- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Phrenic Nerve Hemidiaphragm Assay (MPN)
Published: Vol 8, Iss 5, Mar 5, 2018 DOI: 10.21769/BioProtoc.2759 Views: 10540
Reviewed by: Andrea PuharJordi MolgóAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Electrophysiological Measurements of Isolated Blood Vessels
Samuel A Molina [...] Michael Koval
Mar 20, 2022 3086 Views

An ex vivo Model of Paired Cultured Hippocampal Neurons for Bi-directionally Studying Synaptic Transmission and Plasticity
Ruslan Stanika and Gerald J. Obermair
Jul 20, 2023 2218 Views

Local Iontophoretic Application for Pharmacological Induction of Long-Term Synaptic Depression
Borys Olifirov [...] Pavel Belan
Jun 5, 2025 1939 Views
Abstract
The neuromuscular junction (NMJ) is the specialized synapse by which peripheral motor neurons innervate muscle fibers and control skeletal muscle contraction. The NMJ is the target of several xenobiotics, including chemicals, plant, animal and bacterial toxins, as well as of autoantibodies raised against NMJ antigens. Depending on their biochemical nature, the site they target (either the nerve or the muscle) and their mechanism of action, substances affecting NMJ produce very specific alterations of neuromuscular functionality.
Here we provide a detailed protocol to isolate the diaphragmatic muscle from mice and to set up two autonomously innervated hemidiaphragms. This preparation can be used to study bioactive substances like toxins, venoms and neuroactive molecules of various origin, or to measure the force of skeletal muscle contraction.
The ‘mouse phrenic nerve hemidiaphragm assay’ (MPN) is an established model of ex vivo NMJ and recapitulates the complexity of neuromuscular transmission in a system easy to control and to manipulate, thus representing a valuable tool to study both NMJ physiology and the mechanism of action of toxins and other molecules acting at this synapse.
Background
The neuromuscular junction (NMJ) is the chemical synapse enabling communication between motor neurons and skeletal muscle fibers. This is the best characterized synapse and most of the knowledge on maturation, structure and function of synapses derives from its study (Li et al., 2017). At the NMJ, the action potential running along the motor axon invades the nerve terminal (presynaptic bouton) and induces the fusion of synaptic vesicles with the presynaptic membrane. This triggers the release of acetylcholine (ACh), the neurotransmitter that binds the nicotinic ionotropic ACh receptors (nAChRs) on the postsynaptic muscle fiber. Upon ACh binding to nAChRs, a postsynaptic action potential spreads out along the muscle fiber causing Ca2+ release from the sarcoplasmic reticulum into the cytosol, thereby inducing muscle contraction. At variance from central synapses, NMJs are not protected by anatomical barriers, like the blood brain barrier or the blood nerve barrier, and are exposed to the action of various pathogenic molecules, including chemicals, toxins from plant, animal and bacteria as well as autoantibodies raised against NMJ antigens. Depending on the way they act, these agents produce distinct kinds of injury to the NMJ, eventually leading to impairment of muscle contraction.
The ‘mouse phrenic nerve hemidiaphragm assay’ (MPN) is an established model to study ex vivo NMJ function and offers a valuable tool to investigate the mechanism of action of toxins and molecules that produce NMJ alteration. In addition, MPN can be used to evaluate skeletal muscle contractility and measure diaphragmatic muscle force elicited by electrical stimulation of its phrenic nerve, so providing a model which recapitulates the complexity of the neuromuscular system in a more accessible and isolated environment.
The MPN provided fundamental insights to tackle the mechanism of action of Botulinum Neurotoxins (BoNTs), and it is currently used in many laboratories worldwide to perform qualitative/quantitative analysis of BoNTs. Its employment allows a significant refinement and reduction in the use of animals and results are in good agreement with the classical mouse lethality bioassay (Rasetti-Escargueil et al., 2011; Bigalke and Rummel, 2015).
For this, and because the hemidiaphragm-phrenic nerve intoxicated by BoNTs closely mimics the failure of respiratory muscles occurring in vivo, the MPN is presently listed in the European Pharmacopeia as an alternative method to the mouse bioassay for assaying BoNT/A lots for human use (https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/eur/eph_botat-508.pdf). This system is also a valuable tool to test new BoNTs (Zanetti et al., 2017) and the neutralizing potency of antibodies and inhibitors (Rasetti-Escargueil et al., 2011; Azarnia Tehran et al., 2015; Beske et al., 2017).
Besides BoNTs, the MPN can be used to study many other bioactive substances, comprising toxins like phospholipases, myotoxins and complete venoms, neuroactive molecules like peptides, lipids and drugs (Rigoni et al., 2005; Caccin et al., 2006; Bercsenyi et al., 2014; Yan et al., 2014; Caccin et al., 2015), or to measure muscle force in diaphragms from mice pre-treated with autoantibodies involved in myasthenic syndromes (Klooster et al., 2012) or from animal models of neuromuscular diseases (Nascimento et al., 2014).
Here, we describe a very detailed protocol to successfully dissect the mouse diaphragmatic muscle with both the phrenic nerves completely functional. This is a remarkable advantage as it allows obtaining two autonomously innervated hemidiaphragms to be independently used, either as an internal control or to increment the number of experimental data. The small volume of muscle bath-chambers, the possibility of finely control bath concentration of substances used, the easy manipulability of experimental conditions (temperature, washes, etc.) and the possibility to use the muscles for further analysis (immunofluorescence, Western blot, etc.), represent significant advantages as well.
Materials and Reagents
- 2 µl micropipette
- 200 µl micropipette
- 1,000 µl micropipette
- Tips for 2, 200 and 1,000 µl micropipettes
- Petri dish, 100 x 20 mm, coated with Sylgard (Dow Corning, Sylgard® 184 Silicone Elastomer kit)
- Surgical needles (Rudolf, catalog number: RU 5899-01 )
- Cotton thread
- Mice of desired genotype and age
- Hydrogen chloride (HCl) (Sigma-Aldrich, catalog number: H1758 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014 )
- Magnesium chloride, standard solution 1 M (MgCl2) (Honeywell International, Fluka, catalog number: 63020 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C5080 )
- D-(+)-Glucose (Sigma-Aldrich, catalog number: G7528 )
- Ringer’s solution (see Recipes)
Equipment
- Volumetric flask
- Clamp (Bulldog Clamp, Diethrich) (Rudolf, catalog number: RU 3934-16 ; or similar)
- 2 x scissors (Delicate Surgical Scissors) (Rudolf, catalog number: RU 1503-12 )
- 2 x forceps (Micro Jewelers Forceps, curved) (Rudolf, catalog number: RU 4240-07 )
- Stimulator: 6002 Stimulator (Harvard Apparatus, model: 6002 Basic Stimulator )
- 2 x micromanipulator (three-dimensional coarse manual manipulator) (NARISHIGE, catalog number: M-152 )
- 2 x Tension transducer: isometric transducer (Harvard Apparatus, catalog number: 72-4494 )
- 2 x Lead Screw Positioner (Harvard Apparatus, catalog number: 53-2082W )
- 2 x Support system (to hold both stimulation chamber and transducer sensors)
- 2 x Static stimulation chamber (jacketed, total internal volume 5 ml)
- Thermostatic bath (Isco, catalog number: GTR190 )
- Recording unit: i-WORX 118 system (iWORX, model: iWORX 118 )
- Computer compatible with the software
- Gas tank (95% O2, 5% CO2) equipped with pressure control (Air Liquide, model: HBS 240-1-2 )
Software
- Recording and Analysis: i-WORX 118 system (Dover, NH, USA) interfaced via Labscribe software (iWorx Systems Inc., Dover, NH, USA)
Procedure
- Solutions and setup preparation
- Prepare Ringer’s solution (Recipe 1) and saturate it by aeration with 95% O2, 5% CO2, for at least 15 min to buffer pH at 7.4. During aeration, add 1 mg/ml of glucose (complete Ringer’s solution).
Note: About 50 ml of solution is needed for one standard experiment.
- Switch on all the components of the setup and run Labscribe software (see Figure 1).
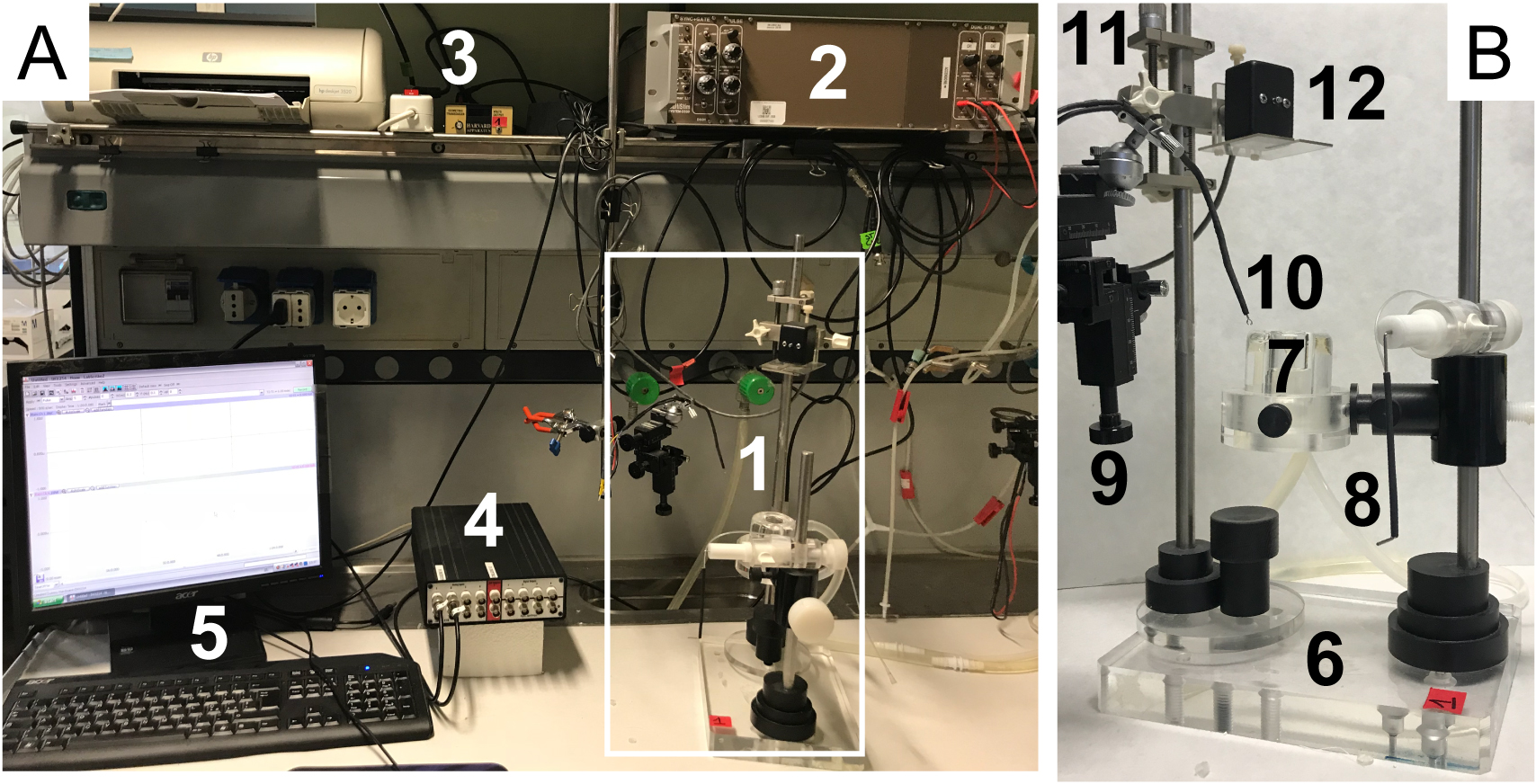
Figure 1. Electrophysiological setup components. A. Setup overview. 1) Muscle chamber; 2) Stimulator; 3) Tension transducer; 4) Data acquisition system; 5) Computer system with Labscribe software. B. Muscle chamber details. 6) Support system; 7) Static stimulation chamber; 8) Muscle support with oxygen cannula; 9) Micromanipulator; 10) Platinum electrodes; 11) Screw Positioner; 12) Transducer sensor.
- Prepare Ringer’s solution (Recipe 1) and saturate it by aeration with 95% O2, 5% CO2, for at least 15 min to buffer pH at 7.4. During aeration, add 1 mg/ml of glucose (complete Ringer’s solution).
- Dissection
- Euthanize the mice, preferably by cervical dislocation.
Notes:- All procedures were performed in accordance with the Italian laws and policies (D.L. no 26 14th March 2014), with the guidelines established by the European Community Council Directive no 2010/63/UE and approved by the veterinary services of the University of Padova (O.P.B.A.-Organismo Preposto al Benessere degli Animali) (protocol 359/2015). All the procedures should be utilized according to the ethical standards of the Institution where experiments are carried out.
- Exsanguination with decapitation can be carried out to avoid the presence of bleeding and clot formations within the rib cage.
- All procedures were performed in accordance with the Italian laws and policies (D.L. no 26 14th March 2014), with the guidelines established by the European Community Council Directive no 2010/63/UE and approved by the veterinary services of the University of Padova (O.P.B.A.-Organismo Preposto al Benessere degli Animali) (protocol 359/2015). All the procedures should be utilized according to the ethical standards of the Institution where experiments are carried out.
- Pin the mouse on a support in a supine position, remove the skin over the chest and the abdomen, and open the peritoneum under the rib cage.
- Remove pectoral muscles to expose the rib cage.
- Hold firmly the lower extremity of the sternum with a clamp, and make a central cut on it, approximately at the level of the second rib; keep on cutting the right and left side of the rib cage to get the diaphragm exposed (see Figure 2A).
Note: While cutting, do not follow the course of the ribs, but chop them.
- Completely remove the superior part of the rib cage (see Figure 2B).
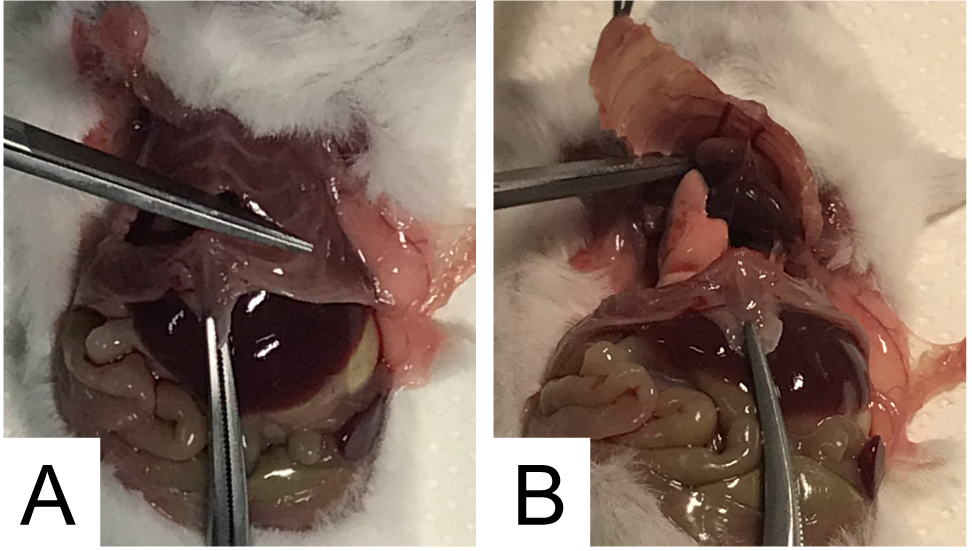
Figure 2. Rib cage opening for diaphragm explantation
- Gently, move away the heart and the pulmonary lobes to spot the left phrenic nerve (it forms a white arch, see Figure 3A). Using forceps, insert the cotton thread under the nerve, in the upper part and make two knots. Carefully, cut the nerve behind the knots; holding the thread (avoid excessive stretching which may cause nerve damage), isolate the nerve removing adherent tissue as much as possibile; it is advisable to start from the knots and gently proceed toward the diaphragm muscle. A cleared nerve is easier to mount and stimolate, yet this requires carefull handling to avoid nerve stretching or damage, particularly close to the nerve insertion point into the muscle. Gently depose the isolated nerve on the muscle (see Figures 3B and 3C). Repeat these operations for the right phrenic nerve. Be careful: the right nerve is very close to blood vessels whose damage may lead to annoying hemorrhages (see Figures 3D and 3E). The result of these steps is shown in Figure 3F.
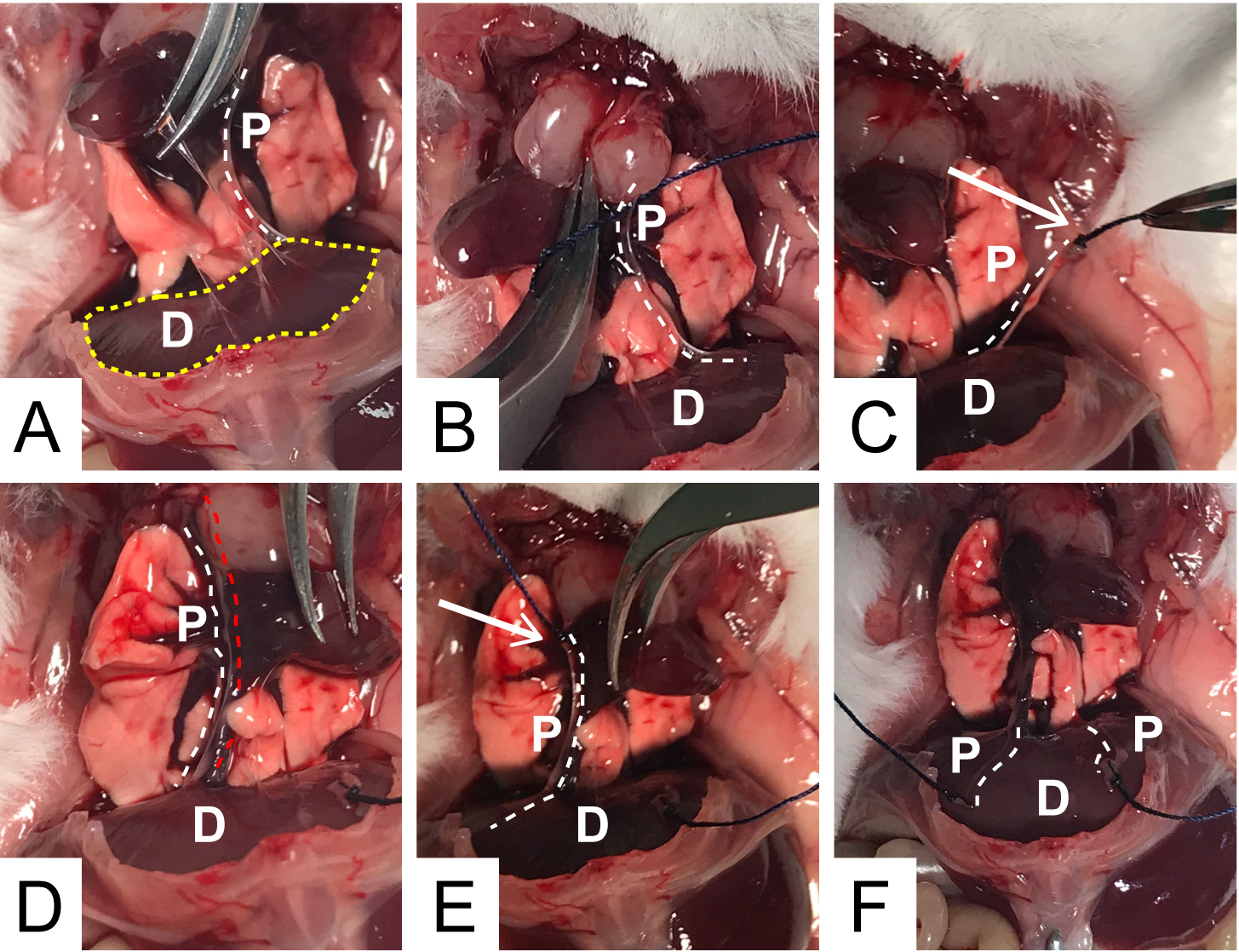
Figure 3. Procedure for isolation and ligation of the two phrenic nerves. D: diaphragm; P: phrenic nerve. White arrows: knots position. White dotted lines: position of the phrenic nerves. Red dotted line: blood vessel running along the right phrenic nerve. Yellow dotted line in A: whole diaphragmatic muscle area.
- Hold the diaphragm using the lower part of the sternum, to avoid damage of muscle fibers; remove the connective tissue adhering the abdominal side of the diaphragm (arrow in Figure 4A). Explant the diaphragm by cutting the dorsal part of the muscle (arrow in Figure 4B).
Note: Pay close attention to spot nerve insertion point and avoid their cleavage.
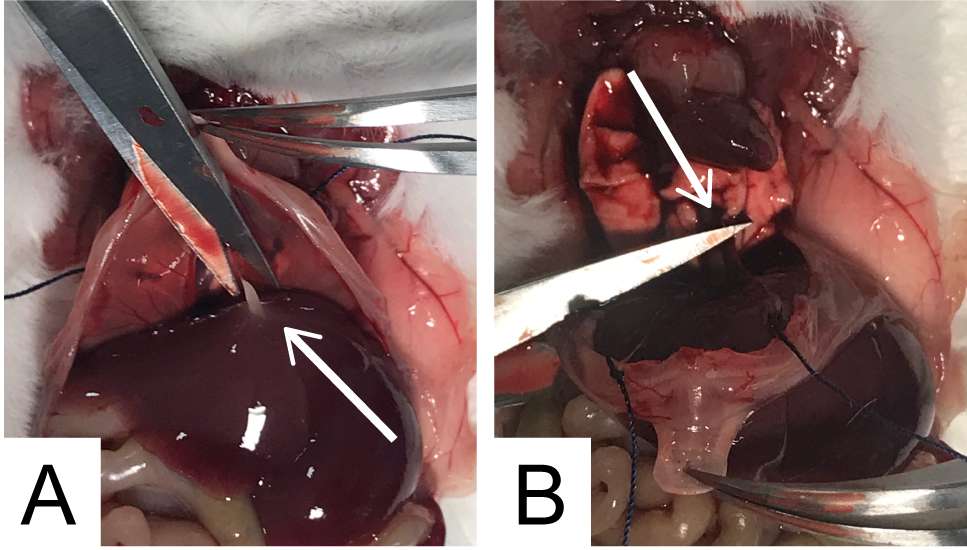
Figure 4. Diaphragm explantation
- Transfer the preparation into a Petri dish (10 cm diameter), bottomed with sylgard and filled with 5-10 ml of complete Ringer’s solution; pin the muscle as shown in Figure 5A.
- Split the whole muscle into two hemidiaphragms, each innervated by its own phrenic nerve, by cutting along the dotted lines shown in Figure 5A and discarding the central wedge with the sternum attachment. The result of this processing is two triangles, one of which is shown in Figure 5B.
Note: With the present dissection procedure, two hemidiaphragms are obtained allowing two parallel but independent analyses, which is a remarkable advantage.
- Tie a cotton thread at the apex of the triangle to connect the preparation to the transducer, making double knots without drilling the tissue. Use the ribs at the base of the triangle to secure the preparation to the support: drill the tissue between the ribs, pass a cotton thread and make a double knot around the lower rib (Figure 5B). Position the support and tie the base of the triangle via a double knot around the holder (Figure 5C).
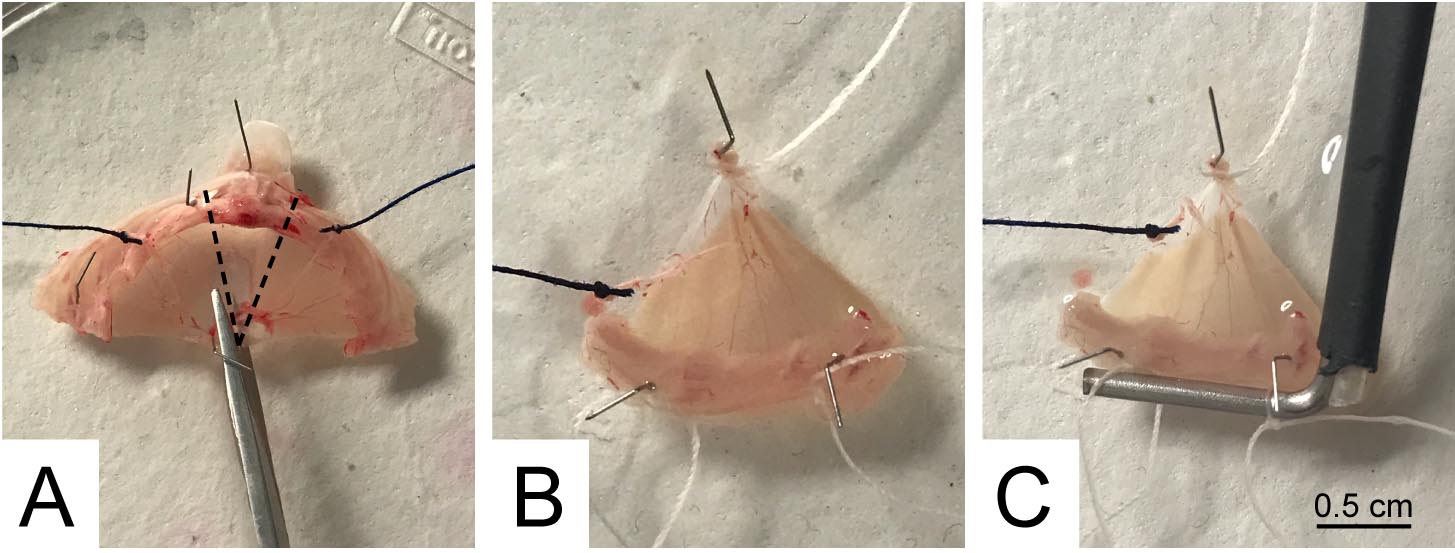
Figure 5. Hemidiaphragms separation and mounting on the tissue holder
- Euthanize the mice, preferably by cervical dislocation.
- Nerve stimulation and acquisition
- Very carefully, transfer the preparation into the stimulation chamber (filled with 4 ml of complete Ringer’s solution, at 37 °C). Set O2 pressure to have a continuous but gentle bubbling of gas. Fix the thread knotted at the triangle apex to the transducer sensor with a double knot (Figure 6A).
Notes:- For a complete force transduction, the muscle must be perpendicular to the axis of the transducer sensor (thread vertical as much as possible); thread contact with other parts of the setup may alter the measurement and must be avoided (Figure 6B). Muscle twitching is recorded by an isometric transducer that translates the mechanical force in electrical signal and commutated into a digital signal displayed by the Labscribe software.
- A calibration curve can be built by applying weights of known value to the transducer and recording the corresponding Volt values; this procedure allows to express the force of muscle contraction in Newton (or in grams). The unit of measurement is irrelevant when results are provided as normalized values (see below).
- For a complete force transduction, the muscle must be perpendicular to the axis of the transducer sensor (thread vertical as much as possible); thread contact with other parts of the setup may alter the measurement and must be avoided (Figure 6B). Muscle twitching is recorded by an isometric transducer that translates the mechanical force in electrical signal and commutated into a digital signal displayed by the Labscribe software.
- Stretch the muscles, using the lead screw positioner (Figure 6A), until its tension reaches about 0.2 V (resting tension).
- Pass the cotton wire connected to the phrenic nerve through the two platinum rings of the stimulating electrode (arrow in Figure 6C). Using the micromanipulator, carefully move the electrode inside the stimulation chamber. Pull the cotton thread until the phrenic nerve gets in contact with the rings (Figure 6C).
Note: The nerve must be completely immersed into the solution and ideally perpendicular to the muscle, avoiding excessive tension. The electrode must not be in contact with the muscle, to prevent direct stimulation of muscle fibers.
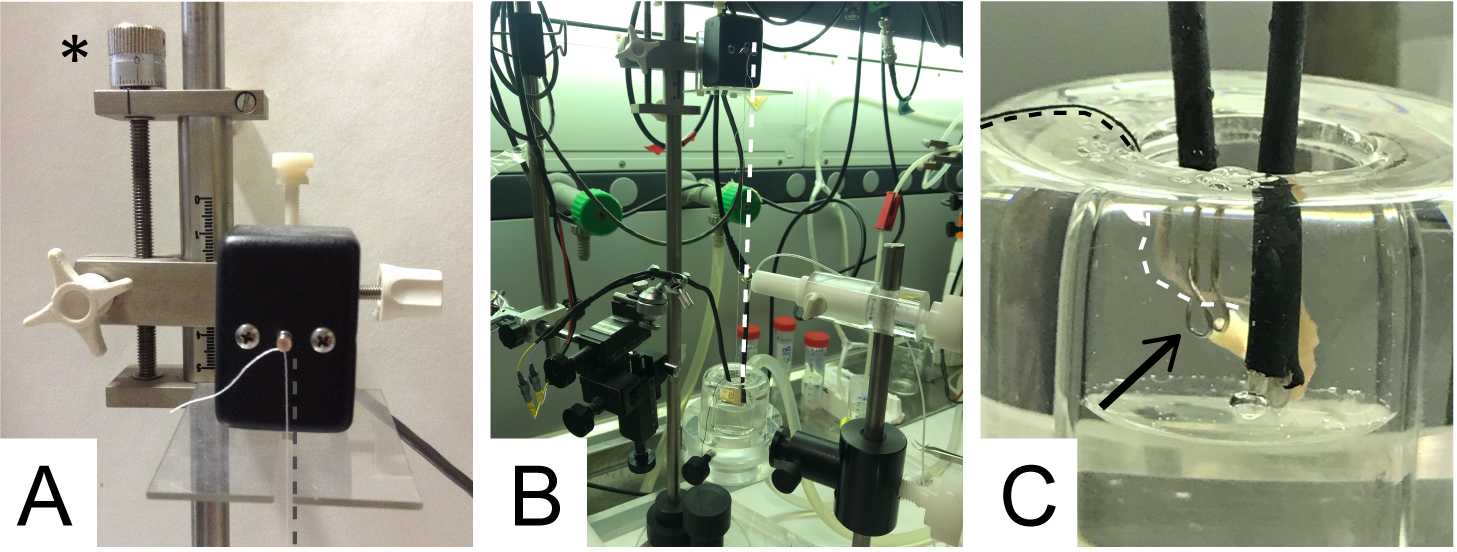
Figure 6. Details of the experimental setup. A. Transducer sensor with the cotton thread knotted. The asterisk indicates the screw positioner. B. Connection between the cotton thread (parallel to the white dotted line) and the hemidiaphragm. C. Final positioning of the phrenic nerve in the chamber. Black arrow: electrode rings with the nerve passing through and contacting it.
- Set the standard parameters for phrenic nerve stimulation. Amplitude: 5 V (supramaximal stimulus); pulse width: 0.1 msec; frequency: 0.1 Hz. Start the stimulation and test for muscle twitching.
Note: In these conditions, a hemidiaphragm muscle preparation can twitch at least 4 h.
- If the muscle contracts properly, adjust the optimal tension for twitch response using the screw positioner. Slowly increase the muscle tension repeated times, alternating with 10-15 min periods of muscle stabilization, until, incrementing the resting tension, twitch amplitude does not increase any more. An optimal resting tension is around 0.3 V.
- Let muscle twitch stabilize for further 20 min at 37 °C before starting the experiment or before adding a treatment.
Note: The mean twitch amplitude measured at the end of this period is used to normalize twitch values upon treatment.
- Add the treatment directly into the stimulation chamber.
- Using i-WORX 118 system with Labscribe software, record muscle twitch for the desired time or until muscle force completely drops.
- Very carefully, transfer the preparation into the stimulation chamber (filled with 4 ml of complete Ringer’s solution, at 37 °C). Set O2 pressure to have a continuous but gentle bubbling of gas. Fix the thread knotted at the triangle apex to the transducer sensor with a double knot (Figure 6A).
Data analysis
- Twitch amplitude is calculated as the difference between the tension peak value after stimulation (an in Figure 7) and the average basal tension before stimulus (mean value of resting tension in the time interval bn in Figure 7). This value, in standard conditions, is maintained for at least 4 h.
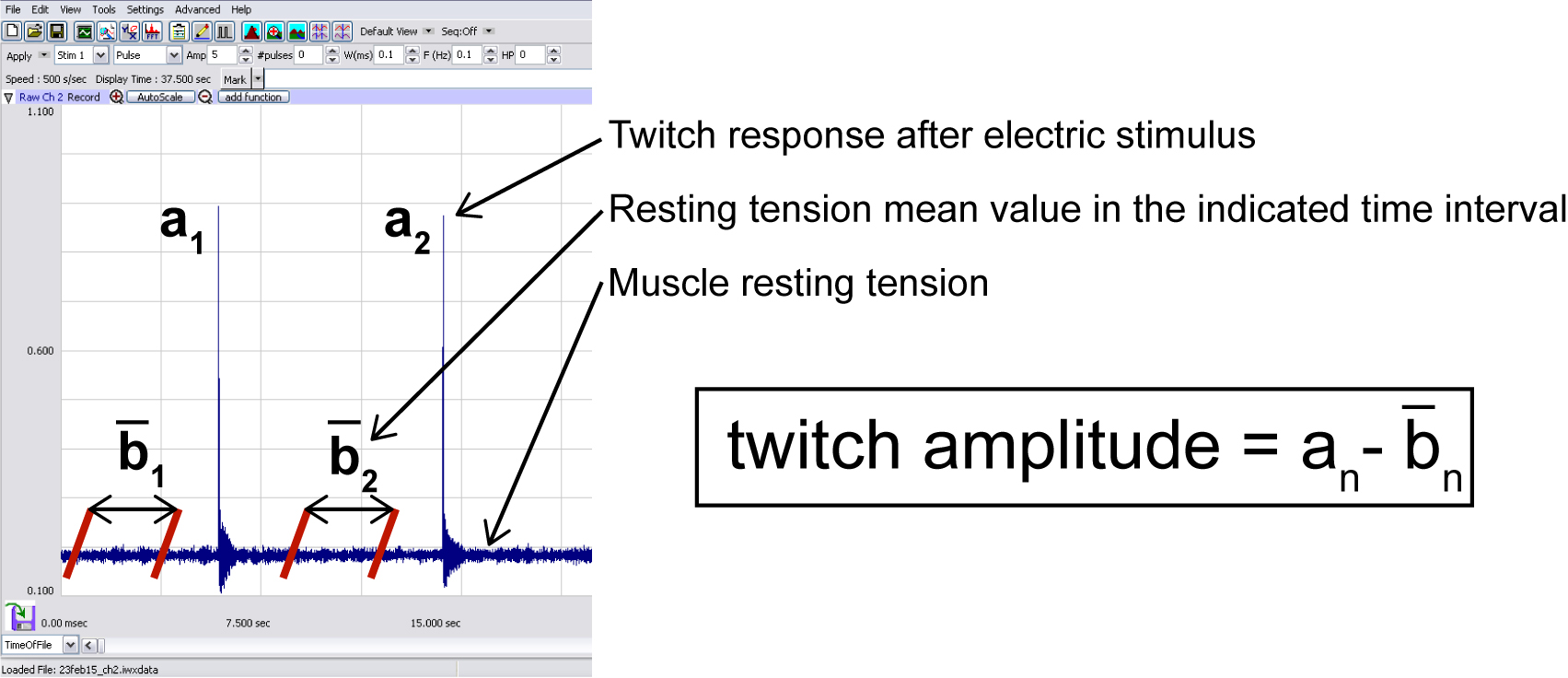
Figure 7. Typical trace displayed by Labscribe software during data recording
- By adding a treatment impinging on neuromuscular transmission (both presynaptic or postsynaptic compartment), twitch amplitude drops over the time (Figure 8A). In case of complete paralysis, the paralytic half-time (T50%), defined as the time required to decrease twitch amplitude to 50% of the initial value (Figure 8B), is commonly used to evaluate the potency of the treatment.
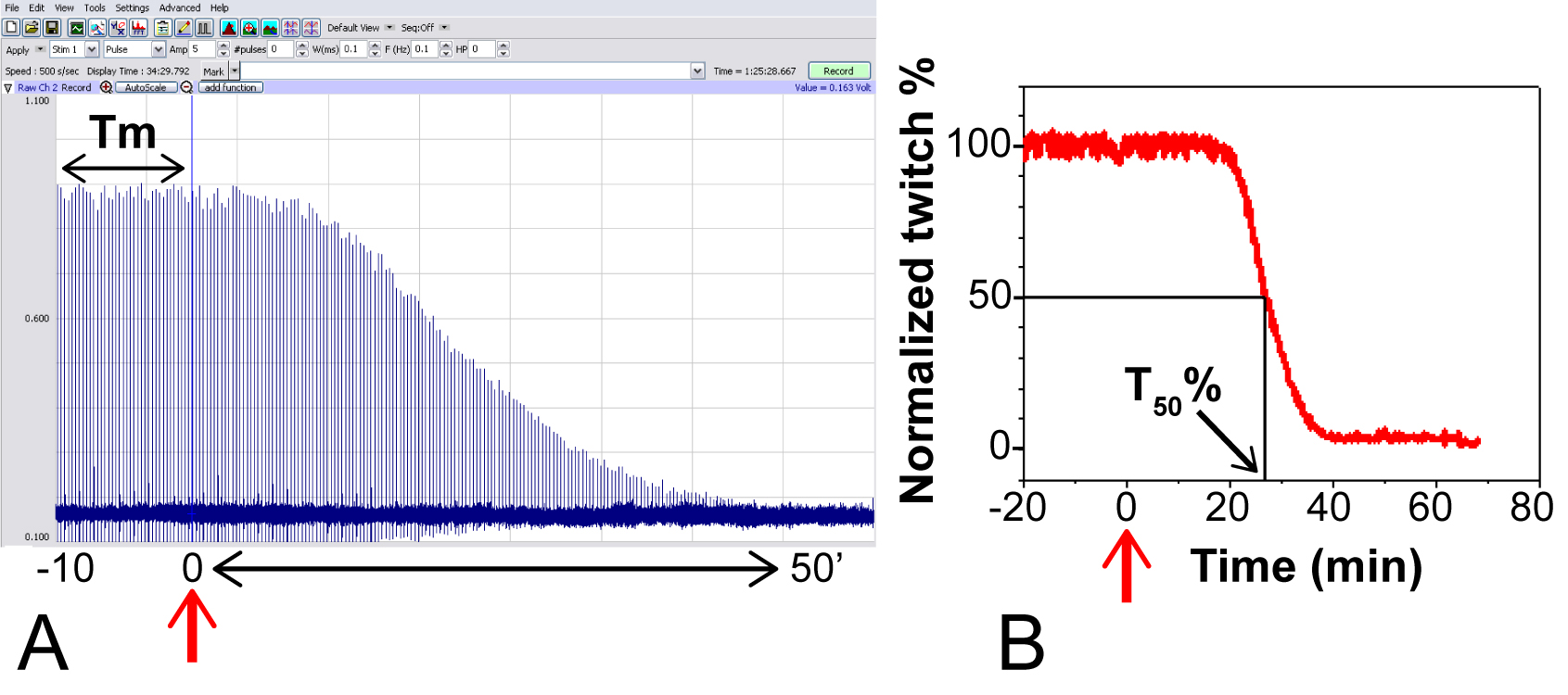
Figure 8. Typical experiment using MPN with Botulinum neurotoxin serotype A. Red arrow: toxin addition (200 pM), assumed as time = 0. A. Raw data recorded with Labscribe Software; the mean value of twitch amplitude in the time interval from -10 to 0’ (Tm), calculated as in Figure 7, is used to normalize subsequent amplitude values. B. Plot of normalized twitch amplitude against function of time, and calculation of paralytic half-time (T50%, black arrow).
- For comparative studies (for example mutant toxin vs. wild-type toxin), it is advisable to perform parallel experiments using the two hemidiaphragms harvested from the same animal to reduce variability, and to report data as paired observations. Alternatively, a potency calibration curve can be carried out with a toxin considered as reference and then used to extrapolate the relative potency of other toxin batches or variants (Bigalke and Rummel, 2015)
- Different toxins (or other substances) could require various experimental conditions (Caccin et al., 2006).
Note: Muscle fibers functionality could be detected using additional electrodes (not shown) to directly stimulate the muscle.
Recipes
- Ringer’s solution

Notes:- Use ddH2O. Glassware must be washed with hydrogen chloride 0.1 N to remove any trace of carbonate and rinsed with ddH2O very well. Do not use solutions in presence of any deposit or opalescence.
- The buffer used is a customized Ringer’s solution; throughout the protocol it is indicated as ‘Ringer’s’ for simplicity.
- Before the analysis, Ringer’s solution is saturated with 95% O2, 5% CO2, by aeration for at least 15 min to obtain pH of 7.4 and supplemented with glucose (final concentration 1 mg/ml, 11 mM) (the buffer after oxygen saturation and glucose supplementation is indicated as ‘complete Ringer’s solution’ in this protocol). Glucose is necessary for recordings longer than 1 h.
- Use ddH2O. Glassware must be washed with hydrogen chloride 0.1 N to remove any trace of carbonate and rinsed with ddH2O very well. Do not use solutions in presence of any deposit or opalescence.
Acknowledgments
This work was supported by the University of Padova, with ‘Senior Research Grant for young people not employed in the University of Padova’ granted to M. Pirazzini and with a ‘junior fellowship’ to S. Negro and by Fondazione Caritro with ‘Bando 2017 per giovani ricercatori coinvolti in progetti di eccellenza’ granted to G. Zanetti. All the procedures were performed in the laboratory of ‘Neurotoxins, Neuroparalysis and Regeneration’ headed by Prof. Cesare Montecucco at the Department of Biomedical Sciences (University of Padova). The authors declare no competing interests. PC performed the procedure for hemidiaphragm preparation. SN and GZ took the pictures to describe all the procedures and prepared the figures. GZ and PC wrote the paper, with the help of SN and MP. All authors reviewed the manuscript and approved the final version.
References
- Azarnia Tehran, D., Zanetti, G., Leka, O., Lista, F., Fillo, S., Binz, T., Shone, C. C., Rossetto, O., Montecucco, C., Paradisi, C., Mattarei, A. and Pirazzini, M. (2015). A novel inhibitor prevents the peripheral neuroparalysis of botulinum neurotoxins. Sci Rep 5: 17513.
- Bercsenyi, K., Schmieg, N., Bryson, J. B., Wallace, M., Caccin, P., Golding, M., Zanotti, G., Greensmith, L., Nischt, R. and Schiavo, G. (2014). Nidogens are therapeutic targets for the prevention of tetanus. Science 346(6213): 1118-1123.
- Beske, P. H., Bradford, A. B., Hoffman, K. M., Mason, S. J. and McNutt, P. M. (2017). In vitro and ex vivo screening of candidate therapeutics to restore neurotransmission in nerve terminals intoxicated by botulinum neurotoxin serotype A1. Toxicon. doi: 10.1016/j.toxicon.2017.10.017.
- Bigalke, H. and Rummel, A. (2015). Botulinum neurotoxins: qualitative and quantitative analysis using the mouse phrenic nerve hemidiaphragm assay (MPN). Toxins (Basel) 7(12): 4895-4905.
- Caccin, P., Rigoni, M., Bisceglie, A., Rossetto, O. and Montecucco, C. (2006). Reversible skeletal neuromuscular paralysis induced by different lysophospholipids. FEBS Lett 580(27): 6317-6321.
- Caccin, P., Scorzeto, M., Damiano, N., Marin, O., Megighian, A. and Montecucco, C. (2015). The synaptotagmin juxtamembrane domain is involved in neuroexocytosis. FEBS Open Bio 5: 388-396.
- Klooster, R., Plomp, J. J., Huijbers, M. G., Niks, E. H., Straasheijm, K. R., Detmers, F. J., Hermans, P. W., Sleijpen, K., Verrips, A., Losen, M., Martinez-Martinez, P., De Baets, M. H., van der Maarel, S. M. and Verschuuren, J. J. (2012). Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 135(Pt 4): 1081-1101.
- Li, L., Xiong, W. C. and Mei, L. (2017). Neuromuscular junction formation, aging, and disorders. Annu Rev Physiol.
- Nascimento, F., Pousinha, P. A., Correia, A. M., Gomes, R., Sebastiao, A. M. and Ribeiro, J. A. (2014). Adenosine A2A receptors activation facilitates neuromuscular transmission in the pre-symptomatic phase of the SOD1(G93A) ALS mice, but not in the symptomatic phase. PLoS One 9(8): e104081.
- Rasetti-Escargueil, C., Liu, Y., Rigsby, P., Jones, R. G. and Sesardic, D. (2011). Phrenic nerve-hemidiaphragm as a highly sensitive replacement assay for determination of functional botulinum toxin antibodies. Toxicon 57(7-8): 1008-1016.
- Rigoni, M., Caccin, P., Gschmeissner, S., Koster, G., Postle, A. D., Rossetto, O., Schiavo, G. and Montecucco, C. (2005). Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science 310(5754): 1678-1680.
- Yan, Y., Li, J., Zhang, Y., Peng, X., Guo, T., Wang, J., Hu, W., Duan, Z. and Wang, X. (2014). Physiological and biochemical characterization of egg extract of black widow spiders to uncover molecular basis of egg toxicity. Biol Res 47: 17.
- Zanetti, G., Sikorra, S., Rummel, A., Krez, N., Duregotti, E., Negro, S., Henke, T., Rossetto, O., Binz, T. and Pirazzini, M. (2017). Botulinum neurotoxin C mutants reveal different effects of syntaxin or SNAP-25 proteolysis on neuromuscular transmission. PLoS Pathog 13(8): e1006567.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zanetti, G., Negro, S., Pirazzini, M. and Caccin, P. (2018). Mouse Phrenic Nerve Hemidiaphragm Assay (MPN). Bio-protocol 8(5): e2759. DOI: 10.21769/BioProtoc.2759.
Category
Neuroscience > Peripheral nervous system > Skeletal muscle
Cell Biology > Cell signaling > Synaptic transmision
Cell Biology > Tissue analysis > Electrophysiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











