- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protocols to Study Declarative Memory Formation in Mice and Humans: Optogenetics and Translational Behavioral Approaches
(*contributed equally to this work) Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2888 Views: 7242
Reviewed by: Edgar Soria-GomezFrancesco PapaleoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2606 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
Declarative memory formation depends on the hippocampus and declines in aging. Two functions of the hippocampus, temporal binding and relational organization (Rawlins and Tsaltas, 1983; Eichenbaum et al., 1992; Cohen et al., 1997), are known to decline in aging (Leal and Yassa, 2015). However, in the literature distinct procedures have been used to study these two functions. Here, we describe the experimental procedures used to investigate how these two processes are related in the formation of declarative memory and how they are compromised in aging (Sellami et al., 2017). First, we studied temporal binding using a one-trial learning procedure: trace fear conditioning. It is classical Pavlovian conditioning requiring temporal binding since a brief temporal gap separates the conditioned stimulus (CS) and unconditioned stimulus (US) presentations. We combined the trace fear condition procedure with an optogenetic approach, and we showed that the temporal binding relies on dorsal (d)CA1 activity over temporal gaps. Then, we studied the interaction between temporal binding and relational organization in declarative memory formation using a two-phase radial-maze task in mice and its virtual analog in humans. The behavioral procedure comprises an initial learning phase where subjects learned the constant rewarding /no rewarding valence of each arm, followed by a test phase where the reward contingencies among the arms remained unchanged but where the arms were recombined to assess flexibility, a cardinal property of declarative memory. We demonstrated that dCA1-dependent temporal binding is necessary for the development of a relational organization of memories that allows flexible declarative memory expression. Furthermore, in aging, the degradation of declarative memory is due to a reduction of temporal binding capacity that prevents relation organization.
Keywords: Trace fear conditioningBackground
Declarative memory formation (i.e., memory of everyday facts and events) is dependent on the hippocampus and declines in aging (Leal and Yassa, 2015). Two fundamental functions of the hippocampus are known to be age-sensitive. First, the hippocampus supports ‘temporal binding’, (Rawlins and Tsaltas, 1983), a function that allows association in memory of discontiguous events. Trace conditioning tasks allow the demonstration of the critical role of hippocampus in temporal binding (Solomon et al., 1986; Clark and Squire, 1998; LaBar and Disterhoft, 1998; Huerta et al., 2000) and its decline in aging (Disterhoft and Oh, 2007). Second, the hippocampus is critical to the formation of ‘relational organization’, a function that links memorized information/events and consequently supports cardinal flexibility of declarative memory, exemplified in the capability to make inferences from memory (Bunsey and Eichenbaum, 1996) or to compare separately acquired information to guide a choice decision in a novel situation (Etchamendy et al., 2003; Mingaud et al., 2007). This capability is compromised in aging (Rapp et al., 1996; Marighetto et al., 1999; Etchamendy et al., 2001; Mingaud et al., 2008). However, temporal binding and relational organization have always been studied separately. Here, we describe procedures to study how these two processes are related. Our previous study showed that these two hippocampal functions are causally related in the formation of declarative memory by using a specific experimental protocol combining behavioral and optogenetics approaches (Sellami et al., 2017). Specifically, temporal binding is a necessary condition for the relational organization of discontiguous events (Figure 1). We found out that the formation of a relational memory is limited by the capability of temporal binding, which depends on dorsal (d)CA1 activity over time intervals and diminishes in aging. Conversely, relational representation is successful, even in aged individuals, when the demand on temporal binding is minimized, showing that the relational/declarative memory per se is not impaired in aging. Thus, bridging temporal intervals by dCA1 activity is a critical foundation of relational representation, and a deterioration of this mechanism is responsible for the age-associated memory impairment.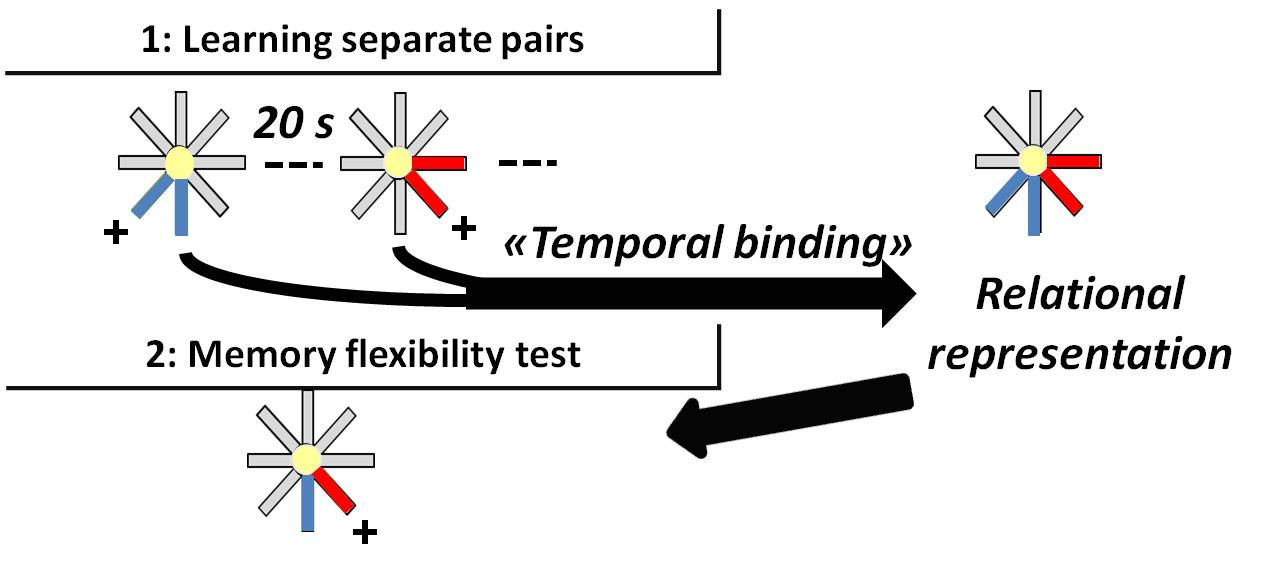
Figure 1. Temporal binding is critical to associate discontiguous events into a relational representation allowing flexible expression of memory
Part I: Virus transfection and fiber implantation
Materials and Reagents
- Glass capillaries (WPI, catalog number: 1B150F-4 )
- Silicone catheter (Dominique DUTSCHER, catalog number: 351070 )
- Syringe 1 ml (Henke-Sass, Wolf, catalog number: 4010.200V0 )
- Syringe 5 ml (Henke-Sass, Wolf, catalog number: 4050.000V0 )
- Petri dish 100 x 15 mm
- Implantable Fiber Optic Cannulae (Thorlabs, catalog number: CFMLC12L02 )
- Optic fiber (Thorlabs, catalog number: FT200EMT )
- Patch cable (Thorlabs, catalog number: M83L01 )
- Ceramic split mating sleeve (Thorlabs, catalog number: ADAL1 )
- Parafilm (Heathrow Scientific, Bemis, catalog number: HEA234526A )
- Surgical blades
- Screws (MicroFastenings, catalog number: M0.6x1.5 )
- Needle 26 G x ½" (Terumo Medical, catalog number: NN-2613R )
- Needle 23 G x 1¼" (Henke-Sass, Wolf, catalog number: 4710006030 )
- Cotton swab (The Lab Depot, catalog number: 394305 )
- Stitching kit and sutures (Péters Surgical, catalog number: 87001F )
- 6-well culture plate (Corning, Falcon®, catalog number: 353046 )
- Microscope slide (Thermo Fisher Scientific, catalog number: LCSF02 )
- Cover slips (Knittel Glass, catalog number: VD12450Y1A.01 )
- Brush (Henry Schein France, catalog number: 878-7825 )
- 1 L Solvent Bottle (Thermo Fisher Scientific, catalog number: 045900 )
- Whatman® paper filter (GE Healthcare, catalog number: 1213125 )
- Young adult (3- to 4-mo-old) and aged (21-to 23-mo-old) C57BL/6 male mice (Charles River)
- AAV vectors:
- AAV-CAMKIIa-hChR2(H134R)-EYFP (University of North Carolina (UNC) Vector Core)
- AAV-CAMKIIa-ArchT-GFP (University of North Carolina (UNC) Vector Core)
- AAV-CAMKIIa-GFP (University of North Carolina (UNC) Vector Core)
- AAV-CAMKIIa-hChR2(H134R)-EYFP (University of North Carolina (UNC) Vector Core)
- Isoflurane 1,000 mg/g (4% induction and 1-2% for maintenance, Iso-Vet)
- Betadine
- Super glue (Loctite)
- Liquid fix glue: Methylmethacrylate (Sigma-Aldrich, catalog number: M55909-25ML )
- Super-Bond C&B dental cement (Sun Medical, catalog number: P021E/0A )
- Metacam: Méloxicam 1.5 mg/ml analgesic (Boehringer Ingelheim)
- Lurocaine: Lidocaïne 20 mg (Vetoquinol)
- 70% ethanol solution
- Lacrigel: eye ointment (Europhta)
- Sulmidol: Sulfapyridine 100 mg (MSD, santé animale)
- Hydrogen peroxide (Sigma-Aldrich, catalog number: H1009 )
- Sodium phosphate dibasic, Na2HPO4 (Sigma-Aldrich, catalog number: S0876 )
- Sodium phosphate monobasic, NaH2PO4 (Sigma-Aldrich, catalog number: S0751 )
- Sodium chloride, NaCl (Sigma-Aldrich, catalog number: 433209 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 441244 )
- Sodium Hydroxide solution 1.0 N, NaOH (Sigma-Aldrich, catalog number: S2770 )
- FluorSave reagent (Merck, catalog number: 345789 )
- 0.1 M PBS (see Recipes)
- 0.2 M PB (see Recipes)
- PFA 4%/0.1 M PB solution (see Recipes)
Equipment
- Small scissors (World Precision Instruments, catalog number: 504615 )
- Bone scraper (World Precision Instruments, catalog number: 503759 )
- Drill (RWD Life Science, catalog number: 78001 )
- Fine tip forceps (World Precision Instruments, catalog number: 501975 )
- Needle holder with Suture Scissors (World Precision Instruments, catalog number: 500023 )
- Screwdriver (World Precision Instruments, catalog number: 501635 )
- Compact Power and Energy Meter Console (Thorlabs, catalog number: PM100D )
- Anesthesia system for isoflurane (Datex Ohmeda ISO Isoflurane Anesthesia Vaporizer Tec 7)
- Pipette puller (Sutter Instrument, model: P97 )
- Picospritzer (Parker Hannifin)
- Hair clipper (PHYMEP, model: Contura Shaver )
- Heating pad (Tem Sega, model: THERM250 )
- Mouse stereotaxic apparatus (KOPF INSTRUMENTS, model: 942 )
- Binocular loupe (Leica Microsystems, model: Leica S6E )
- Perfusion pump (Cole-Parmer, model: Master Flex® L/S® )
- Vibratome (Leica Biosystems, model: Leica VT 1000S )
- Microscope (ZEISS, model: Axio Imager A2 )
- The Mouse Brain in Stereotaxic Coordinates, 2001
- TTL pulse generator (Imetronic, Pessac, France)
Procedure
- Preparation for surgery
- Pull the glass pipette using the puller and then break the tip back to a tip diameter between 10-20 μm.
- Fill the glass pipette with virus solution (2 μl per mouse): Place a drop of virus on parafilm paper then place the pipette on the stereotaxic frame and connect it to a silicone catheter. Under binocular control, gently lower the pipette until it touches the drop and gently aspirate the drop with a syringe of 5 ml, be careful not to aspire in air, and then remove the syringe to stop aspiration.
- Graduate the glass pipette every 1 mm, corresponding to a volume of 1 µl.
- Store the glass pipette filled with the virus and the Super Bond container at 4 °C.
- Pull the glass pipette using the puller and then break the tip back to a tip diameter between 10-20 μm.
- Virus injection procedure
- Place the mouse in the induction box (4% isoflurane) until respiration slows down (5 min). Then transfer it onto a heating pad, and next fix it in the ear bar of the stereotaxic frame connected to the anesthesia mask (1% to 2%); always monitor the breathing rate, if the rhythm increases or decreases abnormally, it is necessary to adjust the flow of the anesthetic gas. Toe pinch the mouse to confirm anesthesia.
- Do intraperitoneal injection of Metacam (0.2 ml diluted at 1:20), inject 0.1 ml of Lurocaine locally under the surface of the scalp, and apply eye ointment.
- Shave the head of the mouse, then use a cotton swab with Betadine to clean the scalp. Make an incision in the scalp horizontally, remove tissues on top of skull with a bone scraper, and dry the skull by applying Hydrogen peroxide with a cotton swab.
- Mark the Bregma and then attach a piece of metal tubing (like a needle) to the stereotaxic probe holder and mark the skull accordingly to the coordinates of the bilateral injections sites that target the:
- dCA1: two bilateral sites: 1.8 and 2.5 mm posterior to the Bregma, 1.3 and 2 mm lateral of the midline respectively.
- dCA2 and dCA3: 2.0 mm posterior to the Bregma, 2.5 mm lateral of the midline.
- dCA1: two bilateral sites: 1.8 and 2.5 mm posterior to the Bregma, 1.3 and 2 mm lateral of the midline respectively.
- Position the glass pipette containing the virus in the stereotaxic frame just above the first hole so that its tip touches the surface of the skull. Very gently lower the pipette down to -1.4 mm for dCA1 and -2 mm for dCA2/dCA3.
- Wait 2 min after the descent of the pipette and then, using a syringe connected to the catheter (Figure 2), inject 0.2 µl of the virus, by applying a positive pressure with hand, check the glass pipette graduations. Wait again for 2 min.
Caution: Remove the syringe just after injections otherwise, the injection continues.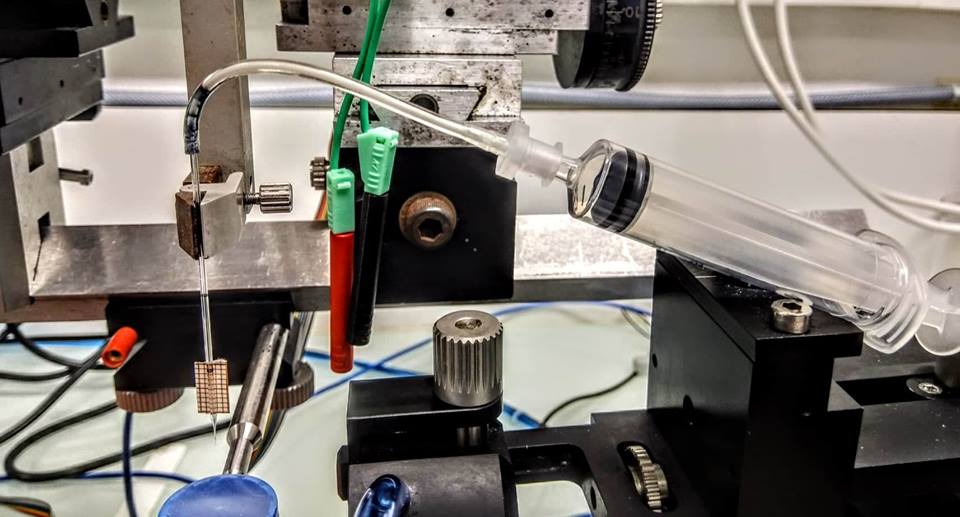
Figure 2. Image of the setup used for the administration for the virus through a glass pipette - Very gently lift the pipette and repeat the procedure with the other 4 coordinates.
- Clean the skull with Betadine then suture the skin with 2-3 stitches using the needle holder. Apply Betadine, Sulmidol healing cream, and put back mouse in its home cage. Monitor each mouse for two weeks until fiber implantation: check the mouse activity and check its weight.
- Place the mouse in the induction box (4% isoflurane) until respiration slows down (5 min). Then transfer it onto a heating pad, and next fix it in the ear bar of the stereotaxic frame connected to the anesthesia mask (1% to 2%); always monitor the breathing rate, if the rhythm increases or decreases abnormally, it is necessary to adjust the flow of the anesthetic gas. Toe pinch the mouse to confirm anesthesia.
- Fiber implantation
Two weeks later, perform a new surgery to implant optic fibers that target dCA1, dCA2 or dCA3.- Steps C1 to C3 of this procedure are the same as for the virus injection procedure (B1-B3).
- Start by implanting screws in order to improve the stability of the fibers:
- Drill 3 small holes away from the coordinates of the fibers: one on the top left, one on middle top left, and one on middle bottom of the skull.
- Place the 3 screws and connect them by applying super glue gel, to solidify different parts of the skull together. Apply a few drops of fix glue to dry the glue.
- Drill 3 small holes away from the coordinates of the fibers: one on the top left, one on middle top left, and one on middle bottom of the skull.
- Place the optic fiber implant in the ‘ceramic split mating sleeve’ to hold the implant and above the first hole in the skull the targets either the:
- dCA1: anteroposterior 1.8 mm, lateral ± 1.3 mm.
- dCA2: anteroposterior 1.8 mm, lateral ± 2.1 mm.
- dCA3: anteroposterior 1.85 mm, lateral ± 2.45 mm.
- dCA1: anteroposterior 1.8 mm, lateral ± 1.3 mm.
- Then clean the coagulated blood and slowly lower the implant to the desired depth; dCA1 (1.4 mm), dCA2 (1.7 mm), and dCA3 (1.9 mm), then apply Super Bond cement and wait 15 min until the cement solidifies. Gently lift the fiber holder and repeat the procedure with the other location by making sure that there is enough cement between the two fibers.
- Suture the skin with 2 stitches using the needle holder. Apply Betadine, Sulmidol healing cream, and put back mouse in its cage. Monitor each mouse for 10 to 12 days until behavioral testing: check the mouse activity and its weight.
- Steps C1 to C3 of this procedure are the same as for the virus injection procedure (B1-B3).
- Injection sites and fiber implantation histological controls
- The mice are euthanized with an intraperitoneal injection of overdose Pentobarbital (0.1 ml/10 g mouse weight).
- Place the mouse on a dissection board, open the rib cage and expose the heart. Insert the needle, attached to a catheter, in the left ventricle of the heart and clamp it.
- Snip the right atrium and turn on ice-cold PFA 4%/PB 0.1 M solution perfusion (100 ml/mouse, at 10 ml/min).
- Open the skull to extract the brain. Pay attention to the dura. Immerse the brain in PFA solution and store at 4 °C for 24 h.
- Make a small incision along the brain to mark the right side and glue the brain to the vibratome plate on the cerebellum side.
- Cut coronal sections of 50 µm from -0.34 mm to -3.6 mm anteroposterior to collect the corresponding sections from the dorsal hippocampus (The Mouse Brain in Stereotaxic Coordinates, 2001).
- Using a brush, transfer the cuts to a slide and mount them with 3-4 drops of the Fluorsave reagent.
- The mice are euthanized with an intraperitoneal injection of overdose Pentobarbital (0.1 ml/10 g mouse weight).
Part II: Optogenetic manipulations of dCA1 or dCA2/dCA3 activity in behaving animals
The behavioral procedure started after a 10- to 12-d recovery.
Materials and Reagents
- Fiber-optic patch cords: diameter, 200 μm (Doric Lenses, catalog number: MFP_50/125/900-0.22_#.#_FC-FC )
- Optic Rotary Joints (Doric Lenses, catalog number: FRJ_1x2i_FC-2FC_0.22 )
- Young adult (3- to 4-mo-old) and aged (21-to 23-mo-old) C57BL/6 male mice (Charles River, catalog number: 027 )
- Ethanol 70%
- Acetic acid 1% (Sigma-Aldrich, catalog number: 1005706 )
- Pasta pellets (PANZANI, model: Perles)
Equipment
- Laser (IkeCool OptoDuet 473/593 nm)
- Fear conditioning chamber equipped for optogenetics (OptoPath platform; IMETRONIC, conditioning and Test in 2 distinct apparatus):
- The first chamber is the conditioning and consists of a Plexiglas chamber (34 x 24 x 22 cm) illuminated with a luminous intensity of 100 lux allowing the mouse to visualize the visual cues (geometric shapes of different colors). The floor of the chamber consists of a grid (60 stainless steel bars of 2 mm in diameter) connected to a generator. This chamber is equipped for optogenetics: TTL pulse generator, a device that activates the laser.
- The second chamber is the neutral context and consists of a round enclosure in Plexiglas (24 cm of diameter) with a rough floor without the presence of a grid.
- The first chamber is the conditioning and consists of a Plexiglas chamber (34 x 24 x 22 cm) illuminated with a luminous intensity of 100 lux allowing the mouse to visualize the visual cues (geometric shapes of different colors). The floor of the chamber consists of a grid (60 stainless steel bars of 2 mm in diameter) connected to a generator. This chamber is equipped for optogenetics: TTL pulse generator, a device that activates the laser.
- Radial maze equipped for optogenetics (OptoPath platform; IMETRONIC) (Figure 4A)
- Rotation assistant for radial maze (OptoPath platform; IMETRONIC)
Software
- Videotracking system (IMETRONIC, model: Polytrack)
- Fear conditioning cage associated software (IMETRONIC)
- Radial maze associated software (IMETRONIC)
Procedure
- Trace fear conditioning
Trace conditioning task (Figure 3A) is a classical Pavlovian conditioning task where a brief temporal gap separates the conditioned stimulus (CS) and unconditioned stimulus (US) presentations.
Animals are continuously recorded on videotape for off-line scoring of freezing behavior by an observer blinded to the experimental groups. Freezing is defined as a lack of all movement except for respiratory-related movements and it is expressed as % time spent freezing over specific periods: tone delivery during the acquisition (3 x 30 sec) and tone test (2 min), the neutral conditioning chamber during the tone test (no-tone period, 2 min), and the conditioning context in the context test (4 min). Mice expressing less than 10% freezing during the last (third) tone in acquisition are excluded of experiment analyses.
Day 1: Acquisition of conditioning for groups with optogenetic manipulations- Handle each mouse for 10 min on each of 2 days before fear conditioning. The handling accustoms the mouse to the contention. Indeed, to connect the mouse to the fiber-optic patch cords in the conditioning chamber, they must be restrained.
- Clean the conditioning chamber with 70% ethanol before placing each animal.
- Connect each animal to the fiber-optic patch cords and then place in the conditioning chamber for 8 min, during which it receives three pairings of a tone CS (65 dB, 1 kHz, 30 sec) and a footshock US (0.3 mA, 50 Hz, 1 sec) separated by a trace interval for either 20 sec (young mice) or 40 sec (old mice). Optogenetic manipulations (~6 mW per implanted fiber bilaterally conducted from the laser) are performed during the acquisition of fear conditioning and at specific moments depending on the group:
- Young mice at trace 20 sec:
- Mice expressing ArchT channel and GFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1 or dCA2 or dCA3: to transitory inhibit the dCA1 neuronal activity, activate the laser (continuous light deliver, 593 nm) during the 20 sec trace interval.
- Mice expressing ArchT channel and GFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1: to transitory inhibit the dCA1 neuronal activity, activate the laser (continuous light deliver, 593 nm) outside the 20 sec trace interval.
- Control mice expressing GFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1: activate the laser (continuous light deliver, 593 nm) during the 20 sec trace interval.
- Mice expressing ArchT channel and GFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1 or dCA2 or dCA3: to transitory inhibit the dCA1 neuronal activity, activate the laser (continuous light deliver, 593 nm) during the 20 sec trace interval.
- Old mice at trace 40 sec:
- Mice expressing ChR2 channel and EYFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1: to transitory activate the dCA1 neuronal activity, activate the laser (light is delivered at 5 Hz: 5 msec laser on, 195 msec laser off) during the 40 sec trace interval.
- Mice expressing ChR2 channel and EYFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1: to transitory activate the dCA1 neuronal activity, activate the laser (light is delivered at 5 Hz: 5 msec laser on, 195 msec laser off) outside the 40-sec trace interval.
- Mice expressing ChR2 channel and EYFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1 without optogenetic manipulation.
- Mice expressing ChR2 channel and EYFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1: to transitory activate the dCA1 neuronal activity, activate the laser (light is delivered at 5 Hz: 5 msec laser on, 195 msec laser off) during the 40 sec trace interval.
- Young mice at trace 20 sec:
Day 2: Retention tests- Mice are first re-exposed to the tone alone in a neutral context, i.e., an unknown chamber (tone test; 6 min with 2 min tone in the middle). Clean the testing chamber with 1% acetic acid before placing each animal to improve discrimination between the neutral and the conditioning context.
- 2 h later, mice are re-exposed to the conditioning chamber without footshock (context test; 4 min). Clean the conditioning chamber with 70% ethanol before placing each animal to reconstruct the conditioning context.
- After the retention tests, mice are euthanized to verify the injections and the fibers implantation sites (see Part I Procedure D).
- Results obtained from the retention tests are presented in Figure 3B for young mice and in Figure 3A for old mice (figures adapted from Sellami et al., 2017).
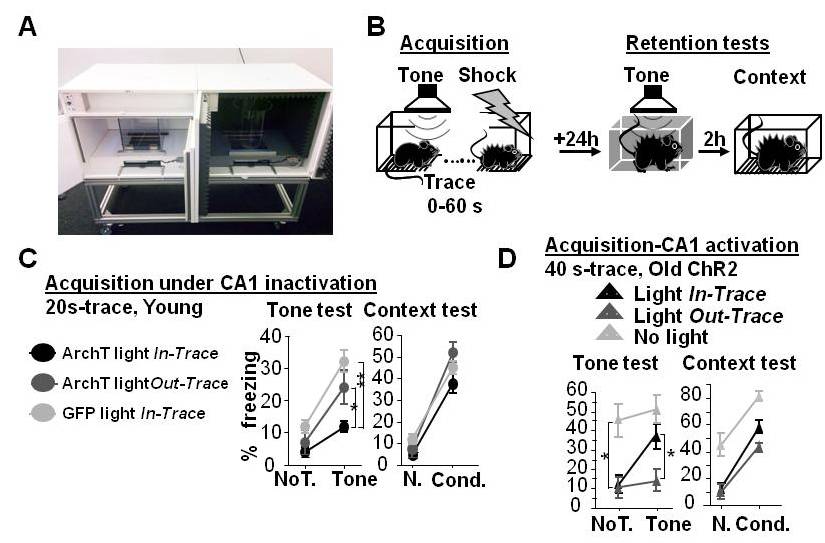
Figure 3. In trace tone–fear conditioning, the formation of long-term memory of the trace CS-US association is sustained by CA1 cell activity across the trace interval, and disrupted in aging. A. Photograph of the conditioning chamber (left) and the neutral context (right). B. Procedure: Young and aged mice were submitted to the acquisition of conditioning: three pairings of a tone (CS) and mild electric foot shock (US) with a time interval between the two (trace) of either 0, 5, 20, 40, or 60 sec depending on the group. C. Retention effects of optogenetic inactivation of CA1 during the acquisition of 20-sec trace conditioning in young mice. The 24-h retention of tone trace conditioning is altered by in-trace inactivation compared with both control conditions. In contrast, the retention of context assessed by freezing difference between the neutral (N.) and conditioning context (Cond.) is similar among the groups. Thus, CA1 activity across the trace interval during conditioning is a necessary condition for successful temporal binding of the CS and US in memory. D. Retention effects of optogenetic activation of CA1 during acquisition of 40-sec trace conditioning in old mice. Without affecting the retention of context conditioning, in-trace (but not out-of-trace) activation enables the retention of tone conditioning, which is normally not retained in old mice. Thus, CA1 activity across temporal gaps is sufficient to restore the age-related defect of temporal binding in memory. Note that the age-related deficit is associated with overall increased levels of freezing, suggesting fear generalization that was normalized by both in-trace and out-of-trace activation. *P < 0.05; **P < 0.01. Data are presented as mean ± SEM.
- Handle each mouse for 10 min on each of 2 days before fear conditioning. The handling accustoms the mouse to the contention. Indeed, to connect the mouse to the fiber-optic patch cords in the conditioning chamber, they must be restrained.
- Memory testing in the radial maze without optogenetic manipulations
The ‘go/no-go’ two-stage discrimination learning paradigm (Figure 4A) comprises an initial learning phase (stage 1) followed by a test phase (stage 2) assessing flexibility, a cardinal property of declarative memory. Flexibility is exemplified in the ability to compare and contrast separately acquired information to guide inferential decision in novel situations. In stage 1, the mice learn the constant locations of food reward through successive and separate exposures to individual arms using a ‘go/no-go’ discrimination procedure. In stage 2, they are challenged with novel arm presentations that require explicit choices among adjacent arms that were experienced separately in stage 1 and are now presented by pairs (simultaneous ‘two-choice’ discrimination test).- Mice are put on a food restriction diet so that their individual body weights are reduced to and maintained at 85-88% of the ad libitum weights. Before behavioral testing, they were habituated to the apparatus over 2 days by allowing them free exploration until all the pasta pellets in the food well of each arm were collected.
- Each mouse was separately assigned six adjacent arms of the radial maze. The reward valence of the arms (three baited, three non-baited) remains constant throughout the experiment, but the manner of presenting the arms to the mouse (i.e., one-by-one vs. by pair presentation) are changed between the two stages of the experiment.
Stage 1 (Acquisition phase): acquisition of successive go/no-go discrimination between rewarded and nonrewarded arms
In the acquisition phase, the arms are opened one by one successively with a waiting-time inter-trial interval (ITI; 0 to 60 sec depending on the group) separating the 24 successive arm visits made within a session, and a 24 h interval between sessions. Training sessions (minimum, 5; maximum, 12) are repeated until reaching the acquisition criterion (when latency to enter non-baited arms is at least 30% longer than for baited ones; see Data analysis). Mice failing to reach the criterion within 12 training sessions are excluded from the experiment.
Stage 2 (Flexibility probe): simultaneous two-choice discrimination assessing mnemonic flexibility
One day after the end of the acquisition phase, the flexibility-test phase is performed. In this test, the arms are opened by pairs (one baited, one non-baited) instead of one by one. Memory flexibility is reflected by the capability to correctly choose the food-containing arm among each pair, indicating that a relational representation was formed of previous separate experiences of individual arms (Figure 4B in Sellami et al., 2017). The test session is made up of 20 pair presentations, and performance is indexed by the percentage of correct choices.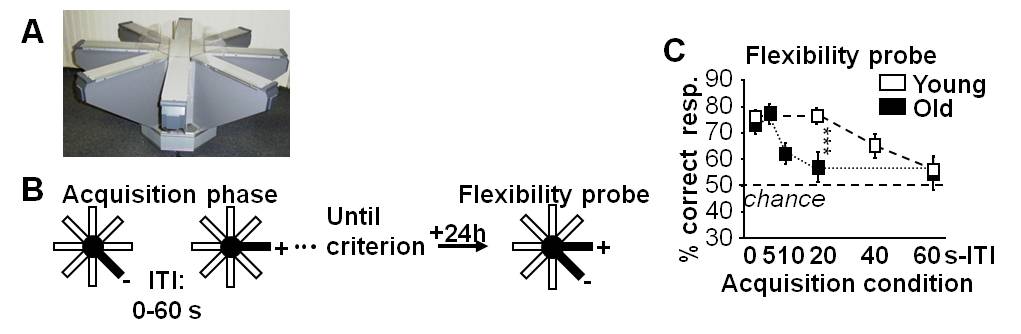
Figure 4. Temporal binding is a critical determinant of declarative memory formation and its age-associated degradation. A. Photography of radial maze; B. Procedure of the ‘go/no-go’ two-stage discrimination learning paradigm in the radial maze. C. Flexibility probe: Performance depends on the ITI condition under which memories were encoded, in an age-specific manner. Thus, flexible memory expression relies on the capability to relate individual arm visits across time intervals, capability limited to less than 60-sec inter-trial interval in young mice and to only 5-sec intervals in aged mice. ***P < 0.001 vs. aged.
- Mice are put on a food restriction diet so that their individual body weights are reduced to and maintained at 85-88% of the ad libitum weights. Before behavioral testing, they were habituated to the apparatus over 2 days by allowing them free exploration until all the pasta pellets in the food well of each arm were collected.
- Memory testing in the radial maze with optogenetic manipulations
In the experiment consisting in optogenetic inactivation of dCA1 in young mice, we chose to use the second version of our radial-maze design, the version which is translated to human subjects using a virtual analog of the radial maze (‘by pair’ two-stage discrimination learning paradigm, Figure 5A), as we wanted to further increase similarities with the study performed in humans. The procedure of the task for mice is similar to the one previously described except that initial acquisition consists of learning three pairs of arms, and the flexibility probe consists of recombination of two initial pairs into a novel pairing.
Stage 1
Acquisition training (20 trials of one pair presentation each day) is performed (during a minimum of 5 to a maximum of 12 days) until reaching the acquisition criterion (overall > 70% correct over 2 days; > 67% correct for each pair) with an ITI of 20 sec.
Note: In order to transitory inhibit the dCA1 neuronal activity, we activated the laser (continuous light deliver, 593 nm) during the ITI of 20 sec, in young mice expressing ArchT channel and GFP in the dorsal hippocampus and chronically implanted with optic fibers in the dCA1.
Stage 2
Flexibility testing is performed the day following reaching the acquisition criterion. The test session consists of 20 trials of one pair presentation (ITI, 20 sec; three pairs). Eight trials with the critical pair made from recombination of two previous pairs are intermixed with two control pairs: one unchanged from acquisition, the other made up of unlearned arms. Performance is measured by the percentage of correct choices of the rewarded arm in each pair. At the end of stage 2, mice are euthanized in order to control the injections and the fibers implantation sites (see Part I Procedure D).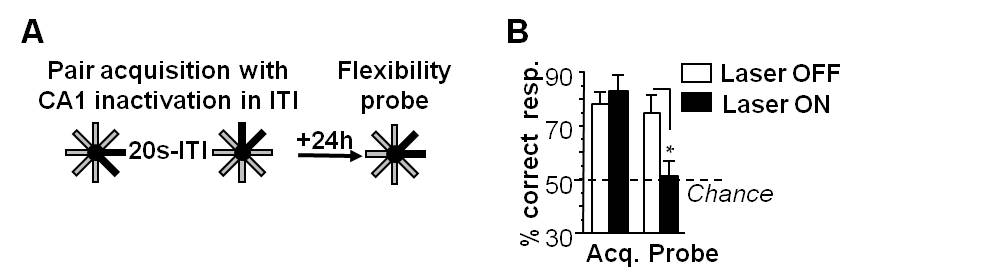
Figure 5. Temporal binding is sustained by the dorsal CA1 activity. A. ‘by pair’ two-stage discrimination learning paradigm with CA1 inactivation in the inter-trial interval (ITI) during the acquisition phase. B. Optogenetic inactivation of CA1 during the 20-sec ITI between events in the acquisition phase also produces a subsequent impairment of performance in the flexibility probe. Here we used the version of our radial-maze design also used in humans (Figure 6). The initial acquisition of separate pairs was spared by CA1 inactivation in 20-sec ITI but performance was severely diminished in the ‘recombined’ test of flexibility in the ‘laser on’ group compared with controls. Thus, CA1 is needed to bridge a temporal gap, and this temporal binding function is crucial for relational organization sustaining the formation of flexible/declarative memory. n = 7 to 8 per group. *P < 0.05. Data are presented as mean ± SEM.
Part III: Memory testing in the virtual radial maze in human
Materials and Reagents
- Young (18- to 25-y-old) and old (59- to 75-y-old) participants well-matched in educational level and sex ratio
Note: The elderly subjects are selected as cognitively normal for their age, based on their score in the Mini Mental Score Evaluation (MMSE > 27/30) and in the Grober & Buschke test of declarative memory (G&B delayed recall > 18/48).
Equipment
- Virtual radial maze (OptoPath; ANR-10-EQX-008- 1; in collaboration with Imetronic)
- Computer (any type of computer is fine)
Software
Procedure
We used a virtual analog of the radial-maze task for mice. The virtual maze was designed and automatized to make the test as similar as possible as the one used with the mice (Figure 6A and Video 1).
Stage 1
In the acquisition phase of the task, each subject is faced with successive presentations of six pairs of adjacent arms and required to visit one of the two arms during each trial. In each pair, one arm always contains a reward (virtual coin) at its end, while the other arm never contains any reward. Training continues until reaching the acquisition criterion (when the number of incorrect choices of the non-rewarded arm is less than 2 over 12 consecutive trials), and a minimum of 6 trials by pair or maximum of 20 trials by pair are performed.
Stage 2
- Once the acquisition criterion is reached, then subject the participant to the probe test in which nothing is modified except that the arms are presented in new sets of pairs (recombination of previous pairs) to assess the flexibility of memory expression.
- Divide the young and aged participants into three inter-trial interval conditions (0, 20, or 40 sec) for the acquisition phase on the basis of their performance in the Grober and Buschke test, such that the mean score in this classical test of declarative memory is similar among the ITI conditions in each age group. Participants who failed to reach the acquisition criterion are rejected from the experiment.
- Results obtained from the flexibility probe are presented in Figure 6B (adapted from Sellami et al., 2017).
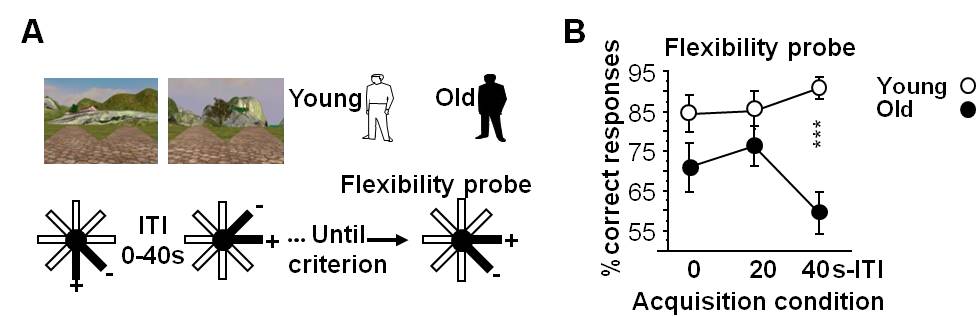
Figure 6. Age-associated decline in flexible/declarative memory depends on temporal binding in humans: the virtual radial-maze task. A. Procedure of the ‘by pair’ two-stage discrimination learning paradigm in the virtual analog of the radial maze; B. In the flexibility probe, there was an age-related impairment dependent on the inter-trial interval (ITI) condition under which the task was acquired. Thus, the age-related loss of flexibility is due to a reduction of temporal binding capability. ***P < 0.001. Data presented are means ± SEM.
Data analysis
- Data were analyzed using one- or two-way ANOVAs. When appropriate, post hoc comparisons were achieved by a Fisher protected least significant difference test with a significance level at P < 0.05.
- For statistical analysis, go/no-go discrimination performance is indexed by a normalized discriminative ratio: (latency for non-baited − latency for baited)/(latency for non-baited + latency for baited).
Recipes
- 0.1 M PBS
Na2HPO4 11.36 g
NaH2PO4 2.4 g
NaCl 0.9 g
Add ddH2O to make 1 L solution and store it at 4 °C - 0.2 M PB
Na2HPO4 22.72 g
NaH2PO4 4.8 g
Add ddH2O to make 1 L solution and store it at 4 °C - PFA 4%/0.1 M PB solution
To store the solution, you need to prepare 8% PFA solution:- Mix 80 g of PFA powder in 600 ml of dH2O and heat to about 70 °C until the powder is dissolved (at least 120 min)
- Add a few drops of NaOH solution to make the solution transparent
- Filter the solution using a Whatman® paper filter
- Adjust the volume to 1 L with dH2O and store it at 4 °C (for maximum 15 d)
- Mix 80 g of PFA powder in 600 ml of dH2O and heat to about 70 °C until the powder is dissolved (at least 120 min)
Acknowledgments
This protocol was used to obtain the data published in PNAS (Sellami et al., 2017).
We thank all of the personnel of the Animal Facility of the Neurocentre Magendie for mouse care, Mathieu Baudonnat and Florian Jacquot for their technical assistance in certain pilot experiments, and Aline Desmedt and Ludovic Calandreau for useful discussions. This work was supported by INSERM, a Bordeaux Neurocampus grant by le Conseil Régional d’Aquitaine, an EquipEx Grant OptoPath (ANR-10-EQX-008-1), Bordeaux Science Agro, CNRS, and National Institute of Mental Health Grant MH095297. We thank the French pension fund ProBTP and its previous director, Paul Grasset, who helped us to recruit healthy aged people to perform our experiments.
Competing interests
The authors declare no conflict of interest.
References
- Bunsey, M. and Eichenbaum, H. (1996). Conservation of hippocampal memory function in rats and humans. Nature 379(6562): 255-257.
- Clark, R. E. and Squire, L. R. (1998). Classical conditioning and brain systems: the role of awareness. Science 280(5360): 77-81.
- Cohen, N. J., Poldrack, R. A. and Eichenbaum, H. (1997). Memory for items and memory for relations in the procedural/declarative memory framework. Memory 5(1-2): 131-178.
- Disterhoft, J. F. and Oh, M. M. (2007). Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 6(3): 327-336.
- Eichenbaum, H., Otto, T. and Cohen, N. J. (1992). The hippocampus--what does it do? Behav Neural Biol 57(1): 2-36.
- Etchamendy, N., Enderlin, V., Marighetto, A., Vouimba, R. M., Pallet, V., Jaffard, R. and Higueret, P. (2001). Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J Neurosci 21(16): 6423-6429.
- Etchamendy, N., Desmedt, A., Cortes-Torrea, C., Marighetto, A. and Jaffard, R. (2003). Hippocampal lesions and discrimination performance of mice in the radial maze: sparing or impairment depending on the representational demands of the task. Hippocampus 13(2): 197-211.
- Huerta, P. T., Sun, L. D., Wilson, M. A. and Tonegawa, S. (2000). Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 25(2): 473-480.
- LaBar, K. S. and Disterhoft, J. F. (1998). Conditioning, awareness, and the hippocampus. Hippocampus 8(6): 620-626.
- Leal, S. L. and Yassa, M. A. (2015). Neurocognitive aging and the hippocampus across species. Trends Neurosci 38(12): 800-812.
- Marighetto, A., Etchamendy, N., Touzani, K., Torrea, C.C., Yee, B.K., Rawlins, J.N., and Jaffard, R. (1999). Knowing which and knowing what: a potential mouse model for age-related human declarative memory decline. Eur J Neurosci 11: 3312-3322.
- Mingaud, F., Le Moine, C., Etchamendy, N., Mormede, C., Jaffard, R. and Marighetto, A. (2007). The hippocampus plays a critical role at encoding discontiguous events for subsequent declarative memory expression in mice. Hippocampus 17(4): 264-270.
- Mingaud, F., Mormede, C., Etchamendy, N., Mons, N., Niedergang, B., Wietrzych, M., Pallet, V., Jaffard, R., Krezel, W., Higueret, P. and Marighetto, A. (2008). Retinoid hyposignaling contributes to aging-related decline in hippocampal function in short-term/working memory organization and long-term declarative memory encoding in mice. J Neurosci 28(1): 279-291.
- Rapp, P. R., Kansky, M. T. and Eichenbaum, H. (1996). Learning and memory for hierarchical relationships in the monkey: effects of aging. Behav Neurosci 110(5): 887-897.
- Rawlins, J. N. and Tsaltas, E. (1983). The hippocampus, time and working memory. Behav Brain Res 10(2-3): 233-262.
- Sellami, A., Al Abed, A. S., Brayda-Bruno, L., Etchamendy, N., Valerio, S., Oule, M., Pantaleon, L., Lamothe, V., Potier, M., Bernard, K., Jabourian, M., Herry, C., Mons, N., Piazza, P. V., Eichenbaum, H. and Marighetto, A. (2017). Temporal binding function of dorsal CA1 is critical for declarative memory formation. Proc Natl Acad Sci U S A 114(38): 10262-10267.
- Solomon, P. R., Vander Schaaf, E. R., Thompson, R. F. and Weisz, D. J. (1986). Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 100(5): 729-744.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sellami, A., Al Abed, A. S., Brayda-Bruno, L., Etchamendy, N., Valério, S., Oulé, M., Pantaléon, L., Lamothe, V., Potier, M., Bernard, K., Jabourian, M., Herry, C., Mons, N. and Marighetto, A. (2018). Protocols to Study Declarative Memory Formation in Mice and Humans: Optogenetics and Translational Behavioral Approaches. Bio-protocol 8(12): e2888. DOI: 10.21769/BioProtoc.2888.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









