is crucial to understand the social interactions between bacteria in naturally occurring biofilm (Flemming et al., 2016). This is also particularly important for the biotechnological application of biofilms in biocatalysis, biosensing and waste treatment (Zhou et al., 2013; Jensen et al., 2016). The biofilm formation always begins with the bacterial adhesion to a substrate, which determines the spatial organization in biofilms (Liu et al., 2016; Nadell et al., 2016). Many strategies have been proposed to control bacterial adhesion such as modifying bacterial surface with bio-orthogonal reactive groups via liposome fusion (Elahipanah et al., 2016), immobilization of adhesive molecules on the substrates (Sankaran et al., 2015; Zhang et al., 2016; Peschke et al., 2017) and conjugating surface tags on bacteria (Poortinga et al., 2000; Rozhok et al., 2005; Lui et al., 2013). Among these, the light responsive approaches provide the highest spatiotemporal control, which is important to precisely control the fine structure of the biofilms. For instance, azobenzene linkers have been used as a photoswitchable tool to reversibly control the bacterial adhesion to substrates by altering the presentation of mannose, which is recognized by the bacterial surface receptor FimH (Voskuhl et al., 2014; Weber et al., 2014; Sankaran et al., 2015). In addition, azobenzene-based molecules have also been used to control bacteria adhesion to mammalian cells (Mockl et al., 2016), bacterial quorum sensing (Van der Berg et al., 2015) and biofilm formation (Hu et al., 2016) with UV-light. One of the major drawbacks of using UV-light is that it is toxic to bacteria. In this protocol, we present a new approach of how to control bacterial adhesion to substrates with blue light based on photoswitchable proteins. Besides being a non-invasive, reversible and tuneable technique to control bacterial adhesion to substrates, it also provides high spatiotemporal control required to form well-defined biofilms. Photoswitchable proteins are commonly used in the field of optogenetics to regulate gene expression, receptor activation and protein localization in cells with visible light (Müller and Weber, 2013; Tischer and Weiner, 2014). These optogenetic systems are very sensitive to visible light, bioorthogonal and noninvasive. Furthermore, these proteins are genetically encoded so they can be sustainably expressed in the cell. Here, we used the blue light responsive proteins, nMag and pMag, as photoswitches control bacterial adhesion. These proteins heterodimerize under blue light (480 nm) and dissociate from each other in the dark (Kawano et al., 2015). The strength and back conversion kinetics of the nMag and pMag interaction are different for the point mutants. The point mutant pMagHigh (and nMagHigh) has a stronger interaction with its binding partners and slower back conversion, while the opposite is true for the mutant pMagFast1 (and nMagFast1) (Zoltowski et al., 2009).
In our method we display the first interaction partner of the photoswitchable proteins, pMagHigh, pMag or pMagFast1 on the surface of E. coli using the circularly permutated OmpX (outer membrane protein X) protein (Daugherty, 2007). The pMag variants are attached through their C-terminal to the OmpX protein. The second interaction partner the photoswitchable protein, nMagHigh, is immobilized through a His6-tag at its C-terminal on a glass substrate with a PEG (polyethylene glycol) coating, which contains a Ni2+-NTA group (Schenk et al., 2014). This setup allows bacteria expressing pMag proteins on their surfaces to adhere to nMagHigh functionalized substrates under blue light when the two proteins interact but not in the dark. (Figure 1)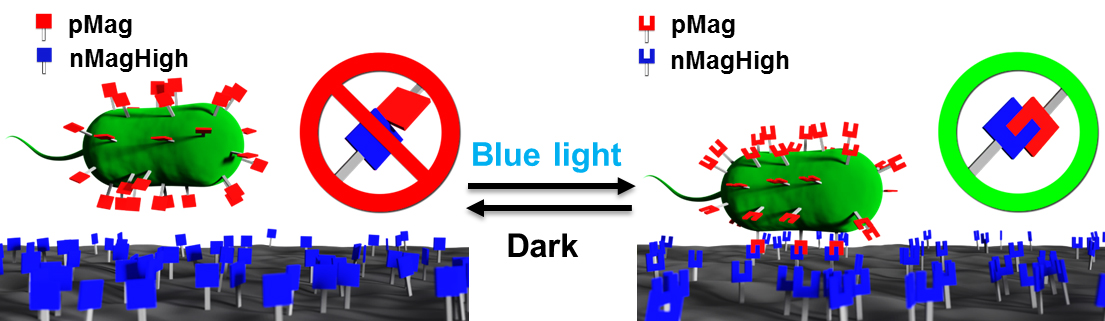
Figure 1. The engineered E. coli that express pMag proteins on their surface adhere to nMagHigh modified substrates under blue light. In the dark, the pMag-nMag interaction is reversed, which leads to the detachment of the bacteria from the substrate. Reproduced with permission from Chen and Wegner (2017).
Materials and Reagents
- Pipette tips (STARLAB, catalog number: S1111-6700 )
- 50 ml Falcon tube (Greiner Bio One International, catalog number: 227261 )
- 1.5 ml Eppendorf tube (Eppendorf, catalog number: 0030120086 )
- 0.2 ml PCR tubes (Thermo Fisher Scientific, catalog number: AB0620 )
- 0.45 µm cellulose filter (Carl Roth, catalog number: KH55.1 )
- Ni-NTA column (GE Healthcare, catalog number: 17524801 )
- 50 ml syringe (VWR, catalog number: 53548-010)
Manufacturer: Air-Tite Products, catalog number: 4850001000 . - 20 x 20 mm glass slides (VWR, catalog number: 631-0122 )
Notes: The glass slide is used as the glass surface for the protein functionalization and bacterial experiments. - Parafilm (Sigma-Aldrich, Bemis, catalog number: P7668 )
- Aluminum foil (Carl Roth, catalog number: 1770.1 )
- 35 mm Petri dish (SARSTEDT, catalog number: 82.1135.500 )
- Dialysis tubing (Repligen, Spectrum, catalog number: 132592 )
- Plasmid pB33eCPX (Addgene plasmid) (Addgene, catalog number: 23336 )
- GFP and mCherry pTrc99A plasmids (Prof. Victor Sourjik lab, Chen and Wegner, 2017)
- nMagHigh pET-21b(+) plasmid (Genescript, Chen and Wegner, 2017)
- nMagHigh-eCPX plasmid (homemade, Chen and Wegner, 2017)
Note: The nMagHigh gene is inserted between the KpnI and SacI cutting sites of pB33eCPX. - pMag-eCPX, pMagHigh-eCPX, pMagFast1-eCPX plasmids (homemade, Chen and Wegner, 2017)
Note: The different pMag variants are generated by point mutagenesis from the nMagHigh-eCPX plasmid using QuikChange II Site-Directed Mutagenesis Kit. - E. coli K12 MG1655 (DSMZ, catalog number: 18039 )
- BL21(DE3) competent E. coli (homemade, Chen and Wegner, 2017)
- DH5α competent E. coli (homemade, Chen and Wegner, 2017)
- PBS Tablets (Thermo Fisher Scientific, GibcoTM, catalog number: 18912014 )
- Mowiol (Sigma-Aldrich, catalog number: 81381 )
- LB medium (Carl Roth, catalog number: X968.3 )
- Ampicillin (Carl Roth, catalog number: HP62.2 )
- IPTG (Sigma-Aldrich, catalog number: I6758 )
- PMSF (Sigma-Aldrich, catalog number: P7626 )
- DTT (Sigma-Aldrich, catalog number: D0632-10G )
- PEG-azide (homemade)
- Triethylamine (Sigma-Aldrich, catalog number: T0886 )
- Toluene, anhydrous (Alfa Aesar, catalog number: 41464-AK )
- EDTA (Sigma-Aldrich, catalog number: 798681 )
- NiCl2 (Sigma-Aldrich, catalog number: 339350 )
- Chloramphenicol (Sigma-Aldrich, catalog number: C0378 )
- L-arabinose (Sigma-Aldrich, catalog number: A3256 )
- Paraformaldehyde, reagent grade (Sigma-Aldrich, catalog number: P6148 )
- Tris Base (Sigma-Aldrich, catalog number: T1503 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Imidazole (Sigma-Aldrich, catalog number: I2399 )
- L-Ascorbic acid (Sigma-Aldrich, catalog number: A5960 )
- NTA-alkyne (homemade, Schenk et al., 2014)
- PEG azide (homemade, Schenk et al., 2014)
- Copper sulfate (CuSO4) (Sigma-Aldrich, catalog number: 451657 )
- 30% H2O2 (Carl Roth, catalog number: 8070.4 )
- H2SO4 (Sigma-Aldrich, catalog number: 30743)
- TEM (Transmission electron microscopy) grid (Ted Pella, catalog number: 1GC150 )
- Centrifuge tubes 500 ml and 50 ml (Thermo Fisher Scientific, catalog numbers: 3141-0500 , 3119-0050 )
- Methanol (Fisher Scientific, catalog number: 10224490 )
- N2 gas (Westfalen)
- Ethyl acetate (VWR, catalog number: 23882.321 )
- Picodent twinsil 22 (Picodent, catalog number: 13001000 )
- Buffer A (see Recipes)
- Buffer B (see Recipes)
- Click reaction solution (see Recipes)
- Riranha solution (see Recipes)
Equipment
- Dumont #7 Tweezers (Carl Roth, catalog number: K344.1 )
Note: Dumont #7 Tweezers is used to pick up the glass slides. - 0.1-2.5 μl, 0.5-10 μl, 10-100 μl, 100-1,000 μl Pipettes (Eppendorf, catalog numbers: 3123000012 , 3123000020 , 3123000047 , 3123000063 )
- Vortexer (neoLab, catalog number: 7-0092 )
- Microcentrifuge (VWR, model: Micro Star 17, catalog number: 521-1646 )
- High-speed centrifuge (Beckman Coulter, model: Avanti® J-26S )
- Rotors for high-speed centrifuge (Beckman Coulter, models: JA-10 , JA-25.50 )
- Incubator (VWR, catalog number: 444-0732 )
- Sonicator (OMNI, model: Sonic Ruptor 400 )
- Invert fluorescence microscope (Leica Microsystems, model: Leica DMi8 )
- Ultrasonic cleaner (BANDELIN electronic, model: Sonorex Super RK 31 )
- Blue LED panel (Albrillo, model: LL-GL003 )
- OD Meter (Biochrom, BioWave, model: WPA CO8000 )
- Nanodrop (Thermo Fisher Scientific, model: NanoDropTM 8000 )
Software
- ImageJ
- Originlab
Note: Oringinlab is used for data analysis.
Procedure
- nMagHigh Protein Expression and purification
- Transform nMagHigh with a C-terminal His6-tag in a pET-21b(+) expression plasmid into BL21(DE3) E. coli cells using a standard protocol.
- Prepare a starter culture from a single colony in 10 ml LB with 50 μg/ml ampicillin. Incubate overnight at 37 °C at 250 rpm.
- Transfer 10 ml overnight culture into 1 L LB with 50 μg/ml ampicillin and grown at 37 °C at 200 rpm till OD600 = 0.6-0.8. When the desired OD600 is reached, add 500 µl IPTG (1 M stock, 500 µM final concentration) to the culture and incubate overnight at 16 °C at 250 rpm.
- Pellet bacteria at 6,371 x g at 4 °C for 8 min using the high-speed centrifuge with the JA-10 rotor (500 ml centrifuge tube). Discard the supernatant. All the following steps must be done on ice.
- Resuspend the pellet in 20 ml Buffer A (Recipe 1) containing supplemented with 1 mM PMSF (100 mM stock in methanol) and 1 mM DTT (1 M stock in water). Transfer the suspension into a 50 ml Falcon tube.
- Lyse bacteria by sonication. Settings for tip sonicator: 50% frequency, 40% power, 10 min. Keep the Falcon on ice during sonication. Transfer lysate into plastic high-speed centrifuge tubes, Centrifuge at 17,418 x g, 4 °C for 30 min using the JA-25.50 rotor (50 ml centrifuge tube).
- Filter the supernatant through 0.45 µm cellulose filter twice.
- Equilibrate Ni-NTA column (column volume = 5 ml) with 20-30 ml Buffer A + 1 mM DTT.
- Load clarified cell lysate on to the Ni-NTA column by passing the lysate twice dropwise using a 50 ml syringe.
- Wash Ni-NTA column with 10 ml Buffer A + 1 mM DTT and 50 ml (47.5 ml Buffer A + 2.5 ml Buffer B + 1 mM DTT) (Recipe 2).
- Elute with 10 ml Buffer B + 1 mM DTT. Discard the first 3 ml and collect the last 7 ml which contain the protein.
- Wash the column once more with Buffer B (50 ml, without DTT) and then Buffer A (50 ml, without DTT). Store Ni-NTA column in the fridge.
- Put the protein solution in dialysis bags (MW cut-off = 3,500 Da) and dialyze in 2 L Buffer A with stirring at 4 °C for 3 h. Replace the Buffer A with a fresh one and dialyze another 3 h at 4 °C. Collect the protein solution and store in -80 °C. Protein concentration is measured by Nanodrop.
- Transform nMagHigh with a C-terminal His6-tag in a pET-21b(+) expression plasmid into BL21(DE3) E. coli cells using a standard protocol.
- Functionalization of glass surfaces with nMagHigh
- Label the upper right corner of glass slides (20 x 20 mm) to distinguish the upper side. Clean glass surfaces in freshly prepared Piranha solution for 1 h, rinse three times with Milli-Q water and dry in an N2 stream.
- For the PEGylation reaction, immerse surfaces in a solution of PEG-azide (10 mg PEG-azide, MW = 3,500 g/mol) and a drop of triethylamine in 50 ml dry toluene and kept at 79 °C overnight under an N2 atmosphere in a closed container. Note that surfaces should not be touching each other.
- Wash the surfaces first with ethyl acetate for 5 min by sonication (Ultrasonic frequency: 35 kHz), then with methanol for 5 min by sonication and dry in an N2 stream.
- Prepare the humid chambers for click reaction by putting wet tissue paper in the lid of a Petri dish and cover it with the clean side of the parafilm.
- Prepare the click reaction solution (for six surfaces) (Recipe 3).
- Put 100 µl of click reaction solution on the parafilm in the Petri dish.
- Place one glass slide upside down on each the click reaction solution droplets (marked surface should be in contact with the click reaction solution). Incubate at room temperature for 2 h.
- After click reaction, wash the glass slides with 1) 50 mM EDTA in Buffer A for 5 min to remove Cu2+, and 2) Buffer A twice for 5 min.
- Incubate the slides with 100 µl 100 mM of NiCl2 on a parafilm at room temperature for 5 min. Ni2+ complexes to the NTA groups on the surface for His-tag binding. Wash the slides twice with Buffer A to remove excess Ni2+.
- Place 100 µl nMagHigh protein solution (10 µM, in Buffer A) droplets on a parafilm and put the glass slides upside down on the droplets. Incubate at room temperature for 30 min.
Note: The glass surfaces not incubated with nMagHigh protein will be used as negative control in D. - Wash the slides twice with sterile PBS and keep them in the PBS before use.
Note: The nMagHigh functionalized glass slides should be freshly prepared for the bacterial experiments.
- Label the upper right corner of glass slides (20 x 20 mm) to distinguish the upper side. Clean glass surfaces in freshly prepared Piranha solution for 1 h, rinse three times with Milli-Q water and dry in an N2 stream.
- Bacteria preparation
- Co-transform E. coli K12 MG1655 with the pMagHigh-eCPX or pMag-eCPX or pMagFast1-eCPX plasmid (chloramphenicol resistant) and the GFP pTrc99A plasmid (ampicillin resistant) and selected on an LB-agar plate with 35 μg/ml chloramphenicol and 50 μg/ml ampicillin.
- Inoculate a single colony into 5 ml LB medium containing 35 μg/ml chloramphenicol and 50 μg/ml ampicillin and incubate overnight at 37 °C at 250 rpm. Wrap all the tubes in aluminum foil.
- Add 500 μl of the overnight cultures into fresh 20 ml LB medium containing 35 μg/ml chloramphenicol and 50 μg/ml ampicillin and culture for 2 h at 37 °C, 250 rpm. Wrap all the tubes in aluminum foil.
- When the OD600 = 0.5, add 0.04% m/v L-arabinose to induce the expression of the pMag-eCPX proteins and 0.5 mM IPTG to induce the production of the fluorescent protein and incubate the cultures at 25 °C, 250 rpm for 4 h.
- Spin down the bacteria at 1,500 x g for 10 min and wash with PBS twice. Finally, re-suspend the bacteria in PBS to OD600 = 1.0.
- Co-transform E. coli K12 MG1655 with the pMagHigh-eCPX or pMag-eCPX or pMagFast1-eCPX plasmid (chloramphenicol resistant) and the GFP pTrc99A plasmid (ampicillin resistant) and selected on an LB-agar plate with 35 μg/ml chloramphenicol and 50 μg/ml ampicillin.
- Bacterial adhesion assays
- Use glass surfaces functionalized with nMagHigh protein by the method mentioned above and keep them in PBS in the dark. Use a surface that was not incubated with nMagHigh protein as a negative control to assure that the functionalization with the PEG worked.
- Place each glass surface in a 35 mm Petri dish. Add 3 ml of bacterial solution (the bacteria express pMagHigh or pMag or pMagFast1) with OD600 = 1.0 in PBS to each Petri dish.
- Place one set of surfaces with bacteria under blue light illumination (blue LED panel, 640 µW/cm2) for 1 h at room temperature. Place the second set of surface in the dark for 1 h.
- Wash the surfaces gently three times with PBS. Fix the bacteria with 4% paraformaldehyde for 20 min and mount them with Mowiol. Acquire nine fluorescent images in the GFP channel for each surface on the inverted fluorescence microscope (DMi8, Leica) through a 20x objective. (Figure 2)
- Analyze the numbers of bacteria on the surfaces using the particle analyzer tool in ImageJ.
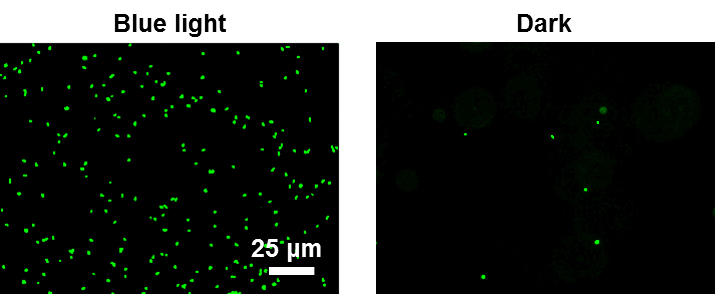
Figure 2. Fluorescence images of E. coli displaying pMagHigh which adhere on nMagHigh functionalized substrates under blue light but not in the dark. The bacteria are labeled with GFP for detection. Reproduced with permission from Chen and Wegner (2017).
- Use glass surfaces functionalized with nMagHigh protein by the method mentioned above and keep them in PBS in the dark. Use a surface that was not incubated with nMagHigh protein as a negative control to assure that the functionalization with the PEG worked.
- Bacterial attachment kinetics
- Functionalize five Glass surfaces with nMagHigh protein by the method mentioned above.
- Place each glass surface in a 35 mm Petri dish. Add 3 ml of pMagHigh-eCPX bacteria in PBS (OD600 = 1.0) to each Petri dish.
- Incubate the surfaces under blue light illumination (blue LED panel, 640 µW/cm2) for different time (10, 30, 60, 120 to 180 min) at room temperature.
- Wash each surface gently three times with PBS. Fix the bacteria with 4% paraformaldehyde and mount them with Mowiol. Acquire nine fluorescent images in the GFP channel (Ex = 488 nm, Em = 510 nm) for each surface on the inverted fluorescence microscope (DMi8, Leica) through a 20x objective.
- Analyze the numbers of bacteria on the surfaces using the particle analyzer tool in ImageJ.
- Quantify the attachment kinetics of the pMag-eCPX and pMagFast1-eCPX bacteria the same way following steps from E1 to E5 with corresponding bacteria.
- Functionalize five Glass surfaces with nMagHigh protein by the method mentioned above.
- Bacterial attachment adjusted by blue light illumination intensity
- Use six glass surfaces functionalized with nMagHigh protein prepared as mentioned above.
- Place each glass surface in a 35 mm Petri dish. Add 3 ml of pMagHigh-eCPX bacteria in PBS (OD600 = 1.0) to each Petri dish.
- Incubate the surfaces under blue light illumination with different intensity (0, 3.2, 32, 320, 640 or 3,200 µW/cm2) for 1 h at room temperature.
- Wash the surfaces gently three times with PBS. Fix the bacteria with 4% paraformaldehyde and mount them with Mowiol. Acquire nine fluorescent images in the GFP channel for each surface on an inverted fluorescence microscope (DMi8, Leica) through a 20x objective. Analyze the number of bacteria on the surfaces using the particle analyzer tool in ImageJ.
- Use six glass surfaces functionalized with nMagHigh protein prepared as mentioned above.
- Bacterial detachment kinetics
- Use six glass surfaces functionalized with nMagHigh protein prepared by the method mentioned above.
- Place each glass surface in a 35 mm Petri dish. Add 3 ml of pMagHigh-eCPX bacteria in PBS (OD600 = 1.0) to each Petri dish and incubate under blue light illumination (blue LED panel, 640 µW/cm2) for 1 h at room temperature.
- Move the Petri dishes to the dark for 0, 10, 30, 60, 120 or 240 min, respectively.
- Wash each surface gently three times with PBS. Fix the bacteria with 4% paraformaldehyde and mount them with Mowiol. Acquire nine fluorescent images in the GFP channel for each surface on an inverted fluorescence microscope (DMi8, Leica) through a 20x objective. Analyze the number of bacteria on the surfaces using the particle analyzer tool in ImageJ.
- Use six glass surfaces functionalized with nMagHigh protein prepared by the method mentioned above.
- Multiple attachment and detachment cycles
- Use five glass surfaces functionalized with nMagHigh protein prepared by the method mentioned above.
- Place each glass surface in a 35 mm Petri dish. Add 3 ml of pMagHigh-eCPX bacteria in PBS (OD600 = 1.0) to each Petri dish and incubate under blue light illumination (blue LED panel, 640 µW/cm2) for 1 h attachment at room temperature.
- Wash the first surface gently three times with PBS. Fix the bacteria with 4% paraformaldehyde and mount them with Mowiol. Acquire images imaged as described above.
- Meanwhile, keep the other four surfaces in the dark for 2 h for bacterial detachment.
- Wash the second surface gently three times with PBS and fix the bacteria with 4% paraformaldehyde.
- Meanwhile, place the remaining three surfaces under blue light (blue LED panel, 640 µW/cm2) for 1 h for attachment.
- Repeat the Steps H3 to H6 for another cycle. For each surface, acquire nine fluorescent images in the GFP channel on an inverted fluorescence microscope (DMi8, Leica) through a 20x objective. Analyze the number of bacteria on the surfaces using the particle analyzer tool in ImageJ.
- Use five glass surfaces functionalized with nMagHigh protein prepared by the method mentioned above.
- Bacterial patterning
- Co-transform E. coli K12 MG1655 with pMagHigh-eCPX (chloramphenicol resistant) and mCherry pTrc99A plasmid (ampicillin resistant) plasmids. Prepare bacteria as described above.
- The glass surface with nMagHigh protein immobilization is stuck to the bottom of a 35 mm Petri dish (with a round hole in 15 mm diameter). 3 ml pMagHigh-eCPX bacteria solution with OD600 = 1.0 is added into the Petri dish and kept in the dark.
Note: Picodent twinsil 22 is used as a glue to stick the glass surface to the Petri dish bottom. - The glass surface is locally illuminated with blue light through photomask (TEM grid without support film) for 1 h on an inverted fluorescence microscope (DMi8, Leica) through a 10x objective.
- The photomask is removed and the glass surface is gently washed by removing 2 ml solution and adding 2 ml PBS into the Petri dish. Repeat the washing steps for three times. Don’t let the surface dry.
- The surface is imaged by the inverted fluorescence microscope (DMi8, Leica) through a 10x objective. (Figure 3)

Figure 3. Photopatterning of pMagHigh displaying bacteria to nMagHigh functionalized glass surfaces. A) Bright field and B) fluorescent images of bacteria patterns. The bacteria are labeled with mCherry for detection. Reproduced with permission from Chen and Wegner (2017).
- Co-transform E. coli K12 MG1655 with pMagHigh-eCPX (chloramphenicol resistant) and mCherry pTrc99A plasmid (ampicillin resistant) plasmids. Prepare bacteria as described above.
Data analysis
Data information can be access via https://pubs.acs.org/doi/abs/10.1021/acssynbio.7b00197.
Notes
- All the experiments are performed in the dark room.
- Bacteria are cultured in the dark to avoid the activation of the photosensitive protein.
Recipes
- Buffer A (1x)
50 mM Tris-HCl, pH 7.4
300 mM NaCl (17.5 g for 1 L buffer) - Buffer B (1x)
Buffer A
250 mM Imidazole (17 g for 1 L buffer) - Click reaction solution (for six surfaces)
473 µl MilliQ water
60 µl 1 M Tris-HCl at pH 8.5
60 µl 1 M L-Ascorbic acid (prepare fresh: 102 mg L-Ascorbic acid in 741 µl MilliQ water)
0.9 µl 25 mM NTA-alkyne
6 µl 100 mM CuSO4 (should be added last; when added, locally a brownish color appears. After mixing, the solution becomes colorless)
Mix the solution by vortexing - Riranha solution
3:1 (v/v) conc. H2SO4:H2O2 (30%)
20 ml H2O2 (30%)
60 ml H2SO4
Acknowledgments
This work is part of the MaxSynBio consortium, which is jointly funded by the Federal Ministry of Education and Research (BMBF) of Germany and the Max Planck Society (FKZ 031A359L). F.C. would like to thank the Chinese Scholarship Council for a doctoral fellowship. The GFP and mCherry plasmids were a gift from Prof. Victor Sourjik and the CPX plasmid was a gift from Prof. Patrick Daugherty (Addgene plasmid # 23336). Our thanks go to Stefan Schumacher for his help with the figures. This protocol is adapted from previous work (Chen and Wegner, 2017). We would like to thank the editor of ACS Synthetic Biology for the permission to reprint the figures.
Competing interests
There are no conflicts of interest.
References
- Chen, F. and Wegner, S. V. (2017). Blue light switchable bacterial adhesion as a key step toward the design of biofilms. ACS Synth Biol 6(12): 2170-2174.
- Daugherty, P. S. (2007). Protein engineering with bacterial display. Curr Opin Struct Biol 17(4): 474-480.
- Elahipanah, S., Radmanesh, P., Luo, W., O'Brien, P. J., Rogozhnikov, D. and Yousaf, M. N. (2016). Rewiring gram-negative bacteria cell surfaces with bio-orthogonal chemistry via liposome fusion. Bioconjug Chem 27(4): 1082-1089.
- Flemming, H. C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S. A. and Kjelleberg, S. (2016). Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9): 563-575.
- Hu, Y., Zou, W., Julita, V., Ramanathan, R., Tabor, R. F., Nixon-Luke, R., Bryant, G., Bansal, V. and Wilkinson, B. L. (2016). Photomodulation of bacterial growth and biofilm formation using carbohydrate-based surfactants. Chem Sci 7(11): 6628-6634.
- Jensen, H., Biggs, C. A. and Karunakaran, E. (2016). The importance of sewer biofilms. WIREs Water 3 (4): 487-494.
- Kawano, F., Suzuki, H., Furuya, A. and Sato, M. (2015). Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat Commun 6: 6256.
- Liu, W., Roder, H. L., Madsen, J. S., Bjarnsholt, T., Sorensen, S. J. and Burmolle, M. (2016). Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization. Front Microbiol 7: 1366.
- Lui, L. T., Xue, X., Sui, C., Brown, A., Pritchard, D. I., Halliday, N., Winzer, K., Howdle, S. M., Fernandez-Trillo, F., Krasnogor, N. and Alexander, C. (2013). Bacteria clustering by polymers induces the expression of quorum-sensing-controlled phenotypes. Nat Chem 5(12): 1058-1065.
- Mockl, L., Muller, A., Brauchle, C. and Lindhorst, T. K. (2016). Switching first contact: photocontrol of E. coli adhesion to human cells. Chem Commun (Camb) 52(6): 1254-1257.
- Müller, K. and Weber, W. (2013). Optogenetic tools for mammalian systems. Mol Biosyst 9(4): 596-608.
- Nadell, C. D., Drescher, K. and Foster, K. R. (2016). Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol 14(9): 589-600.
- Peschke, T., Rabe, K. S. and Niemeyer, C. M. (2017). Orthogonal surface tags for whole-cell biocatalysis. Angew Chem Int Ed Engl 56(8): 2183-2186.
- Poortinga, A. T., Bos, R. and Busscher, H. J. (2000). Controlled electrophoretic deposition of bacteria to surfaces for the design of biofilms. Biotechnol Bioeng 67(1): 117-120.
- Rozhok, S., Shen, C. K., Littler, P. L., Fan, Z., Liu, C., Mirkin, C. A. and Holz, R. C. (2005). Methods for fabricating microarrays of motile bacteria. Small 1(4): 445-451.
- Sankaran, S., Kiren, M. C. and Jonkheijm, P. (2015). Incorporating bacteria as a living component in supramolecular self-assembled monolayers through dynamic nanoscale interactions. ACS Nano 9(4): 3579-3586.
- Sankaran, S., van Weerd, J., Voskuhl, J., Karperien, M. and Jonkheijm, P. (2015). Photoresponsive cucurbit[8]uril-mediated adhesion of bacteria on supported lipid bilayers. Small 11(46): 6187-6196.
- Schenk, F. C., Boehm, H., Spatz, J. P. and Wegner, S. V. (2014). Dual-functionalized nanostructured biointerfaces by click chemistry. Langmuir 30(23): 6897-6905.
- Tischer, D. and Weiner, O. D. (2014). Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol 15(8): 551-558.
- Van der Berg, J. P., Velema, W. A., Szymanski, W., Driessen, A. J. M. and Feringa, B. L. (2015). Controlling the activity of quorum sensing autoinducers with light. Chem Sci 6(6): 3593-3598.
- Voskuhl, J., Sankaran, S. and Jonkheijm, P. (2014). Optical control over bioactive ligands at supramolecular surfaces. Chem Commun (Camb) 50(96): 15144-15147.
- Weber, T., Chandrasekaran, V., Stamer, I., Thygesen, M. B., Terfort, A. and Lindhorst, T. K. (2014). Switching of bacterial adhesion to a glycosylated surface by reversible reorientation of the carbohydrate ligand. Angew Chem Int Ed Engl 53(52): 14583-14586.
- Zhang, R., Heyde, K. C., Scott, F. Y., Paek, S. H. and Ruder, W. C. (2016). Programming surface chemistry with engineered cells. ACS Synth Biol 5(9): 936-941.
- Zhou, M. H., Wang, H. Y., Hassett, D. J. and Gu, T. Y. (2013). Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J Chem Technol Biot 88(4): 508-518.
- Zoltowski, B. D., Vaccaro, B. and Crane, B. R. (2009). Mechanism-based tuning of a LOV domain photoreceptor. Nat Chem Biol 5(11): 827-834.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, F. and Wegner, S. V. (2018). Implementation of Blue Light Switchable Bacterial Adhesion for Design of Biofilms. Bio-protocol 8(12): e2893. DOI: 10.21769/BioProtoc.2893.
Category
Microbiology > Microbial biofilm > Biofilm culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









