- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Boron Uptake Assay in Xenopus laevis Oocytes
Published: Vol 8, Iss 5, Mar 5, 2018 DOI: 10.21769/BioProtoc.2755 Views: 8493
Reviewed by: Tie LiuHarrie van ErpAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification of S-locus F-box Protein Sequences in Diploid Potato, Solanum okadae, via Degenerate PCR
Amar Hundare [...] Timothy P. Robbins
Jun 5, 2025 1995 Views

Quantitative Analysis of the Arabidopsis Leaf Secretory Proteome via TMT-Based Mass Spectrometry
Sakharam Waghmare [...] Rucha Karnik
Nov 20, 2025 2020 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 543 Views
Abstract
Boron (B) is essential for plant growth and taken up by plant roots as boric acid. Under B limitation, B uptake and translocation in plants are dependent on the boric acid channels located in the plasma membrane. Xenopus leavis oocyte is a reliable heterologous expression system to characterize transport activities of boric acid channels and related major intrinsic proteins (aquaporins). Here, we outline the protocols for expression of boric acid channels and boric acid uptake assay in Xenopus leavis oocytes.
Keywords: Boric acidBackground
Boron is essential for plant growth but is toxic when it is accumulated. Boron has structural functions in the cell wall through cross-linking of pectic polysaccharides at rhamnogalacturonan II regions (Funakawa and Miwa, 2015). In solution, B exists primarily as boric acid at physiological pH [B(OH)3 + H2O ⇆ B(OH)4- + H+ (pKa = 9.24)]. Boric acid is a small neutral molecule and thus shows significant passive diffusion across biological membranes (Dordas et al., 2000). In addition, plants utilize boric acid channels belonging to major intrinsic protein (MIP, aquaporin) family and borate exporters (BOR family) to maintain B homeostasis (Takano et al., 2008).
Initially, maize PIP1 was expressed in Xenopus leavis oocytes and shown to facilitate boric acid uptake by 30% over the water-injected oocytes (Dordas et al., 2000). Then, Arabidopsis NIP5;1 was shown to facilitate boric acid uptake five to nine-fold over the water-injected oocytes (Takano et al., 2006). Together with the finding that Arabidopsis mutants lacking NIP5;1 activity showed lower B uptake into roots and severe growth defects under low B conditions, NIP5;1 was identified as a boric acid channel. NIP5;1 homologs, NIP6;1 and NIP7;1 from Arabidopsis, Lsi1 (NIP2;1) from rice, NIP2;1 from barley, and TLS1 (NIP3;1) from maize were also shown to facilitate boric acid uptake using Xenopus oocytes (Tanaka et al., 2008; Li et al., 2011, Mitani-Ueno et al., 2011; Schnurbusch et al., 2010; Durbak et al., 2014). Importantly, OsLsi1 transported boric acid, silicic acid, arsenite, and water, and AtNIP5;1 transported boric acid, arsenite, and water, but not silicic acid in Xenopus oocytes (Mitani-Ueno et al., 2011). Xenopus oocytes were also used for characterization of barley Bot1, a borate exporter for high-B tolerance, by electrophysiology (Nagarajan et al., 2015).
As another heterologous expression system, yeast Saccharomyces cerevisiae has been used for transport studies of B. In this system, Arabidopsis BOR1 and yeast ScBOR1 decreased B concentrations in the cells, and thus were characterized as borate exporters (Takano et al., 2002; Takano et al., 2007). In contrast, expression of AtNIP5;1 and its rice ortholog OsNIP3;1 increased boric acid uptake (Hanaoka et al., 2014). As a convenient but indirect assay, survival assay has been employed using Saccharomyces cerevisiae. Expression of BOR1 homologs conferred tolerance to toxic B conditions (Nozawa et al., 2006; Miwa et al., 2007; Sutton et al., 2007; Cañon et al., 2013; Hayes et al., 2015) and expression of NtXIP1;1, AtNIP4;1, HvPIP1;3 and HvPIP1;4 increased sensitivity (Fitzpatrick and Reid 2009; Bienert et al., 2011; Di Giorgio et al., 2016).
Nevertheless, Xenopus oocytes expression system is advantageous for ‘quantitative’ transport studies (Miller and Zhou, 2000; Yesilirmak and Sayers, 2009). It has apparently low intrinsic transport activity for many substrates. It tends to produce comparable levels of membrane proteins in the plasma membrane dependent on the amount of injected cRNA. Recently we utilized these merits for analysis of AtNIP5;1 variants. AtNIP5;1 is localized on the soil-side plasma membrane domain of the outermost root cell layers of roots (Takano et al., 2010). We found that the phosphorylated state of conserved threonine residues in ‘TPX’ repeat in N-terminal region of AtNIP5;1 is associated with the polar localization of NIP5;1 (Wang et al., 2017). Using the AtNIP5;1 variant in which conserved threonines were substituted to alanine, we showed that the polar localization is important for efficient B translocation in plant roots. Importantly, the direct boric acid uptake assay in X. laevis oocytes clarified that the threonine residues are not involved in the boric acid uptake activity of NIP5;1 channel (Wang et al., 2017).
Here, we describe the protocol of boric acid transport assay using Xenopus oocytes (Figure 1) and inductively coupled plasma-mass spectrometry (ICP-MS), which is applicable for various other metalloids and metals.
Materials and Reagents
- Glass capillaries (Drummond Scientific, catalog number: 3-000-203-G/X )
- Slide glass
- Parafilm M (LMS, catalog number: 94-2542-5 )
- Petri dishes (Diameter: 3.5 cm and 9.0 cm, LMS, Japan)
- Pipette tips
- 2 ml Eppendorf tube (Eppendorf, catalog number: 022363344 )
- Eppendorf safe-lock tubes,1.5 ml (Eppendorf, catalog number: 0030120086 )
- Metal-free tube without lids (DigiTUBEs, GL Sciences, catalog number: 8520-50112 )
- Syringe with needle
- Polypropylene mesh (0.8 mm, Spectrum, catalog number: 146492 )
- DigiFILTER 0.45 μm (SCP SCIENCE, catalog number: 010-500-070 )
- Xenopus leavis oocytes treated with collagenase (Kitagawa Institute, hita, Japan)
Note: Protocol of harvesting of ovaries from female Xenopus laevis is available (Shi and Carattino, 2017). - pXBG-ev1 vector (Preston et al., 1992; Figure 2)
- Gel extraction kit (Sigma-Aldrich, catalog number: NA1111 )
- TE buffer
- Ultra pure water produced using the MILLI-Q ADVANTAGE A10 purification system (Millipore)
Note: To prepare samples for ICP-MS, do not use glassware made by borosilicate to avoid contamination of B. - mMESSAGE mMACHINE T3 Transcription Kit (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM1348)
- Mineral oil (NACALAI TESQUE, CAS number: 8020-83-5)
- Boric acid (Wako Pure Chemical Industries, catalog number: 029-02191 )
- Nitric acid (HNO3) (For boron determination, Wako Pure Chemical Industries, catalog number: 140-05415 )
- Boron standard solution (B 1,000, Wako Pure Chemical Industries, catalog number: 025-16581 )
- Modified Barth’s Saline (MBS) buffer with or without Ca2+ (see Recipes)
- Sodium chloride (NaCl, Wako Pure Chemical Industries, catalog number: 191-01665 )
- Potassium chloride (KCl, Wako Pure Chemical Industries, catalog number: 163-03545 )
- Sodium bicarbonate (NaHCO3, NACALAI TESQUE, catalog number: 31212-25 )
- Tris-HCl (pH 7.6, Sigma-Aldrich, Roche Diagnostics, catalog number: 10708976001 )
- Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, Wako Pure Chemical Industries)
- Calcium chloride dihydrate (CaCl2·2H2O, Wako Pure Chemical Industries, catalog number: 039-00431 )
- Magnesium sulfate heptahydrate (MgSO4·7H2O, Wako Pure Chemical Industries, catalog number: 131-00405 )
- Sodium penicillin (Wako Pure Chemical Industries, catalog number: 168-23191 )
- Streptomycin sulfate (Wako Pure Chemical Industries, catalog number: 196-08511 )
- Sodium chloride (NaCl, Wako Pure Chemical Industries, catalog number: 191-01665 )
Equipment
- Dual-Stage Glass Micropipette (NARISHIGE, model: PC-10 )
- Confocal laser scanning microscope (Leica Microsystems, model: Leica TCS SP8 )
- Fluorescent microscope (Olympus, model: MVX-10 )
- Nanoliter injector (Drummond Scientific, model: Nanoject II )
- Stereo microscope (Nikon, model: SMZ-2T )
- Incubator (Advantec, model: THS030PA )
- Heat digestion system (GL Sciences, model: DigiPREP Jr )
- ICP-MS (PerkinElmer, model: ELAN DRC-e )
- Autosamplers (PerkinElmer, model: ESI )
- Ultrapure water system (Merck, model: MILLI-Q® ADVENTAGE A10 )
Procedure
An outline of procedures to express boric acid channels in oocytes is illustrated in Figure 1.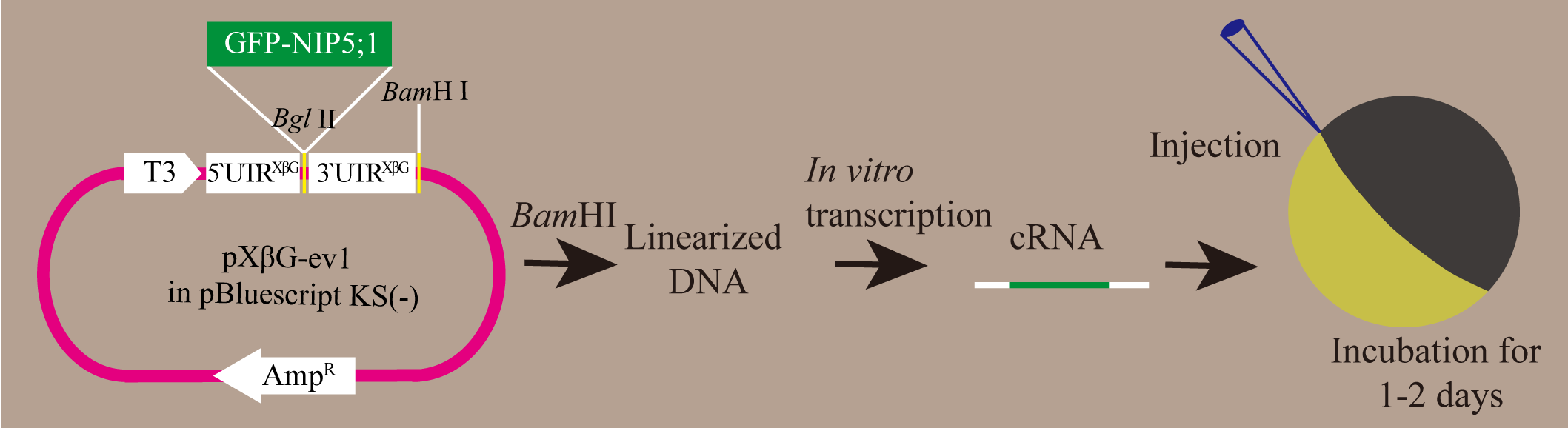
Figure 1. Procedures to express boric acid channels in oocytes. The GFP-NIP5;1 ORF sequence was subcloned into pXBG-ev1 vector using the BglII site. The vector was linearized by BamHI and used for in vitro cRNA transcription. GFP-NIP5;1 cRNA was injected into oocytes for expression and subsequent boron transport assay.
- cRNA synthesis of NIP5;1
- Subclone ORF sequence of GFP-NIP5;1 in pXBG-ev1 vector (Figure 2) using BglII site.
Note: The GFP was used to select oocytes expressing NIP5;1 in a later step (Procedure C).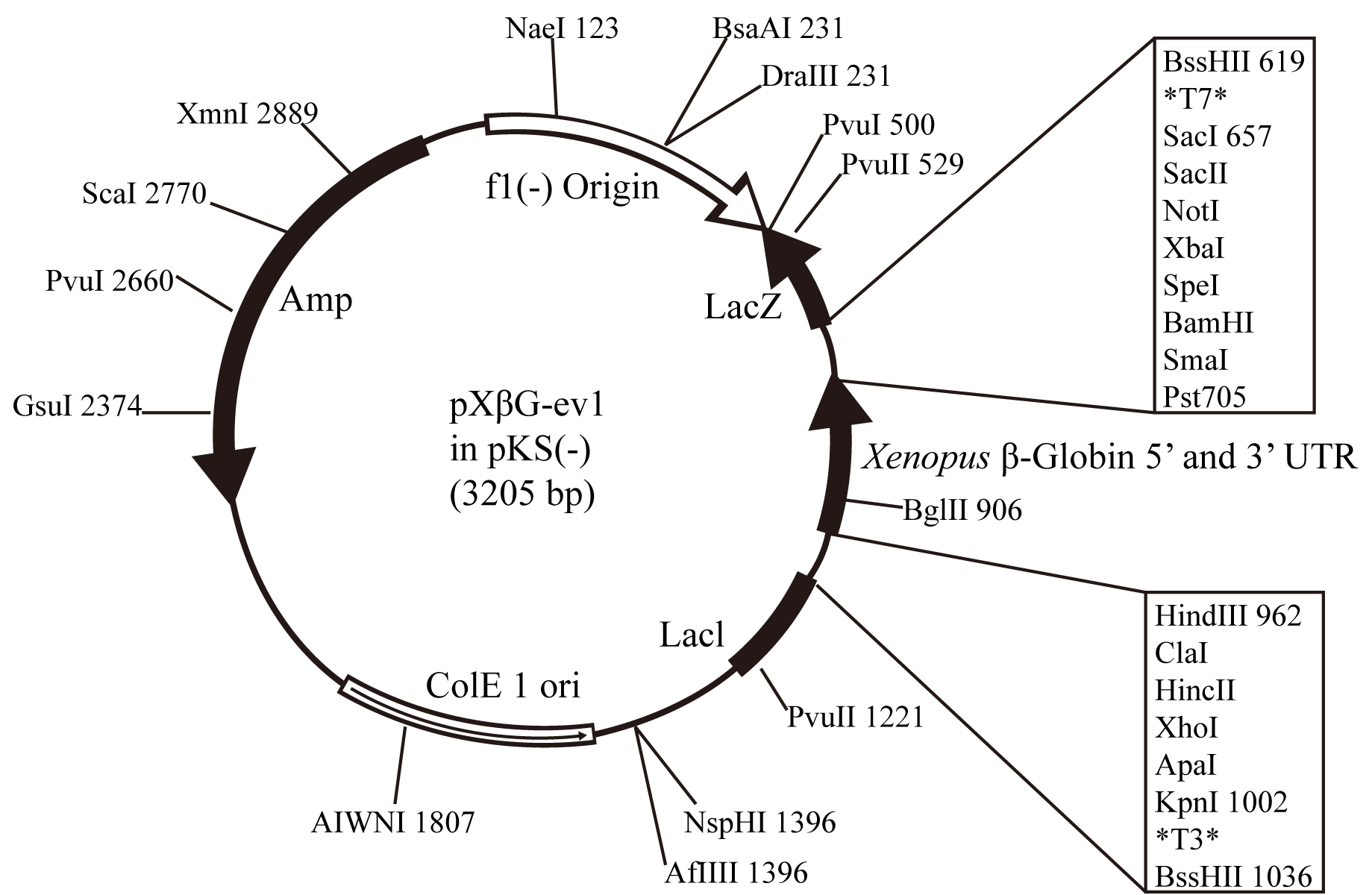
Figure 2. A map of the pXBG-ev1 vector for in vitro transcription (Preston et al., 1992). An ORF sequence of gene of interest can be subcloned between the 5’ untranslated region (UTR) and 3’ UTR using BglII site. - Linearize the plasmid by endonuclease such as BamHI. Purify DNA fragment with a gel extraction kit (Sigma-Aldrich) and dilute to be the concentration of 500 ng/µl in TE buffer or nuclease-free water.
- Run capped transcription reaction and recover cRNA following the manufacturer’s instruction. We use mMESSAGE mMACHINE T3 Transcription Kit (Thermo Fisher Scientific).
Note: The manufacturer’s guide for cRNA synthesis could be found at https://www.thermofisher.com/order/catalog/product/AM1348.
- Subclone ORF sequence of GFP-NIP5;1 in pXBG-ev1 vector (Figure 2) using BglII site.
- cRNA injection of NIP5;1
- Prepare sharp glass capillary by dual-stage glass micropipette puller PC-10 (Figure 3).
Note: Fix a capillary and set the parameters in PC-10 as follows: No.1 Heater level: 60, No.2 Heater level: 58. Press the ‘start’ button to process automatically.
Figure 3. Preparation of glass capillaries by the puller PC-10. Fixation (A), heating and extension (B) of a glass capillary on PC-10. A glass capillary with sharp tip end (C). - Break tip end by the cut-surface of a slide glass under a stereo microscope and check the sharpness of the glass tip under a stereo microscope (Figures 4A-4C).
Note: Check the tip-end by taking MilliQ water. Usually the glass capillary made by PC-10 cannot be used directly because of the sealed tip-end (Figure 4D).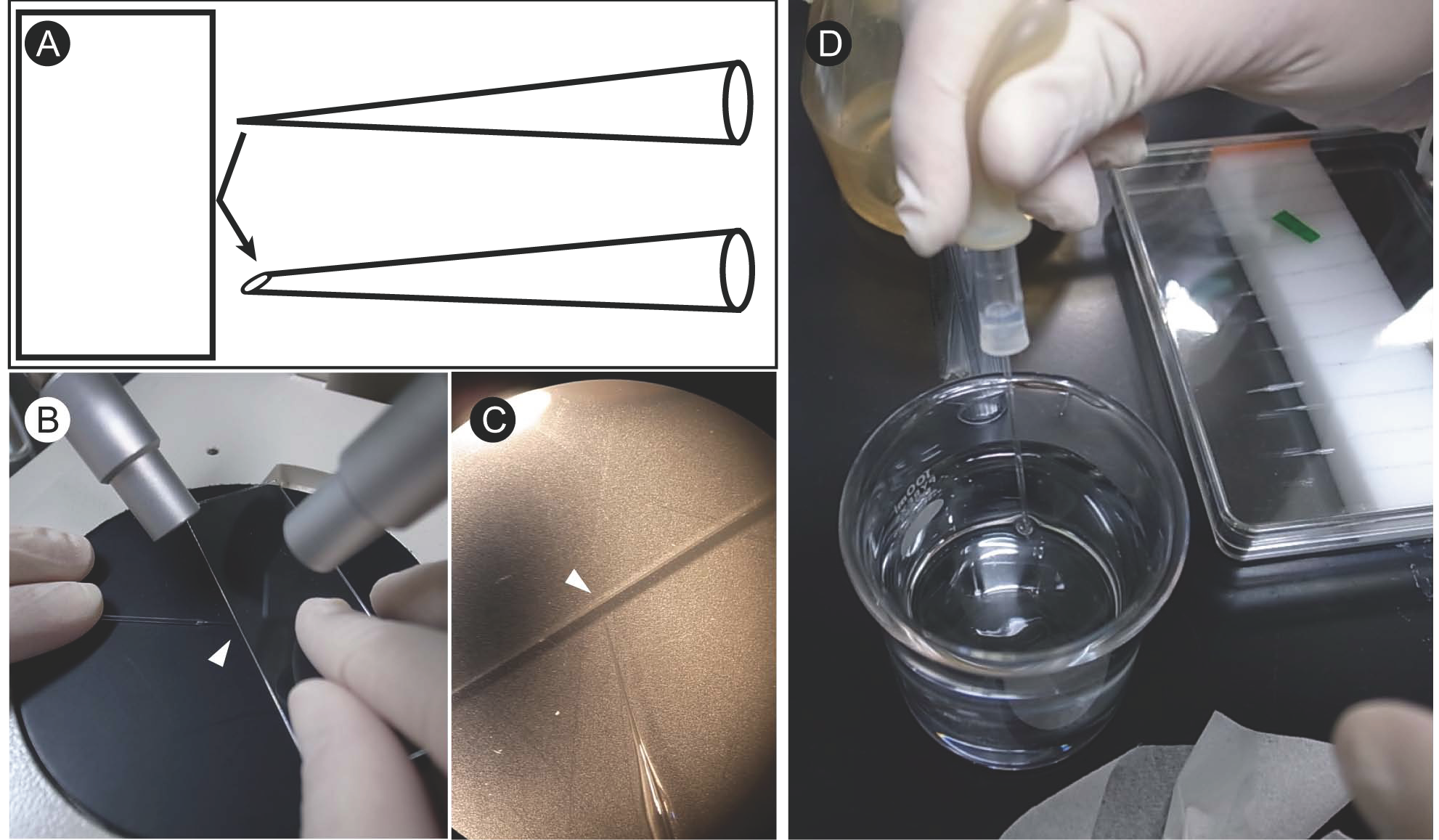
Figure 4. Examination of the glass capillary. A. The process of breaking tip end by the cut-surface of a glass slide; B. Observation of tip end of glass capillary in stereo microscopy; C. A close-up view of interface of slide and tip end indicated by the white arrowhead; D. Examination of the hole at the tip end of glass capillary by taking water. - Fill mineral oil up capillary using a syringe with needle, and equip the capillary on the injector NANOJECT II (Figures 5A-5C).

Figure 5. Filling the glass capillary with mineral oil. Filling the glass capillary with mineral oil by a syringe (A) and setting it on NANOJECT II (B, C). The white circle in B indicates the position of glass capillary on NANOJECT II. - Incubate oocytes in MBS with Ca2+ buffer (see Recipes) at 18 °C for 6 h prior to injection.
Note: Select healthy oocytes with clear black and yellow halves for injection (Sigel, 2010). - For each cRNA sample, prepare 35 oocytes and inject 50 nl of cRNA solution for one oocyte by NANOJECT II (Figure 6).
Note: Press ‘empty’ button to push out metal needle to the position at ~0.5 cm from tip-end of glass capillary. Place 4 µl of cRNA on Parafilm (Figure 6B) and take up by pushing ‘fill’ button (Figure 6A). Transfer several oocytes on polypropylene mesh placed in a new Petri dish by 1 ml end cut pipette tips (Figure 6C). Move microinjector close to an oocyte and gently insert glass capillary. Press the ‘inject’ button of NANOJECT II for injection. Detach the glass capillary from the oocyte and sequentially inject next one. Change capillary when you deal with different cRNA.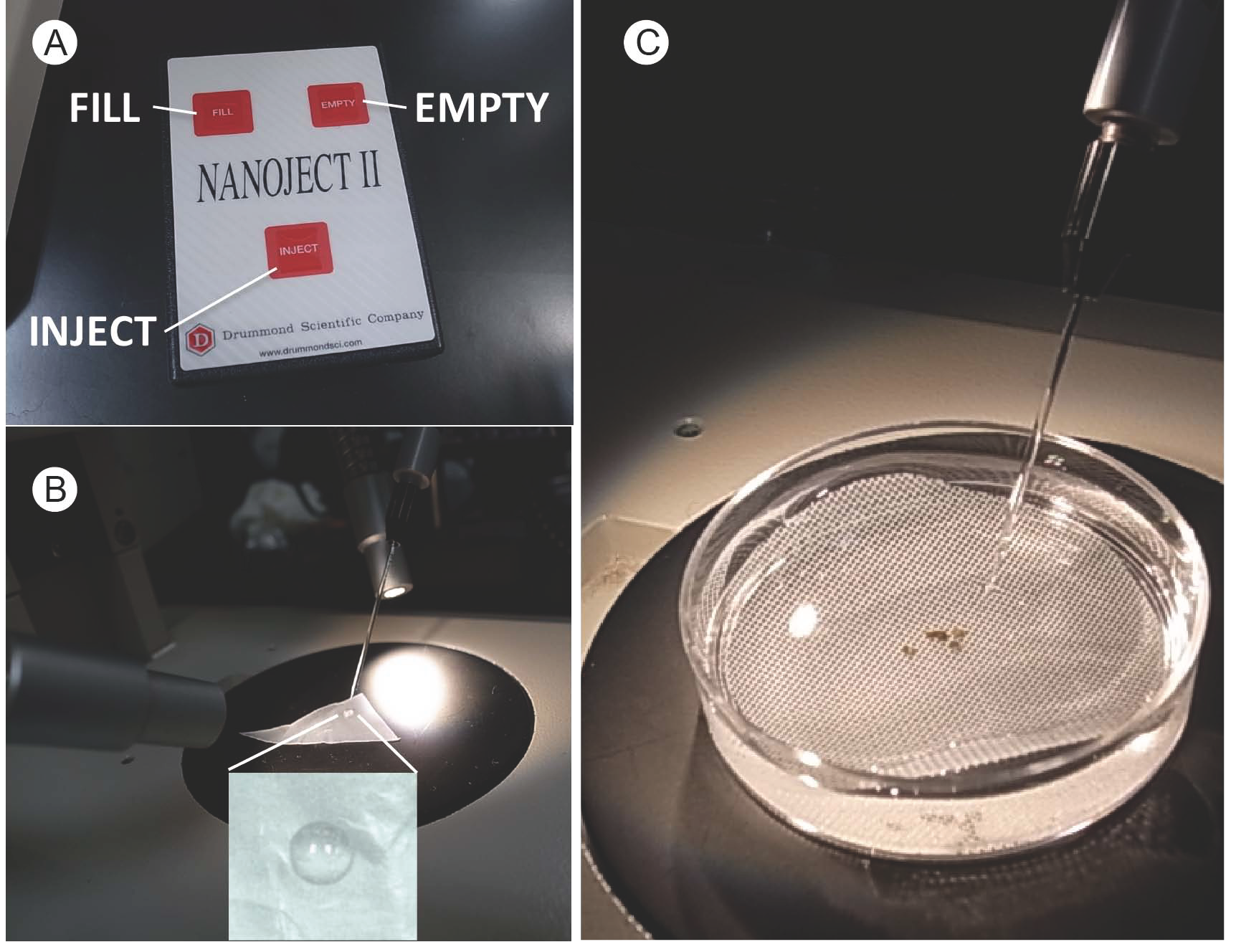
Figure 6. cRNA injection into oocytes by NANOJECT II. A. Control panel of NANOJECT II; B. Filling a glass capillary with cRNA; C. cRNA injection into oocytes by NANOJECT II.
- Prepare sharp glass capillary by dual-stage glass micropipette puller PC-10 (Figure 3).
- Incubation of oocytes with boric acid
- Observe expression of GFP-fused proteins in injected oocytes under a fluorescent stereo microscopy after 1-2 days incubation in MBS with Ca2+ buffer (see Recipes) at 18 °C (Figures 7A-7C).
- Image localization of GFP-fused protein in the oocyte by confocal laser scanning microscopy (Figures 7D-7F).
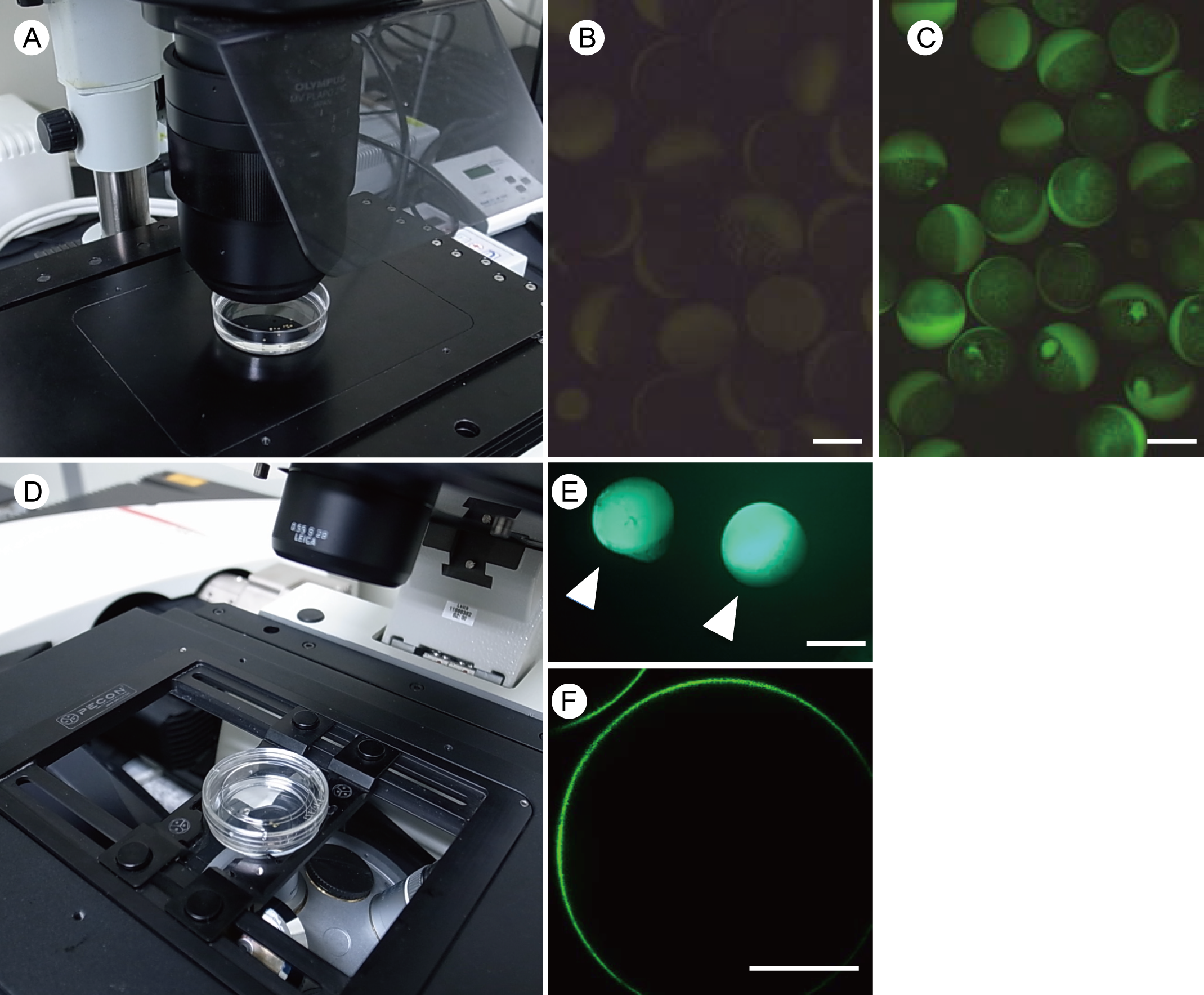
Figure 7. Observation of GFP in oocytes. A-C. Observation of GFP signals in oocytes injected with cRNA by a fluorescent microscope. Oocytes injected with TE buffer (B) and GFP-NIP5;1 cRNA (C), respectively. D. Observation of oocytes by a confocal laser scanning microscopy; E and F. A surface image of oocytes (white arrowheads) (E) and a medial-optical section of oocyte (F) injected with GFP-NIP5;1 cRNA. Scale bars = 1.0 mm (7B, 7C, 7E), 0.5 mm (7F). - Collect 7-8 oocytes as one pool in a 2 ml Eppendorf tube using 1 ml end cut pipette tip. Preparing 4 or more replicas is recommended.
- Remove the MBS buffer and add 1 ml of MBS containing 5 mM boric acid into each tube. Incubate for 30 min at 18 °C.
- Remove the MBS buffer and wash oocytes for 5 times using 1 ml of fresh MBS buffer without boric acid.
- Observe expression of GFP-fused proteins in injected oocytes under a fluorescent stereo microscopy after 1-2 days incubation in MBS with Ca2+ buffer (see Recipes) at 18 °C (Figures 7A-7C).
- B measurement by ICP-MS
- Transfer one pool of oocytes into a new metal-free tube without lids (we used DigiTUBEs, GL Sciences). Add 500 µl concentrated HNO3 to digest 20 min, followed by incubation at 100 °C in the DigiPREP Jr equipment (GL Sciences, Tokyo). Shake DigiTUBEs every 2 min to dry the solution completely. Resuspend the sample with 165 µl concentrated HNO3 and then add ultrapure water up to 5 ml to make 2% HNO3 solution.
- Add an appropriate volume of 2% HNO3 to dilute samples for ICP-MS measurement. We prepared 2% nitric acid containing solution and measure the boron concentration.
Note: Occasionally, undissolved materials remain in DigiTUBEs. They can be eliminated by DigiFILTER 0.45 μm (GL Sciences). 1 to 5% HNO3 can be used dependent on ICP-MS instruments. - Measure 11B using 0 to 100 ppb total B (natural abundance) as standards by ICP-MS (Figure 8). We used ICP-MS ELAN DRC-e equipped with ESI Autosamplers and software suit (PerkinElmer, Waltham, Massachusetts, USA) We calculated the total B concentration assuming that there is no isotopic discrimination during the transport (10B:11B = 20:80).
Note: The guide for ICP-MS operation could be found at http://helpwiki.evergreen.edu/wiki/index.php/PerkinElmer_ELAN_DRC-E_ICP-MS_Manuals.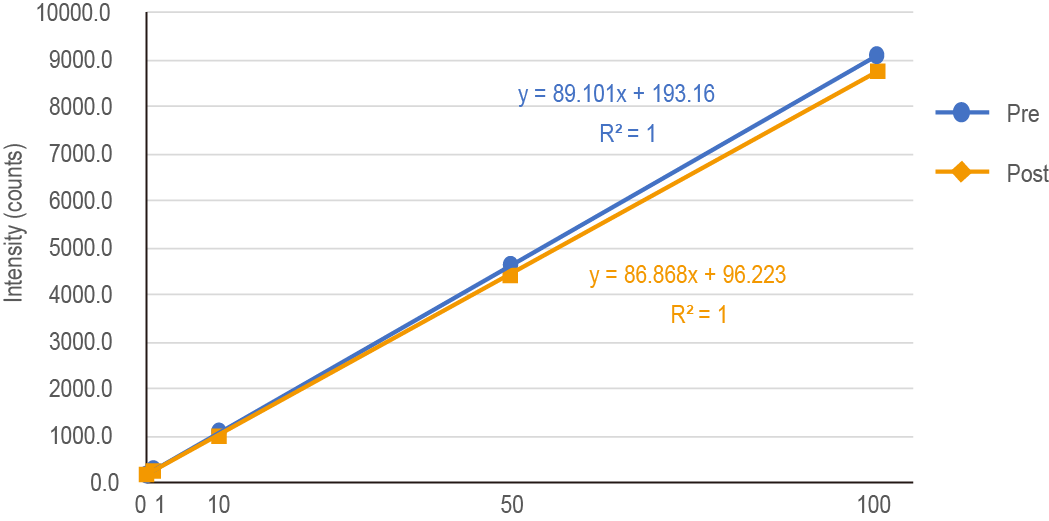
Figure 8. Linear equation of B standards and intensity measured by ICP-MS. Pre: Measurement of standards prior to measurement of samples. Post: Measurement of standards after measurement of samples.
- Transfer one pool of oocytes into a new metal-free tube without lids (we used DigiTUBEs, GL Sciences). Add 500 µl concentrated HNO3 to digest 20 min, followed by incubation at 100 °C in the DigiPREP Jr equipment (GL Sciences, Tokyo). Shake DigiTUBEs every 2 min to dry the solution completely. Resuspend the sample with 165 µl concentrated HNO3 and then add ultrapure water up to 5 ml to make 2% HNO3 solution.
Data analysis
We calculated the total B content in each oocyte as nmol/oocyte (Table 1). Significantly higher B content in GFP-NIP5;1 expressing oocytes than in TE injected control indicates that GFP-NIP5;1 has boron transport activity (Figure 9).
Table 1. Example of data analysis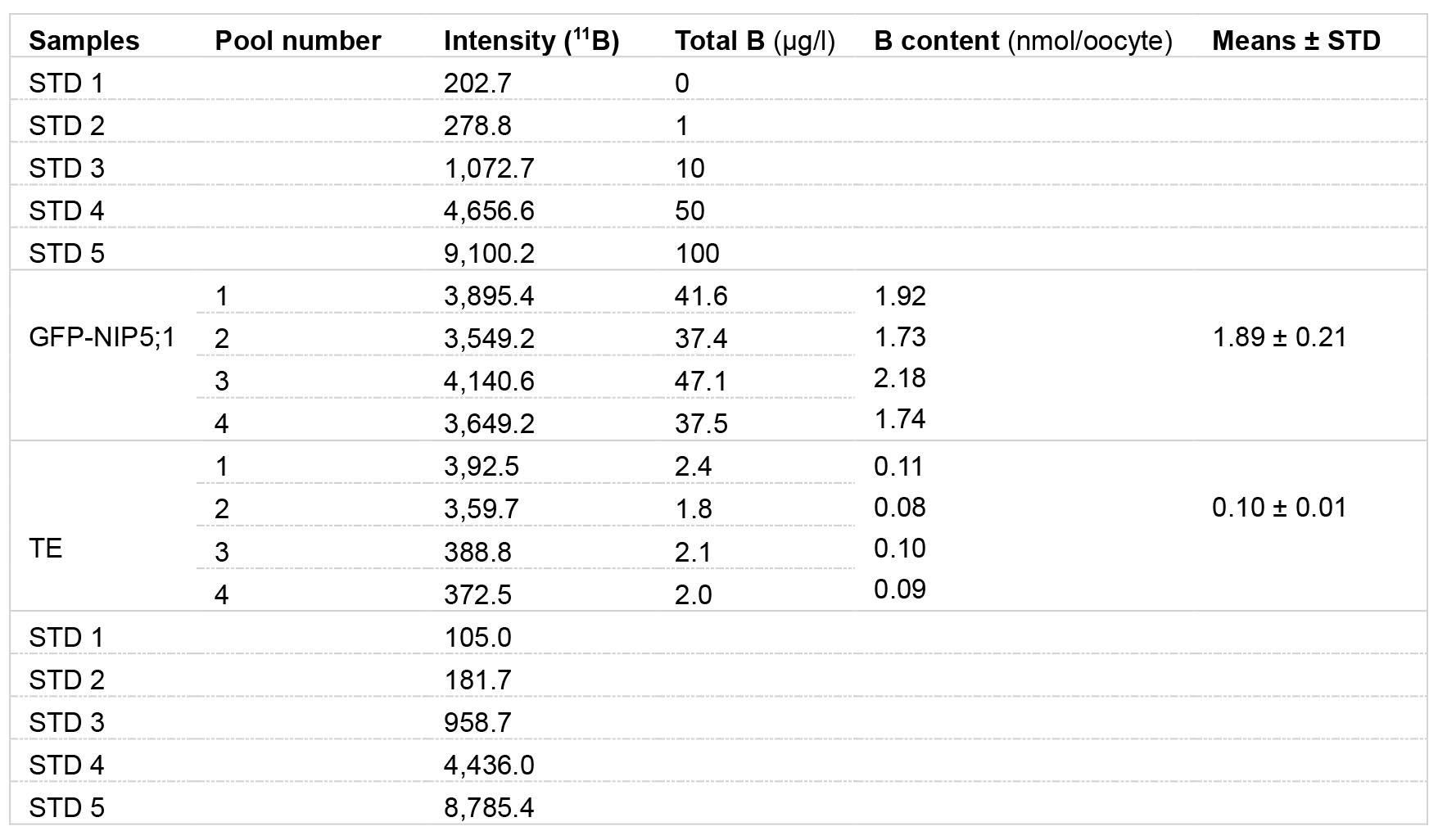
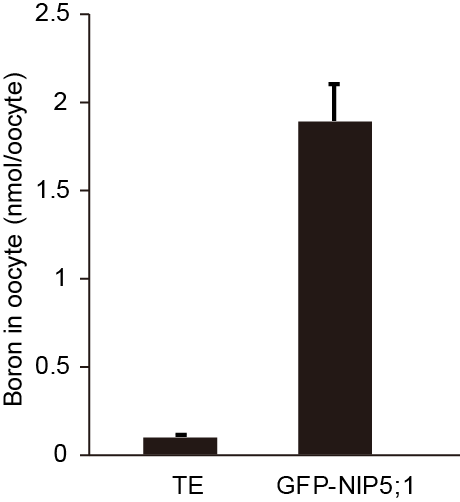
Figure 9. Boron content in oocyte after incubation with 5 mM boric acid for 30 min
Recipes
- Modified Barth’s Saline (MBS) buffer with or without Ca2+ (Table 2)
- High salt stock
64 g NaCl
1 g KCl
2.5 g NaHCO3
22.5 g Tris-base
pH 7.6 by concentrated HCl
Fill up to 500 ml with MilliQ water and store at 4 °C - Ca2+ stock
0.95 g Ca(NO3)2·4H2O
0.755 g CaCl2·2H2O
Fill up to 100 ml with MilliQ water and store at 4 °C - Mg2+ stock
2.5 g MgSO4·7H2O
Fill up to 100 ml with MilliQ water and store at 4 °C - Penicillin stock
Sodium penicillin 10 mg/ml (0.1 g/10 ml) - Streptomycin stock
Streptomycin sulfate 10 mg/ml (0.1 g/10 ml)
Table 2. Preparation of MBS buffer with or without Ca2+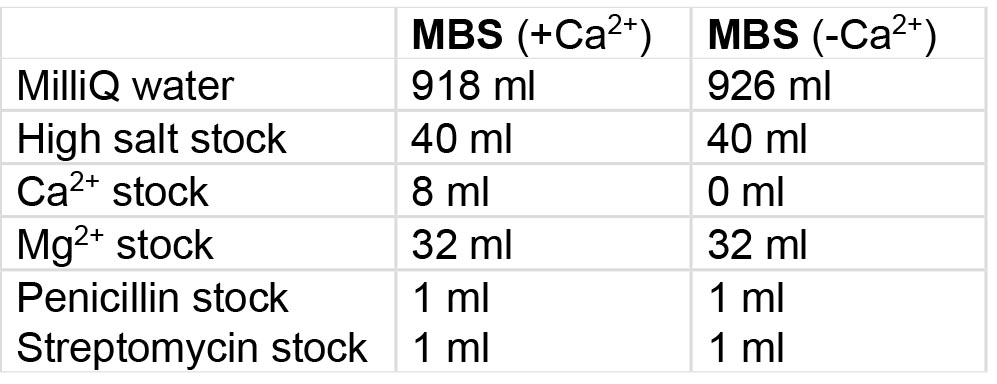
- High salt stock
Acknowledgments
We thank Jian Feng Ma, Naoki Yamaji (Okayama University), Toshihiro Watanabe and Tomoko Shimizu (Hokkaido University) for their help in fluorescent confocal microscopy and ICP-MS measurement. This work was supported by the NEXT program (GS001) and the Grant-in-Aid for Young Scientists (A) (26712007) from the Japan Society for the Promotion of Science, the Young Investigators Grant from the Human Frontier Science Program (RGY0090/2011), and a research grant from the Naito foundation to J.T. The authors declare no conflicts of interest or competing interests.
References
- Bienert, G. P., Bienert, M. D., Jahn, T. P., Boutry, M. and Chaumont, F. (2011). Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J 66(2): 306-317.
- Cañon, P., Aquea, F., Rodriguez-Hoces de la Guardia, A. and Arce-Johnson, P. (2013). Functional characterization of Citrus macrophylla BOR1 as a boron transporter. Physiol Plant 149(3): 329-339.
- Dordas, C., Chrispeels, M. J. and Brown, P. H. (2000). Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124(3): 1349-1362.
- Durbak, A. R., Phillips, K. A., Pike, S., O'Neill, M. A., Mares, J., Gallavotti, A., Malcomber, S. T., Gassmann, W. and McSteen, P. (2014). Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 26(7): 2978-2995.
- Fitzpatrick, K. L. and Reid, R. J. (2009). The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ 32(10): 1357-1365.
- Funakawa, H. and Miwa, K. (2015). Synthesis of borate cross-linked rhamnogalacturonan II. Front Plant Sci 6: 223.
- Di Giorgio, J. A., Bienert G. P., Ayub. N., Yaneff, A., Barberini, M. L., Mecchia, M. A., Amodeo, G., Soto, G. and Muschietti, J. P. (2016). Pollen-specific aquaporins NIP4;1 and NIP4;2 are required for pollen development and pollination in Arabidopsis thaliana. Plant Cell 28(5): 1053-1057.
- Hanaoka, H., Uraguchi, S., Takano, J., Tanaka, M. and Fujiwara, T. (2014). OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J 78: 890-902.
- Hayes, J. E., Pallotta, M., Garcia, M., Öz, M. T., Rongala, J. and Sutton, T. (2015). Diversity in boron toxicity tolerance of Australian barley (Hordeum vulgare L.) genotypes. BMC Plant Biol 15: 231.
- Li, T., Choi, W. G., Wallace, I. S., Baudry, J. and Roberts, D. M. (2011). Arabidopsis thaliana NIP7;1: an anther-specific boric acid transporter of the aquaporin superfamily regulated by an unusual tyrosine in helix 2 of the transport pore. Biochemistry 50(31): 6633-6641.
- Miller, A. J. and Zhou, J. J. (2000). Xenopus oocytes as an expression system for plant transporters. Biochim Biophys Acta 1465(1-2): 343-358.
- Mitani-Ueno, N., Yamaji, N., Zhao, F. J. and Ma, J. F. (2011). The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot 62(12): 4391-4398.
- Miwa, K., Takano, J., Omori, H., Seki, M., Shinozaki, K. and Fujiwara, T. (2007). Plants tolerant of high boron levels. Science 318(5855): 1417.
- Nagarajan, Y., Rongala, J., Luang, S., Singh, A., Shadiac, N., Hayes, J., Sutton, T., Gilliham, M., Tyerman, S., McPhee, G., Voelcker, N. H., Mertens, H. D. T., Kirby, N., Lee, J. and Yingling, Y. G. (2015). Na+-dependent anion transport by a barley efflux protein revealed through an integrative platform. The Plant Cell 28: 202-218.
- Nozawa, A., Takano, J., Kobayashi, M., von Wiren, N. and Fujiwara, T. (2006). Roles of BOR1, DUR3, and FPS1 in boron transport and tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett 262(2): 216-222.
- Preston, G. M., Carroll, T. P., Guggino, W. B. and Agre, P. (1992). Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256(5055): 385-387.
- Schnurbusch, T., Hayes, J., Hrmova, M., Baumann, U., Ramesh, S. A., Tyerman, S. D., Langridge, P. and Sutton, T. (2010). Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiol 153(4): 1706-1715.
- Shi, S. and Carattino, M. D. (2017). Expression and analysis of flow-regulated ion channels in Xenopus oocytes. Bio-protocol 7(8) e2224.
- Sigel, E. (2010). Microinjection into Xenopus Oocytes. In: Encyclopedia of Life Sciences (ELS). John Wiley & Sons.
- Sutton, T., Baumann, U., Hayes, J., Collins, N. C., Shi, B. J., Schnurbusch, T., Hay, A., Mayo, G., Pallotta, M., Tester, M. and Langridge, P. (2007). Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318(5855): 1446-1449.
- Takano, J., Kobayashi, M., Noda, Y., Fujiwara, T. (2007). Saccharomyces cerevisiae Bor1p is a boron exporter and a key determinant of boron tolerance. FEMS Microbiology Letters 267(2), 230–235.
- Takano, J., Miwa, K. and Fujiwara, T. (2008). Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci 13(8): 451-457.
- Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., Hayashi, H., Yoneyama, T. and Fujiwara, T. (2002). Arabidopsis boron transporter for xylem loading. Nature 420(6913): 337-340.
- Takano, J., Tanaka, M., Toyoda, A., Miwa, K., Kasai, K., Fuji, K., Onouchi, H., Naito, S. and Fujiwara, T. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci U S A 107(11): 5220-5225.
- Takano, J., Wada, M., Ludewig, U., Schaaf, G., von Wiren, N. and Fujiwara, T. (2006). The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18(6): 1498-1509.
- Wang, S., Yoshinari, A., Shimada, T., Hara-Nishimura, I., Mitani-Ueno, N., Feng Ma, J., Naito, S. and Takano, J. (2017). Polar localization of the NIP5;1 boric acid channel is maintained by endocytosis and facilitates Boron transport in Arabidopsis roots. Plant Cell 29(4): 824-842.
- Yesilirmak, F. and Sayers, Z. (2009). Heterelogous expression of plant genes. Int J Plant Genomics 2009: 296482.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, S., Mitani-Ueno, N. and Takano, J. (2018). Boron Uptake Assay in Xenopus laevis Oocytes. Bio-protocol 8(5): e2755. DOI: 10.21769/BioProtoc.2755.
Category
Plant Science > Plant molecular biology > Protein
Plant Science > Plant physiology > Nutrition
Cell Biology > Cell-based analysis > Transport
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










