- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of the Composition Dynamics of a Maize Root-associated Simplified Bacterial Community and Evaluation of Its Biological Control Effect
Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2885 Views: 10489
Reviewed by: Joëlle SchlapferMeng WuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Capturing Z-stacked Confocal Images of Living Bacteria Entering Hydathode Pores of Cauliflower
Aude Cerutti and Alain Jauneau
Oct 20, 2017 9184 Views

Tracking Root Interactions System (TRIS) Experiment and Quality Control
Hassan Massalha [...] Asaph Aharoni
Apr 20, 2019 7337 Views

A Quick Method for Screening Biocontrol Efficacy of Bacterial Isolates against Bacterial Wilt Pathogen Ralstonia solanacearum in Tomato
Heena Agarwal [...] Niraj Agarwala
Nov 20, 2020 5549 Views
Abstract
Besides analyzing the composition and dynamics of microbial communities, plant microbiome research aims to understanding the mechanism of plant microbiota assembly and their biological functions. Here, we describe procedures to investigate the role of bacterial interspecies interactions in root microbiome assembly and the beneficial effects of the root microbiota on hosts by using a maize root-associated simplified seven-species (Stenotrophomonas maltophilia, Ochrobactrum pituitosum, Curtobacterium pusillum, Enterobacter cloacae, Chryseobacterium indologenes, Herbaspirillum frisingense and Pseudomonas putida) synthetic bacterial community described in our previous work. Surface-sterilized maize seeds were grown in a gnotobiotic system based on double-tube growth chambers after being soaked in suspensions containing multiple species of bacteria. The dynamics of the composition of the bacterial communities colonized on maize roots were tracked by a culture-dependent method with a selective medium for each of the seven strains. The impact of bacterial interactions on the community assembly was evaluated by monitoring the changes of community structure. The plant-protection effects of the simplified seven-species community were assessed by quantifying (1) the growth of a fungal phytopathogen, Fusarium verticillioides on the surfaces of the seeds and (2) the severity of seedling blight disease the fungus causes, in the presence and absence of the bacterial community. Our protocol will serve as useful guidance for studying plant-microbial community interactions under the laboratory conditions.
Keywords: MaizeBackground
In natural settings, plants are associated with myriad microorganisms of extremely high diversity. These microbes exploit the niches provided by plant hosts and form complex microbial communities (Bulgarelli et al., 2012; Ofek-Lalzar et al., 2014; Cardinale et al., 2015; Edwards et al., 2015; Beckers et al., 2016; de Souza et al., 2016; Niu et al., 2017). Such plant-associated microbiomes are able to affect the development and health of the hosts profoundly (Berendsen et al., 2012). Recently, huge amounts of data describing plant microbiome compositions and their dynamics have been obtained by using advanced DNA sequencing technologies and data analysis methods. Much has been learned about the community structure of plant microbiota (Ofek-Lalzar et al., 2014; Bai et al., 2015; Ritpitakphong et al., 2016). However, due to the great complexity, currently it is nearly impossible to directly define experimentally the mechanisms underlying the dynamics of plant microbiome assembly and their beneficial effects on hosts. The establishment of simplified plant-associated microbial communities under controlled laboratory conditions is an approach to overcome the challenges in analyzing the properties of plant microbiota (Bodenhausen et al., 2014; Bai et al., 2015; Lebeis et al., 2015). Testing of hypotheses by targeted manipulation in gnotobiotic systems with simplified synthetic communities become a lot easier (Vorholt et al., 2017).
Previously, through host-mediated selection, we assembled a greatly simplified, yet representative, synthetic bacterial community consisting of seven strains (Stenotrophomonas maltophilia, Ochrobactrum pituitosum, Curtobacterium pusillum, Enterobacter cloacae, Chryseobacterium indologenes, Herbaspirillum frisingense and Pseudomonas putida) (Niu et al., 2017). We found that the removal of E. cloacae caused dramatic changes of the community composition and that this seven-species community protects maize from colonization by a fungal pathogen, Fusarium verticillioides. These results suggest that this synthetic seven-species community has the potential to serve as a useful system to explore how bacterial interspecies interactions affect root microbiome assembly and to dissect the beneficial effects of the root microbiota on hosts under laboratory conditions (Niu et al., 2017). This protocol has been developed to set up a gnotobiotic system for cultivating maize seedlings colonized by the root-associated simplified communities, to track the dynamics of the composition of the simplified communities and to evaluate the in vivo biological control effects of the seven-species community against F. verticillioides.
Materials and Reagents
- Consumables
- Disposable Petri dishes (VWR, catalog number: 89022-320 )
- Pipette tips (Corning, Axygen®, catalog number: T1005WBCRS ; Biotix, catalog number: M-0200-1RCNS )
- Parafilm (VWR, catalog number: 52858-000)
Manufacturer: Bemis, catalog number: PM996 . - Inoculation loops (Globe Scientific, catalog number: 130118 )
- Centrifuge tubes 2.0 ml (Corning, Axygen®, catalog number: MCT-200-C-S )
- Centrifuge tubes 1.5 ml (VWR, catalog number: 20170-038 )
- Centrifuge tubes 50 ml (Corning, catalog number: 352098 )
- Scalpel blades (Integra LifeSciences, catalog number: 4-110 )
- Glass beads (Propper, catalog number: 03000600 )
- Paper wipers (KCWW, Kimberly-Clark, catalog number: 34155 )
- 96-well plates (Corning, catalog number: 351172 )
- Cell scrapers (VWR, catalog number: 89260-222 )
- Trays (Thermo Fisher Scientific, Nunc, catalog number: 242811 )
- Disposable Petri dishes (VWR, catalog number: 89022-320 )
- Plants
Zea mays cv. Sugar Buns F1 (se+) (Johnny’s Selected Seeds, catalog number: 267 ) - Bacterial strains
Stenotrophomonas maltophilia ZK5342, Ochrobactrum pituitosum ZK5343,
Curtobacterium pusillum ZK5344, Enterobacter cloacae ZK5345,
Chryseobacterium indologenes ZK5346, Herbaspirillum frisingense ZK5347 and
Pseudomonas putida ZK5348 (Niu and Kolter, 2017)
These strains can be requested via e-mail: ben_niu@nefu.edu.cn or roberto_kolter@hms.harvard.edu - Fungal strain
Fusarium verticillioides MRC826 (Hinton and Bacon, 1995) - Chemical reagents
- Ethanol (Decon Labs, catalog number: V1001 )
- Bleach (Janitorial Supplies, Clorox®, catalog number: CLO30966CT )
- BactoTM Tryptic Soy Broth without Dextrose (BD, catalog number: 286220 )
- Soyabean Casein Digest Agar (HiMedia Laboratories, catalog number: GM290-500G )
- Agar (BD, catalog number: 214010 )
- 10x Phosphate buffered saline (PBS) (Lonza, catalog number: 17-517Q )
- Murashige and Skoog Basal Salt Mixture (MS) (Sigma-Aldrich, catalog number: M5524-50L )
- Nalidixic acid (Sigma-Aldrich, catalog number: N8878-5G )
- Colistin (Sigma-Aldrich, catalog number: C4461-100MG )
- Lincomycin (Sigma-Aldrich, catalog number: 62143-1G )
- Chlortetracycline (Sigma-Aldrich, catalog number: C4881-5G )
- Erythromycin (Sigma-Aldrich, catalog number: E5389-1G )
- Vancomycin (Sigma-Aldrich, catalog number: 75423-5VL )
- Sodium chlorite (VWR, catalog number: BDH9286-500G )
- Novobiocin (Sigma-Aldrich, catalog number: N1628-1G )
- Tobramycin (Sigma-Aldrich, catalog number: T4014-100MG )
- Glucose (VWR, catalog number: BDH9230-500G )
- Ethanol (Decon Labs, catalog number: V1001 )
- Media and buffers (see Recipes)
- Tryptone soya agar medium
- 0.1x Tryptone soya agar medium
- Tryptic soy broth medium
- 1x Phosphate buffered saline (PBS)
- ½ Murashige and Skoog (MS) agar medium
- Selective medium for S. maltophilia ZK5342
- Selective medium for O. pituitosum ZK5343
- Selective medium for C. pusillum ZK5344
- Selective medium for E. cloacae ZK5345
- Selective medium for C. indologenes ZK5346
- Selective medium for H. frisingense ZK5347
- Selective medium for P. putida ZK5348
- Potato dextrose agar medium
- Water agar medium
- Tryptone soya agar medium
Equipment
- Forceps
- Pipettes (Gilson, models: P20, P200 and P1000, catalog numbers: F123600 , F123601 and F123602 ; Thermo Fisher Scientific, model: F1-ClipTipTM, catalog numbers: 4661140N and 4661130N )
- Class II biological safety cabinet (Thermo Fisher Scientific, model: HerasafeTM KS9 )
- Centrifuge (Eppendorf, model: 5424 )
- Vortex (Scientific Industries, model: Vortex-Genie 2, catalog number: G560 )
- Spectrophotometer (Beckman Coulter, model: DU 640 )
- Sonicator (Qsonica, model: Q125 , catalog number: Q125-110)
- Balance (Mettler-Toledo International, catalog number: AG135 )
- Hemacytometer (Hausser Scientific, catalog number: 1492 )
- Microscope (ZEISS, model: Axioscop 2 plus )
- Stereoscope (ZEISS, model: Stemi SV 6 )
Software
- RStudio (version 0.99.903)
- QIIME (version 1.6.0)
- PRISM (version 6.0c)
Procedure
- Surface sterilization and germination of maize seeds
- Pick ten intact maize seeds of no disease symptom and put them in a Petri dish (9-cm diameter) (Figure 1A) with tweezers.
- Immerse the seeds in 70% (v/v) ethanol for three minutes then remove the ethanol.
- Immerse the seeds in 5% (v/v) bleach for three minutes then remove the bleach.
- Rinse the seeds with sterile distilled water three times.
- Take 250 μl water from the third rinse and spread onto tryptone soya agar (TSA) plates in order to check for contamination.
- Incubate the TSA plates at 30 °C overnight.
- Continue with the following steps if there is no microbial colony presenting on the TSA plates, otherwise, discard the maize seeds and repeat Steps A1 to A6 until no colony is detected on the TSA plates.
- Remove the distilled water from the Petri dish containing maize seeds.
- Keep the embryos of the seeds up and fill the Petri dish with 7 ml sterile distilled water (Figure 1B).
- Put the Petri dish containing surface-sterilized seeds in the dark at 30 °C.
- After an incubation of 24 h, take 250 μl water from the Petri dish and spread onto TSA plates to check for contamination.
- Incubate the TSA plates at 30 °C overnight.
- Continue with the following steps if there is no microbial colony presenting on the TSA plates, otherwise, discard the maize seeds and repeat Steps A1 to A12 until no colony is detected on the TSA plates.
- Remove the water from the Petri dish and refill with 7 ml sterile distilled water.
- Put the Petri dish back in the dark at 30 °C.
- After an incubation of 50 to 55 h in total, choose the germinated maize seeds (Figure 1C) with a root of 1-2 cm for the following steps.
Note: Perform Steps A2 to A17 in a biological safety cabinet.
- Pick ten intact maize seeds of no disease symptom and put them in a Petri dish (9-cm diameter) (Figure 1A) with tweezers.
- Inoculation of bacterial community on maize seedlings
- Streak the seven bacterial strains (Stenotrophomonas maltophilia ZK5342, Ochrobactrum pituitosum ZK5343, Curtobacterium pusillum ZK5344, Enterobacter cloacae ZK5345, Chryseobacterium indologenes ZK5346, Herbaspirillum frisingense ZK5347 and Pseudomonas putida ZK5348) on 0.1x TSA plates and incubate at 30 °C for 24-48 h.
- Inoculate a single colony of each strain in 5 ml of tryptic soy broth (TSB) and shake at 120 rpm at 30 °C overnight.
- Transfer 50 μl of overnight culture of each strain into 5 ml fresh TSB and shake at 30 °C for another 8 h.
- Collect the cells in 2.0-ml tubes by centrifuge at 2,940 x g for 10 min at 4 °C.
- Resuspend the cells in 1x phosphate buffered saline (PBS) and dilute the cell suspensions of each strain to ~108 cells per milliliter (Table 1).
Table 1. The OD600 value for each strain corresponding to ~108 cells/ml
- Mix the cell suspension of each strain in a 50-ml Falcon tube in equal volume to prepare the multiple species (seven-species or six-species resulting from the removal of each of the seven species, respectively) bacterial suspensions.
- Soak no more than 30 surface-sterilized and germinated maize seeds with primary roots of 1-2 cm (Figure 1C) in 30 ml multiple species bacterial suspensions in a Petri dish (Figure 1D) without shaking at room temperature for 0.5-1.0 h. Move the seeds to make sure the roots are completely submerged in the suspensions. Soak another 10 surface-sterilized and germinated seeds in 1x PBS buffer for 0.5-1.0 h and use as a control.
- Transfer the maize seeds adhered by bacteria and sterile seeds onto 20 ml ½ Murashige and Skoog (MS) agar (0.8%) in glass tubes (16 x 150 mm) by sterile forceps. Press the seeds gently with the forceps to insert the primary roots into the agar (Figure 1E). Use the sterile empty glass tubes of the same size to close the tubes containing the seeds in a mouth-to-mouth way. Connect and fix the two tubes by parafilm (Figure 2A).
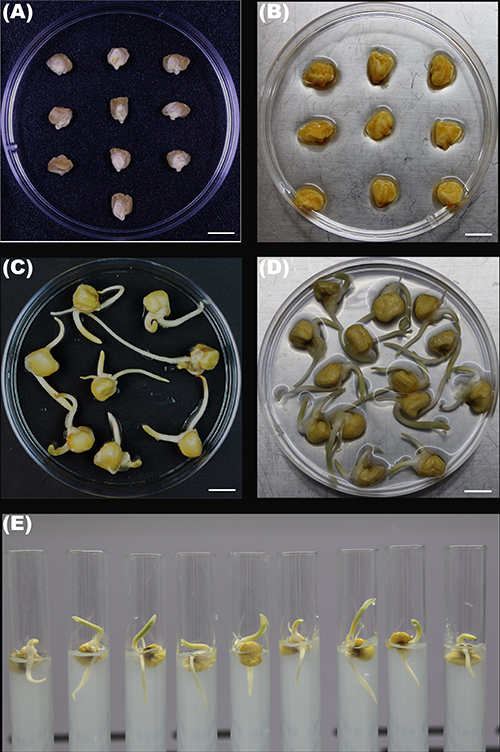
Figure 1. Maize seeds. A. Dry seeds; B. The seeds after surface-sterilization; C. Surface-sterilized and germinated seeds; D. Surface-sterilized and germinated seeds soaked in suspensions of multiple bacterial species in a Petri dish; E. Surface-sterilized and germinated seeds with/without bacteria sitting in ½ MS agar. Scale bars = 1 cm.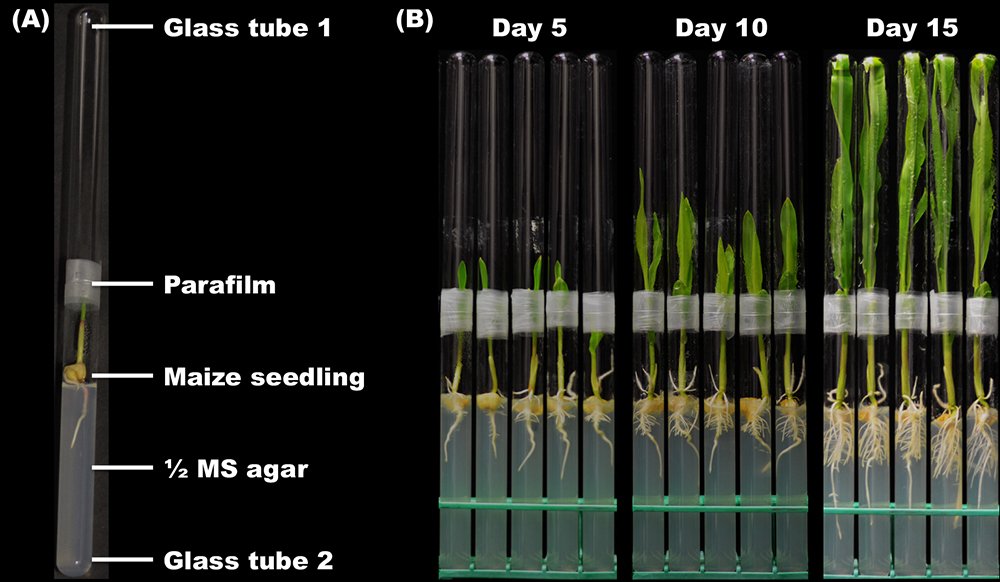
Figure 2. Growth of axenic maize seedlings. A. The double-tube growth chamber. The parafilm is used to hold the two glass tubes together. B. The axenic maize seedlings of different ages grown in the double-tube growth chambers. - Place the maize seedlings in double-tube chambers under the following conditions: 16 h of light (day) and 8 h of dark (night), 4,000 lx, 25 °C and a relative humidity of 54%. Keep the maize seedlings under the above conditions for 15 days.
Note: Perform the Steps B1 to B8 in a biological safety cabinet or close to a flame.
- Streak the seven bacterial strains (Stenotrophomonas maltophilia ZK5342, Ochrobactrum pituitosum ZK5343, Curtobacterium pusillum ZK5344, Enterobacter cloacae ZK5345, Chryseobacterium indologenes ZK5346, Herbaspirillum frisingense ZK5347 and Pseudomonas putida ZK5348) on 0.1x TSA plates and incubate at 30 °C for 24-48 h.
- Quantification of the compositions of the bacterial communities colonized on maize roots
- Sample three to five maize seedlings inoculated with each of the eight bacterial communities (one seven-species and seven six-species communities) at day 5, day 10 and day 15 after inoculation.
- Cut the root from each maize seedling with a sterile scalpel blade (Video 1).Video 1. Sampling root fragments from maize seedlings
- Rinse the root in sterile 1x PBS buffer quickly to remove the agar adheres to the root surface.
- Harvest a 1-cm-long primary root fragment below maize kernel by cutting the primary root with a sterile scalpel blade.
- Remove the lateral roots on the root fragment with the sterile scalpel blade.
- Transfer the root fragment into a 1.5-ml centrifuge tube with two sterile 200-μl pipette tips.
- Put six glass beads (diameter: 3 mm) in the tubes and add 1 ml sterile 1x PBS buffer.
- Dislodge the bacterial cells colonized on the root surfaces by sonicating (amplitude: 30%; pulse: on 01 sec, off 01 sec; time: 30 sec) for 1 min, then by vortexing for another 1 min.
- Repeat the Step C8 twice. Put the tube on ice for 1 min.
- Add 180 μl sterile 1x PBS buffer in one lane (8 wells) of a 96-well plate (Video 2).Video 2. Diluting bacterial suspension and plating
- Put 20 μl bacterial suspension obtained in Step C9 in the first well of the lane and mix by sucking and excluding the suspension with a pipette.
- Take the root fragment out with a pair of forceps, dry the fragment with paper wipers, then weigh it on a balance and record the weight of the root fragment.
- Transfer 20 μl well mixed bacterial suspension from the first to the eighth well sequentially to get 10 to 108 times dilutions of the bacterial suspension.
- Take 10 μl diluted bacterial suspension from each of the eight wells and spot on three selective 0.1x TSA plates (Table 2) for each strain with a multichannel pipette.
Table 2. Supplements in the selective medium and incubation time for each strain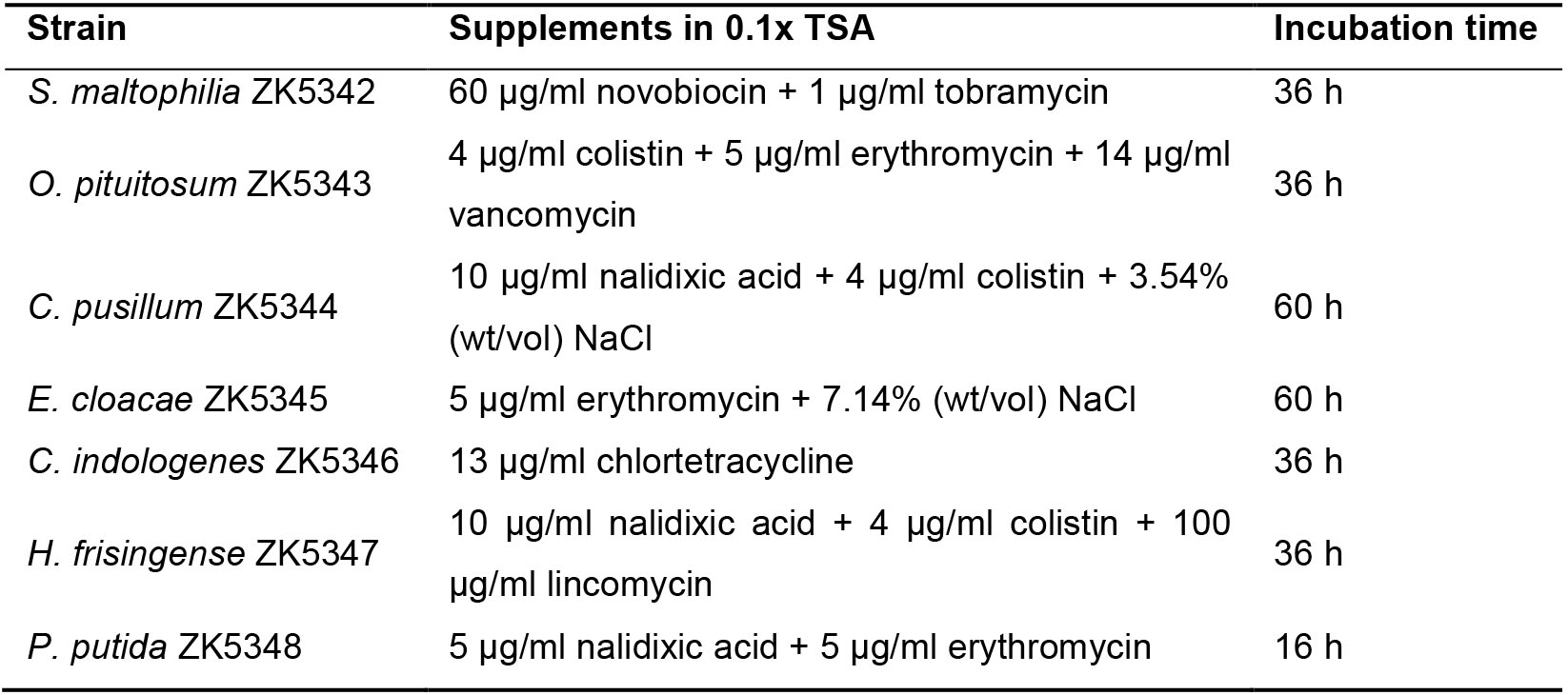
- Tilt the plates to make the bacterial suspension drops move toward one direction to spread the cells on agar surfaces.
- Air-dry the selective 0.1x TSA plates spread with the bacterial suspensions.
- Incubate the plates at 30 °C in the dark for 16 to 60 h (Table 2).
- Count and record the numbers of the Colony Formation Units (CFUs) (Figure 3A) on the selective plates.
- Use the CFU numbers between 10 and 200 (Figure 3B) to calculate the bacterial abundances:
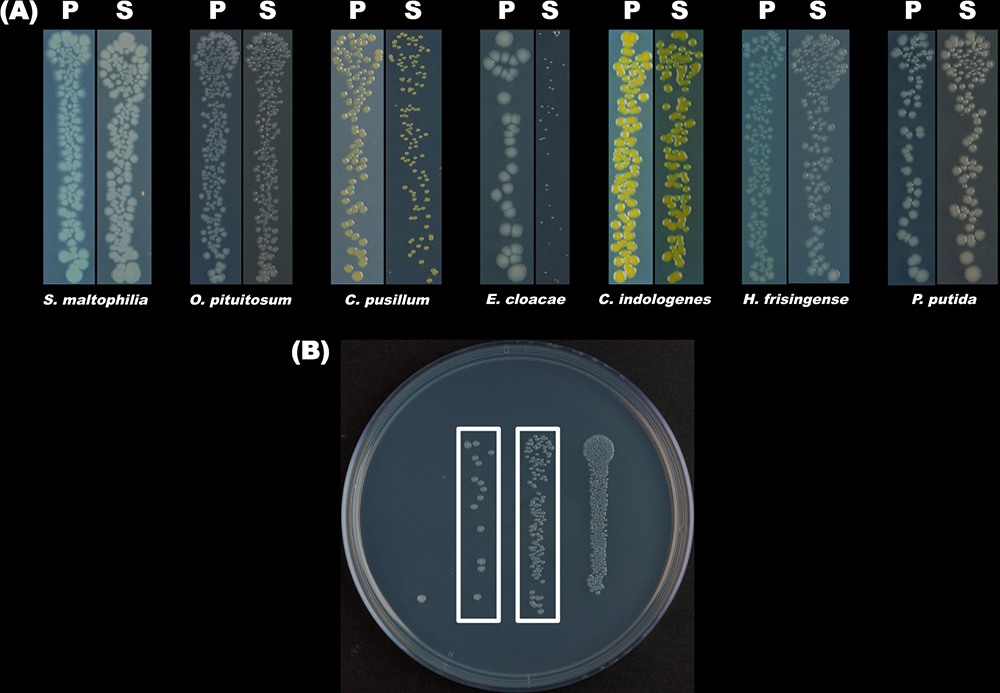
Figure 3. Colonies of the seven bacterial strains of the simplified community. A. Morphology of the colonies of each of the seven bacterial strains grown on the plain and selective 0.1x TSA plates. ‘P’ and ‘S’ designate the plain 0.1x TSA plates and the selective 0.1x TSA plates, respectively. B. Colonies of O. pituitosum grown on the selective plates. From left to right, the four lanes of colonies were formed through the growth of the cells in 104-, 103-, 102- and 10-times diluted bacterial suspensions, respectively. The CFUs (Colony Formation Units) numbers of the two lanes framed are fit for the quantification of bacteria. - Calculate the relative abundance of each species in the communities.
- Perform the Steps C2 to C16 in a biological safety cabinet or close to a flame.
- Watch Video 1 for details of Steps C2 to C7.
- Watch Video 2 for details of Steps C10 to C16.
- Perform the Steps C2 to C16 in a biological safety cabinet or close to a flame.
- Sample three to five maize seedlings inoculated with each of the eight bacterial communities (one seven-species and seven six-species communities) at day 5, day 10 and day 15 after inoculation.
- In vivo assay for the inhibitory effect of bacterial community against F. verticillioides
Note: Perform the Steps D1 to D10 in a biological safety cabinet or close to a flame.- Place an F. verticillioides MRC826 mycelial disk of 0.5-cm diameter at the center of a potato dextrose agar (PDA) plate and incubate at 28 °C for 7 days until the mycelia cover the whole plate.
- Put 20 ml sterile 1x PBS buffer in the F. verticillioides colony grown on a PDA agar plate.
- Harvest the fresh spores with a cell scraper.
- Filter the F. verticillioides spore suspension through eight layers of sterile gauze.
- Dilute the spore suspension and count the spores using a hemacytometer under a microscope.
- Adjust the concentration of spores to ~108 spores per milliliter sterile 0.01% (vol/vol) Tween 20. Store the suspension at 4 °C.
- Dilute the suspension to ~106 spores per milliliter sterile 1x PBS buffer.
- Inoculate the spores by spreading on the trays (~103 CFU/cm2) containing 20 ml 2.25% (wt/vol) water agar.
- Soak 10 surface-sterilized maize seeds (Figure 1B) in a 30 ml suspension of the seven-species bacterial community, each single species of the community, or Escherichia coli DH5α in a Petri dish for 0.5-1.0 h. Soak another 10 surface-sterilized seeds in sterile 1x PBS for 0.5-1.0 h and use as a control.
- Put the seeds on the surfaces of water agar in trays with sterile forceps.
- Put the trays in the dark at 23 °C for 10 days.
- Count and record the number of seeds showing visible fungal mycelia (Figure 4A) in each treatment every day. Calculate the fungi colonization rate as:
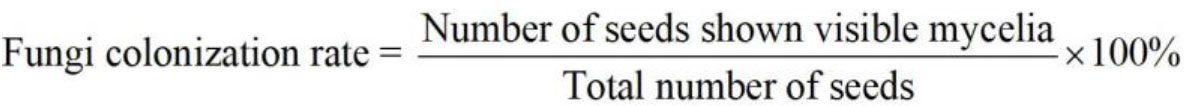
- Take photographs of mycelial growth on the surface of each seed on day 4 and day 10 after incubation using a dissecting microscope (Figure 4B).
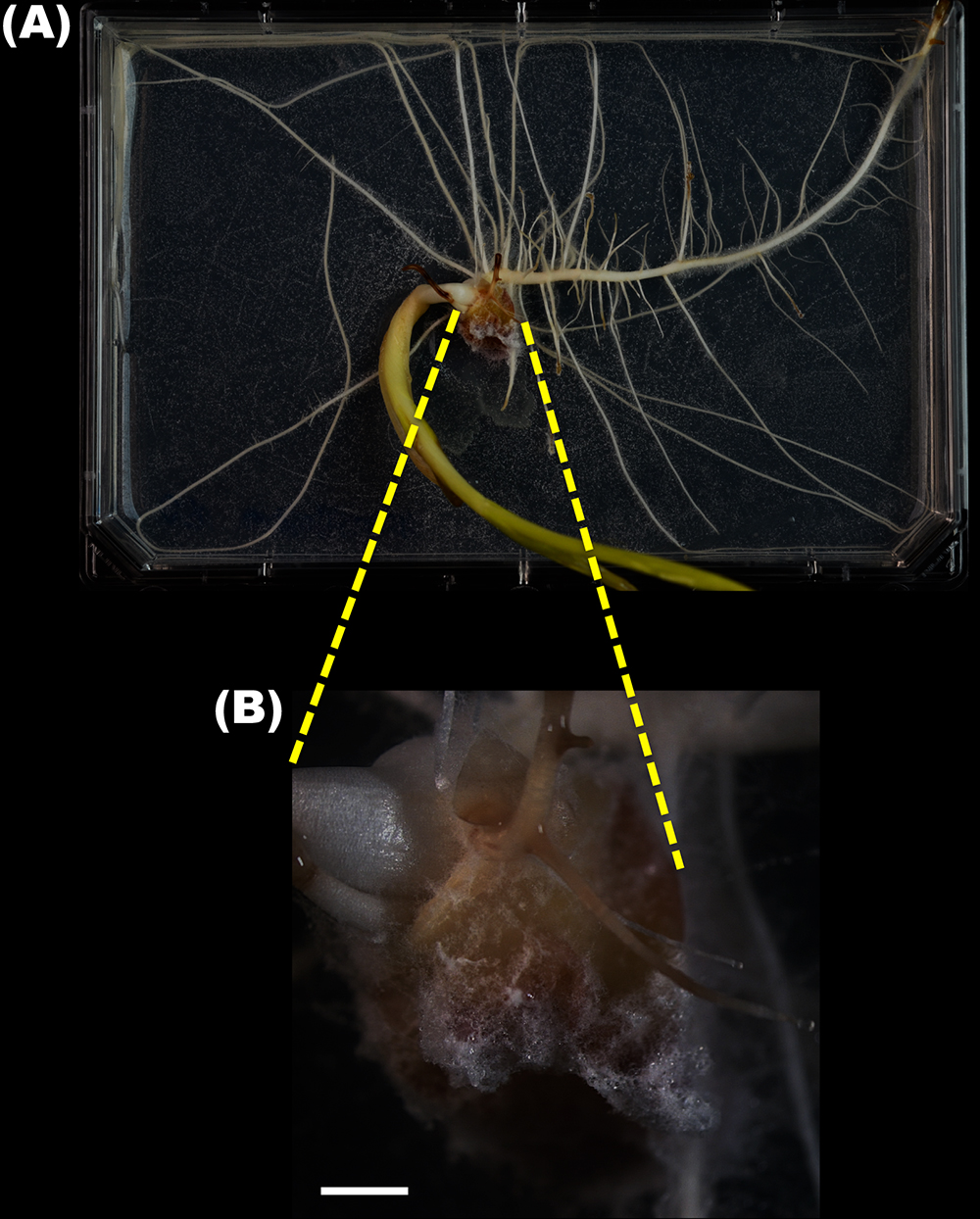
Figure 4. The maize seedling co-inoculated with the seven-species simplified bacterial community and F. verticillioides. A. A ten-day-old maize seedling inoculated with the bacterial community grown on water agar spread with fungal spores. The white spots on the agar are the mycelia developed from the spores. B. The enlargement of the seed in (A) indicated by the two yellow dash lines. The white hairs on the seed are mycelia colonized on the surface of seed (scale bar = 2 mm). - Evaluate the severities of maize seedling blight disease for each treatment on day 10 by calculating the disease severity indices based on the ranks described previously (Niu et al., 2017) following the formula (Sherwood and Hagedorn, 1958):
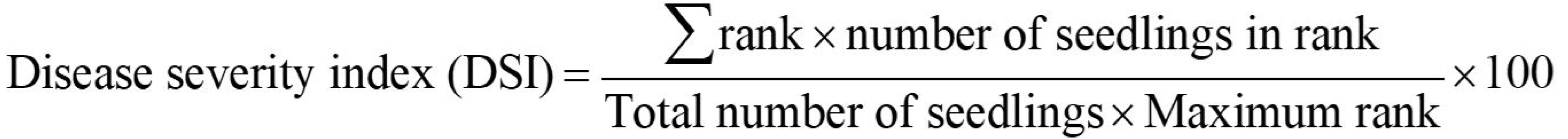
The disease ranks were established as follows:
rank 1 = no visible fungal mycelia grow on the surfaces of kernels, and tan lesions are present on the roots;
rank 2 = the surfaces of kernels are partially covered by fungal mycelia, and tan lesions are present on the roots;
rank 3 = the surfaces of kernels are partially covered by fungal mycelia, and tan to brown lesions are present on the roots;
rank 4 = the surfaces of kernels are fully covered by fungal mycelia, and tan to brown lesions are present on the roots;
rank 5 = the surfaces of kernels are fully covered by fungal mycelia, and reddish brown lesions are present on the roots (Niu et al., 2017).
- Place an F. verticillioides MRC826 mycelial disk of 0.5-cm diameter at the center of a potato dextrose agar (PDA) plate and incubate at 28 °C for 7 days until the mycelia cover the whole plate.
Data analysis
Bray-Curtis (BC) dissimilarity indexes were calculated based on the relative abundance values of each species by the function of the package ‘Vegan’ of RStudio (version 0.99.903). The dissimilarity matrix was then used to generate corresponding cluster dendrograms by hierarchical clustering using the function ‘hclust’ of the R package ‘gplots.’ The BC distance between the community on the maize root inoculated with each six-species community and the community on the root inoculated with the seven-species model community was calculated using QIIME (version 1.6.0). The Fisher’s LSD test (PRISM, version 6.0c) was used to compare: 1). BC distances between each six-species community and the seven-species model community plant by plant, 2). fungi colonization rates and disease severity indices of maize seedlings treated with F. verticillioides alone, jointly with F. verticillioides and the seven-species model community, jointly with F. verticillioides and each of the seven species and jointly with F. verticillioides and E. coli DH5α, respectively.
Recipes
- Tryptone soya agar medium
20 g Tryptone Soya Agar (HiMedia Laboratories)
1,000 ml distilled water
Autoclave at 121 °C for 20 min - 0.1x tryptone soya agar medium
1.38 g Tryptic Soy Broth without Dextrose (BD)
7.5 g agar (BD)
500 ml distilled water
Autoclave at 121 °C for 20 min - Tryptic soy broth medium
13.75 g Tryptic Soy Broth without Dextrose (BD)
500 ml distilled water - 1x phosphate buffered saline (PBS)
100 ml PBS (10x) without calcium or magnesium
900 ml distilled water
Autoclave at 121 °C for 20 min - ½ Murashige and Skoog (MS) agar medium
2.15 g Murashige and Skoog Basal Salt Mixture (Sigma-Aldrich)
4 g agar (BD)
500 ml distilled water
Autoclave at 121 °C for 20 min - Selective medium for S. maltophilia ZK5342
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min. Cool down the sterilized medium to around 60 °C. Then add 300 μl novobiocin (Sigma-Aldrich) (100 mg/ml) and 12.8 μl tobramycin (Sigma-Aldrich) (39.1 mg/ml) - Selective medium for O. pituitosum ZK5343
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min
Cool down the sterilized medium to around 60 °C. Then add 200 μl colistin (Sigma-Aldrich) (10 mg/ml), 125 μl erythromycin (Sigma-Aldrich) (20 mg/ml) and 70 μl vancomycin (Sigma-Aldrich) (100 mg/ml) - Selective medium for C. pusillum ZK5344
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min
Cool down the sterilized medium to around 60 °C. Then add 1,132 μl nalidixic acid (Sigma-Aldrich) (5 mg/ml), 226.4 μl colistin (Sigma-Aldrich) (10 mg/ml) and 66 ml NaCl (VWR International) (30%, wt/vol) - Selective medium for E. cloacae ZK5345
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min. Cool down the sterilized medium to around 60 °C. Then add 163.77 μl erythromycin (Sigma-Aldrich) (20 mg/ml) and 155.07 ml NaCl (VWR International) (30%, wt/vol) - Selective medium for C. indologenes ZK5346
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min. Cool down the sterilized medium to around 60 °C. Then add 120.19 μl chlortetracycline (Sigma-Aldrich) (10 mg/ml) - Selective medium for H. frisingense ZK5347
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min. Cool down the sterilized medium to around 60 °C. Then add 1000 μl nalidixic acid (Sigma-Aldrich) (5 mg/ml), 200 μl colistin (Sigma-Aldrich) (10 mg/ml) and 1,000 μl lincomycin (Sigma-Aldrich) (50 mg/ml) - Selective medium for P. putida ZK5348
1.38 g Tryptic Soy Broth without Dextrose
7.5 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min. Cool down the sterilized medium to around 60 °C. Then add 500 μl nalidixic acid (Sigma-Aldrich) (5 mg/ml) and 125 μl erythromycin (Sigma-Aldrich) (20 mg/ml) - Potato dextrose agar medium
Potato extracts from 200 g of potato tuber
17 g agar (BD)
20 g glucose (VWR International)
1,000 ml distilled water
Autoclave at 121 °C for 20 min - Water agar medium
11.25 g agar
500 ml distilled water
Autoclave at 121 °C for 20 min
Acknowledgments
We thank Yue Liu for helps in shooting the videos; Bo Shen for helps in culturing the maize seedlings; and members of the Kolter Laboratory for valuable advice. This work was supported by NIH Grant No. GM58213 (to R.K.) and Start-up Scientific Foundation of Northeast Forestry University JQ2017-02 (to B.N.). This protocol was adapted from Niu et al. (2017).
Competing interests
The authors have no conflict of interest or competing interests to declare.
References
- Bai, Y., Muller, D. B., Srinivas, G., Garrido-Oter, R., Potthoff, E., Rott, M., Dombrowski, N., Munch, P. C., Spaepen, S., Remus-Emsermann, M., Huttel, B., McHardy, A. C., Vorholt, J. A. and Schulze-Lefert, P. (2015). Functional overlap of the Arabidopsis leaf and root microbiota. Nature 528(7582): 364-369.
- Beckers, B., Op De Beeck, M., Weyens, N., Van Acker, R., Van Montagu, M., Boerjan, W. and Vangronsveld, J. (2016). Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc Natl Acad Sci U S A 113(8): 2312-2317.
- Berendsen, R. L., Pieterse, C. M. and Bakker, P. A. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci 17(8): 478-486.
- Bodenhausen, N., Bortfeld-Miller, M., Ackermann, M. and Vorholt, J. A. (2014). A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet 10(4): e1004283.
- Bulgarelli, D., Rott, M., Schlaeppi, K., Ver Loren van Themaat, E., Ahmadinejad, N., Assenza, F., Rauf, P., Huettel, B., Reinhardt, R., Schmelzer, E., Peplies, J., Gloeckner, F. O., Amann, R., Eickhorst, T. and Schulze-Lefert, P. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488(7409): 91-95.
- Cardinale, M., Grube, M., Erlacher, A., Quehenberger, J. and Berg, G. (2015). Bacterial networks and co-occurrence relationships in the lettuce root microbiota. Environ Microbiol 17(1): 239-252.
- de Souza, R. S., Okura, V. K., Armanhi, J. S., Jorrin, B., Lozano, N., da Silva, M. J., Gonzalez-Guerrero, M., de Araujo, L. M., Verza, N. C., Bagheri, H. C., Imperial, J. and Arruda, P. (2016). Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci Rep 6: 28774.
- Edwards, J., Johnson, C., Santos-Medellin, C., Lurie, E., Podishetty, N. K., Bhatnagar, S., Eisen, J. A. and Sundaresan, V. (2015). Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci U S A 112(8): E911-920.
- Hinton, D. M. and Bacon, C. W. (1995). Enterobacter cloacae is an endophytic symbiont of corn. Mycopathologia 129(2): 117-125.
- Lebeis, S. L., Paredes, S. H., Lundberg, D. S., Breakfield, N., Gehring, J., McDonald, M., Malfatti, S., Glavina del Rio, T., Jones, C. D., Tringe, S. G. and Dangl, J. L. (2015). PLANT MICROBIOME. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349(6250): 860-864.
- Niu, B. and Kolter, R. (2017). Complete genome sequences of seven strains composing a model bacterial community of maize roots. Genome Announc 5(36).
- Niu, B., Paulson, J. N., Zheng, X. and Kolter, R. (2017). Simplified and representative bacterial community of maize roots. Proc Natl Acad Sci U S A 114(12): E2450-E2459.
- Ofek-Lalzar, M., Sela, N., Goldman-Voronov, M., Green, S. J., Hadar, Y. and Minz, D. (2014). Niche and host-associated functional signatures of the root surface microbiome. Nat Commun 5: 4950.
- Ritpitakphong, U., Falquet, L., Vimoltust, A., Berger, A., Metraux, J. P. and L'Haridon, F. (2016). The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol 210(3): 1033-1043.
- Sherwood, R. and Hagedorn, D. (1958). Determining common root rot potential of pea fields. Agricultural Experiment Station, University of Wisconsin, Madison, WI.
- Vorholt, J. A., Vogel, C., Carlstrom, C. I. and Muller, D. B. (2017). Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 22(2): 142-155.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
牛, �. and Kolter, R. (2018). Quantification of the Composition Dynamics of a Maize Root-associated Simplified Bacterial Community and Evaluation of Its Biological Control Effect. Bio-protocol 8(12): e2885. DOI: 10.21769/BioProtoc.2885.
Category
Microbiology > Microbe-host interactions > In vivo model > Plant
Microbiology > Community analysis > Gnotobiotic system
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










