- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assaying Thermo-nociceptive Behavior in Drosophila Larvae
Published: Vol 8, Iss 4, Feb 20, 2018 DOI: 10.21769/BioProtoc.2737 Views: 8686
Reviewed by: Jay Z ParrishAdler R. DillmanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assaying Mechanonociceptive Behavior in Drosophila Larvae
Nina Hoyer [...] Peter Soba
Feb 20, 2018 10082 Views

Accessing Olfactory Habituation in Drosophila melanogaster with a T-maze Paradigm
Ourania Semelidou [...] Efthimios M.C. Skoulakis
Jun 5, 2019 7851 Views

Rearing and Shipping of Uranotaenia lowii, a Frog-Biting Mosquito
Richa Singh [...] Ximena E. Bernal
Jun 5, 2024 1425 Views
Abstract
Thermo-nociception, the detection and behavioral response to noxious temperatures, is a highly conserved action to avoid injury and ensure survival. Basic molecular mechanisms of thermal responses have been elucidated in several model organisms and are of clinical relevance as thermal hypersensitivity (thermos-allodynia) is common in neuropathic pain syndromes. Drosophila larvae show stereotyped escape behavior upon noxious heat stimulation, which can be easily quantified and coupled with molecular genetic approaches. It has been successfully used to elucidate key molecular components and circuits involved in thermo-nociceptive responses. We provide a detailed and updated protocol of this previously described method (Tracey et al., 2003) to apply a defined local heat stimulus to larvae using a fast-regulating hot probe.
Keywords: DrosophilaBackground
Drosophila larvae respond to thermal stimuli above 40 °C with an escape response (Tracey et al., 2003), likely to prevent cell damage and injury. The activation of nociceptive sensory neurons, class IV dendritic arborization (C4da) neurons, is necessary and sufficient for this response (Hwang et al., 2007). Applying a local thermo-nociceptive stimulus using a heated probe (> 40 °C, Figures 1, 5, 6) typically triggers a stereotyped behavior consisting of a 360° rolling motion along the larval body axis and increased speed of locomotion. Previous studies have shown that the transient receptor potential (Trp) channel painless (Tracey et al., 2003) and TrpA1 (Neely et al., 2011; Zhong et al., 2012) are the sensory channels responding to noxious heat in this system, as their expression and function in C4da neurons is required for nociceptive escape response.
Although mechano- and thermos-nociceptive stimulation of larvae result in very similar rolling escape responses (Hwang et al., 2007; Tracey et al., 2003; Zhong et al., 2012), the involved neuronal networks seem to differ. Despite the need to touch the animal with the heated probe, gentle touch-sensitive neurons (C2da and C3da) do not play a role in the behavioral response to noxious temperatures, but are essential for mechano-nociceptive responses (Hu et al., 2017). Activation of C4da neurons with noxious heat elicits in neuronal burst firing (Terada et al., 2016), which might be sufficient to elicit strong downstream network responses to induce escape behavior. Moreover, thermo-sensitive TrpA1 expressing neurons in the CNS respond to temperature gradients and are sufficient to induce rolling behavior (Luo et al., 2017). Thus thermo- and mechano-nociception might employ distinct subsets of the nociceptive network.
Using thermo-nociceptive assays together with genetic approaches in this system has led to the identification of several molecular components (Neely et al., 2010; Honjo et al., 2016), including pathways regulating thermal sensitivity (Babcock et al., 2011; Im et al., 2015). This makes the larval nociceptive system an attractive model to identify key components and circuit mechanisms regulating thermo-nociception.
Here, we provide a detailed protocol to conduct hot probe thermo-nociception assays as used in our recent work (Hu et al., 2017), which is based on the previously developed and described method by Tracey et al. (2003). We built a custom hot probe (Figure 1) with fast control of temperature required for consistent behavioral responses. Similar setups and protocols have also been employed in various other studies with comparable results (Neely et al., 2011; Chattopadhyay et al., 2012; Zhong et al., 2012). Our protocol and setup allow assaying behavioral responses to a locally applied hot stimulus depending on C4da and downstream neuron function (Figure 6).
Materials and Reagents
- Petri dishes 10 cm Ø (SARSTEDT, catalog number: 82.1473 )
- Fly stocks
Chromosome, Bloomington stock center No.:
w1118 (X, BL 6326)
w*; ppk-Gal4 (X, 3rd, BL 32079)
w*;UAS-TNTE (X, 3rd, BL 28997) - Agar Kobe I (Carl Roth, catalog number: 5210.4 )
- Agar plates (see Recipes)
- Fly food (see Recipes)
- Agar (strings) (Gewürzmühle Brecht, Eggenstein, Germany, catalog number: 00262 )
- Corn flour (Davert, Newstartcenter, catalog number: 17080 )
- Soy flour (Davert, Newstartcenter, catalog number: 46985 )
- Brewer’s yeast (ground) (Gewürzmühle Brecht, Eggenstein, Germany, catalog number: 03462 )
- Malt syrup (MeisterMarken – Ulmer Spatz, Bingen am Rhein, Germany, catalog number: 728985 )
- Treacle (molasses) (Grafschafter Krautfabrik, Meckenheim, catalog number: 01939 )
- Nipagin (Methyl 4-hydroxybenzoate) (Sigma-Aldrich, catalog number: 54752-1KG-F )
- Propionic acid (Carl Roth , catalog number: 6026.3 )
- Agar (strings) (Gewürzmühle Brecht, Eggenstein, Germany, catalog number: 00262 )
Equipment
- Brush (Size 1, Boesner, model: Da Vinci Nova Serie 1570 , catalog number: D15701)
- Forceps Dumont #3 (Fine Science Tools, catalog number: 11231-30 )
- Camera Basler ace acA2040-25gc (Basler, catalog number: 105716 )
- Stereoscope ZEISS Stemi-2000C (Pulch und Lorenz, catalog number: 455053-0000-000)
Manufacturer: ZEISS, model: Stemi-2000C . - External light souce LED Schott KL 1500 LCD (Pulch und Lorenz, catalog number: 150.200)
Manufacturer: SCHOTT, model: KL 1500 LCD . - Hot probe, custom made (see below, Figure 1):
- Thermometer Greisinger electronic GTH1170 (Conrad, catalog number: 100599-62 )
- Electrolube polyurethane (Electrolube, catalog number: UR5634RP250G )
- Electronic parts (see supplementary material)
In principle, a commercially available and precise temperature-controlled soldering iron as used in several other studies (Neely et al., 2011; Tracey et al., 2003; Zhong et al., 2012) should provide sufficient accuracy to perform this assay. Here, we designed a temperature control device to keep the temperature very precisely at a constant value of 46 °C (Figure 1). One problem is the high temperature loss when the probe dips into the water film on the agar plate, which results in lower and variable temperatures on the larva. To prevent this caveat, the device has to regulate the temperature very fast to keep the previously set values. One such design has been previously developed and employed (Babcock et al., 2009) by using a custom-built temperature controller.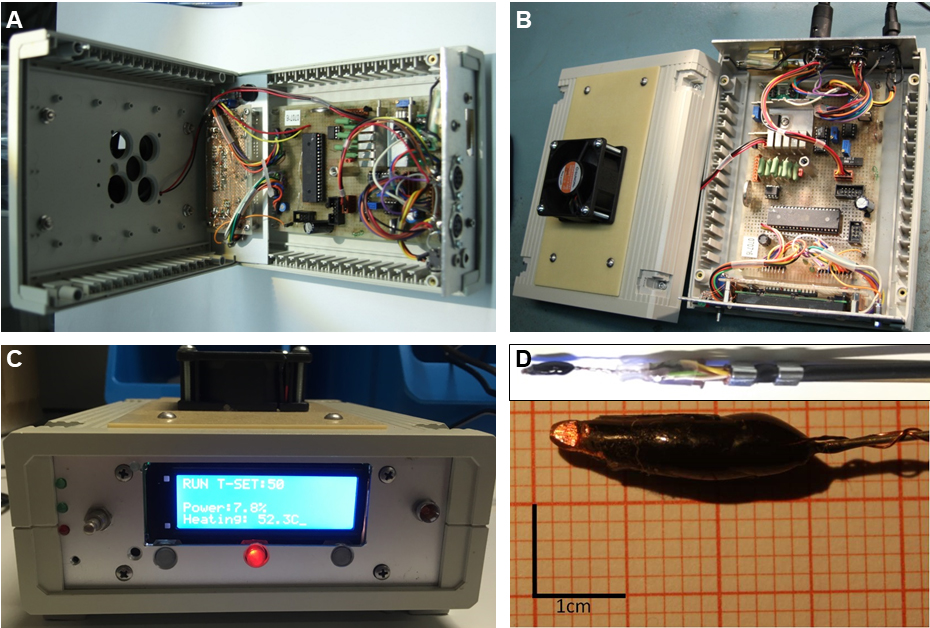
Figure 1. Hot probe setup and design. A-C. Custom built controller for setting the temperature with connected probe. D. The temperature-controlled hot probe consists of a diode (Z-diode or regular diode) with one wire shortened and shaped as a probe tip (facing left). The second wire is connected to the electronic controller and the diode is sealed and insulated with a polyurethane resin to prevent excessive temperature leakage. An external temperature sensor (thermocouple, e.g., Greisinger electronic GTH1170) can be optionally included by soldering the sensor to the probe for external temperature monitoring.
For our hot probe design, our in-house workshop employed a universal diode (1N4001) or alternatively, a Z-diode as a heating element. The diode is heated by a constant and precisely controlled direct current. The forward voltage is independent of the current (which is kept constant), but only depends on the temperature of the barrier layer of the diode. This allows using the occurring voltage for precise measurement and regulation of the barrier layer temperature.
One of the two conducting diode wires functions as the hot probe and should be shaped as follows: The protruding wire is shortened to 4-5 mm and 1.6 by 0.9 mm width, similarly to the previously described size and shape (Tracey et al., 2003). The tip of the hot probe should be spatula shaped and should not have any sharp edges. When the temperature of the tip is below 40 °C, touching the larva should not cause rolling behavior. It should also be as short as possible (4-5 mm) to prevent unnecessary heat loss and large temperature differences between the tip and the diode barrier layer, where the temperature is measured.
Voltage measurement and temperature regulation are achieved using an appropriate electronic system (built in-house and custom programmed) connected to the second diode wire. It displays the current temperature and adjusts the power at the diode tip to maintain the desired constant temperature. The precise circuit and block diagram, electronic parts and custom programming are provided as supplementary material.
Calibration of hot probe diode:
The insulated diode is placed in a 37 °C water reservoir (~1 L bottle with a magnetic stirrer, the temperature measured with a high precision reference thermometer). A very short impulse (2-10 msec only to prevent significant heating of the diode) is used to measure the voltage of the diode barrier layer at 37 °C. The same forward voltage measurement is performed at 55 °C. The forward voltage change between the two calibration points linearly correlates with the temperature and thus allows using the diode voltage for temperature measurement and control within this temperature range. - Thermometer Greisinger electronic GTH1170 (Conrad, catalog number: 100599-62 )
Software
- Video software
- Stream Pix6 (Norpix Inc., Montreal, Quebec, ordered from Rauscher GmbH, Germany, art. no. Streampix STP-6-S-STD)
- Windows VLC Media Player (www.videolan.org)
- Stream Pix6 (Norpix Inc., Montreal, Quebec, ordered from Rauscher GmbH, Germany, art. no. Streampix STP-6-S-STD)
Procedure
- All fly stocks were maintained at 25 °C and 70% humidity with a 12 h dark/light cycle on standard fly food. All experiments were performed using 3rd instar larvae at 96 h (hours) after egg laying (AEL). In order to ensure that all larvae were about the same age, the egg laying was restricted to 4-6 h. Experimental crosses were raised on standard fly food at 25 °C with 70% humidity and a 12 h light/12 h dark cycle.
- Prepare your genetic crosses 2 days before staging with approx. 20-30 virgins and 10-15 males each (more if you are using weak genotypes). After 2 days, transfer flies to a fresh food vial for timed egg-laying for 4-6 h at 25 °C (Figure 2A). Transfer the adult flies to a fresh vial (for another round of staging on the same or next day). The original vial is maintained at 25 °C until 96 ± 3 h AEL. All larvae should be in the third instar (L3) foraging stage and not yet leave the food (Figure 2B).
Note: Precise staging is important, as nociceptive responses of larvae are reduced after 120 h AEL, likely due to the transition to the wandering stage and preparation for pupariation. The density of larvae in the food will also affect staging: too few animals cannot efficiently process the food, while having too many larvae will result in competition for food, both of which is affecting developmental progression and will broaden the developmental stage of the larval population.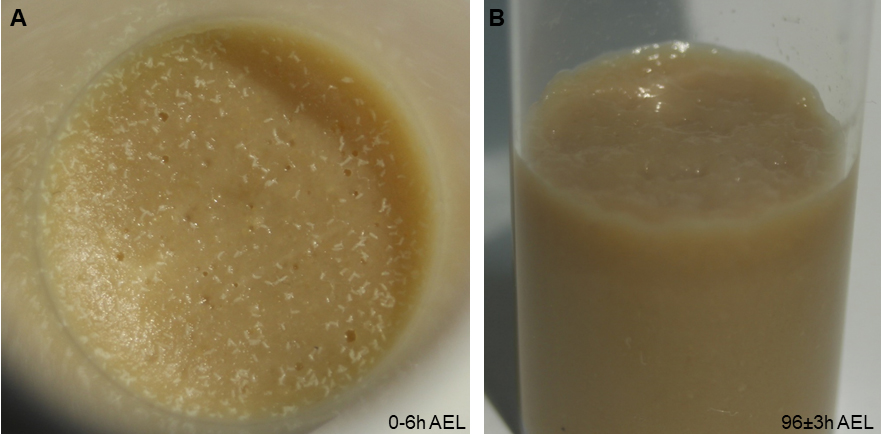
Figure 2. Correct staging and larval density. A. Photograph of appropriate embryo numbers for the used vial size (approx. 100-150 embryos laid within 4-6 h). B. At 96 h AEL, larvae should have processed the food well and still be in the foraging stage. - Prepare the hot probe by dialing at the desired temperature (generally 46 °C) and wait until it stabilizes (5 min).
- Distribute 250 µl of dH2O on a 2% agar plate to create a thin water film, which enables the animals to crawl freely and perform their rolling behavior (Figure 3C). Without water, the larvae are not fully mobile and do not display consistent behavioral responses.
Note: the water film evaporates over time which can affect the behavioral response. Replace the plate after every assay. The setup should be placed in a vibration free environment under the stereoscope, as vibration can affect nociceptive behavior.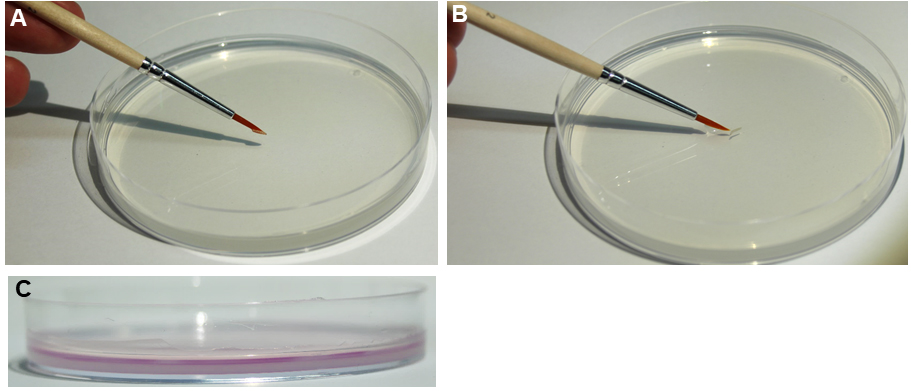
Figure 3. Larval transfer and water film on agar surface. A and B. The assay is prepared by gently placing 10-20 staged 3rd instar larvae on a 10 cm 2% agar plate using a brush. C. A thin water film of 0.25 ml (colored magenta for illustration) is necessary for consistent behavioral responses. - Prepare about 20 staged larvae by washing in dH2O to remove any residual food and place them gently on the agar plate using a brush (Figures 3A and 3B). Let the animals acclimatize for at least 1 min.
- Place the agar plate with the larvae under a stereoscope connected to a camera. Start videotaping using the mounted camera and a capturing software (e.g., Streampix) using avi-format (25 frames per second) so the reaction to the hot probe can be analyzed offline by video (Figures 4A and 4B).
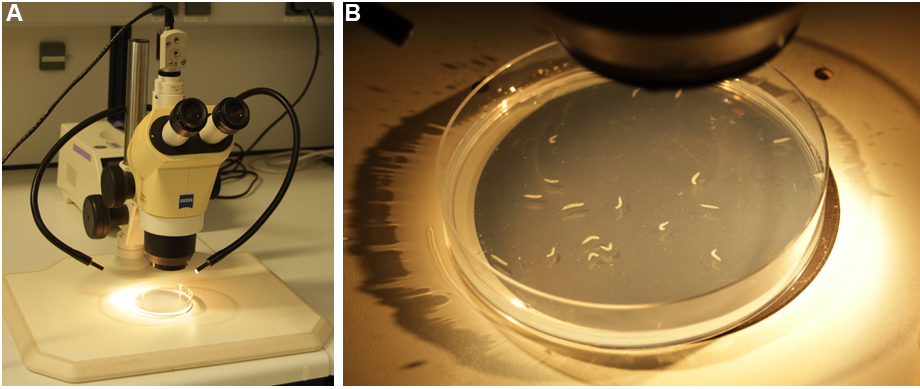
Figure 4. Thermo-nociception setup. A. The thermo-nociception assay requires a stereoscope, connected to a camera and white light illumination light source. B. Larvae are placed on a freshly prepared agar dish with water and tested for thermo-nociceptive responses using the hot probe. - Touch a forward locomoting larva with the 46 °C hot probe laterally on abdominal segment 4-6 at a 45° angle (Figures 5A and 5B). Typically, larvae react with a stereotyped rolling escape response within less than 10 sec. Keep the hot probe in direct contact with the larva for at least 10 sec or until it performs the rolling behavior.
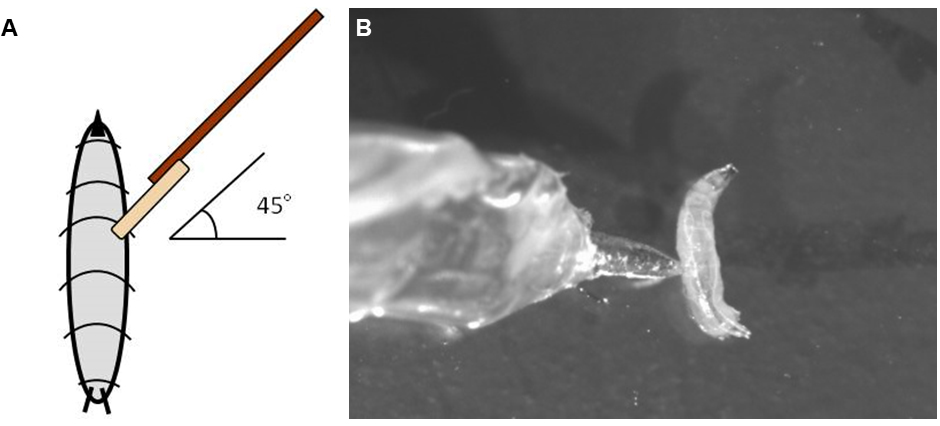
Figure 5. Procedure for hot probe positioning. A. Schematic depiction of how to apply the hot probe laterally on abdominal segment 4-6 at a 45° angle. B. Still frame of an actual video showing the position where the hot probe should touch the animal. - Remove the animal directly after testing to avoid double sampling.
- Stop videotaping and proceed with the next batch of animals or video analysis. For each experiment, sample a minimum of 60 larvae to get sufficient statistical power.
Data analysis
- Play the avi video using a media player (e.g., VLC media player (https://www.videolan.org/, free download). For each experimental animal, analyze the reaction to the hot probe by calculating the latency (in seconds) between the first contact of the hot probe and the beginning of the first 360° roll. You can slow down playback to ensure accuracy.
Note: All behavioral experiments should be double-blinded and randomized to avoid unwanted bias. The genotypes for the hot probe assay should be coded (e.g., numbers or letters) and the experimenter assigns the appropriate genotype code to each video. The videos are ideally analyzed by a second person unaware of both the genotype and code. - Plot the percentage of animals displaying a response latency between 1-9 sec and > 10 sec to visualize the distribution.
- Statistical analysis: the distribution of response latencies measured here typically does not satisfy the criteria for normality (i.e., Gaussian distribution of data points) as they are asymmetrically distributed. This generally precludes the use of parametric tests (e.g., Student’s t-test) which assume normality. For comparison of two groups, a non-parametric Wilcoxon Rank-Sum Test can be used instead. When comparing more than two genotypes, the Kruskal-Wallis Test (extension of Wilcoxon Rank-Sum Test) can be applied as a non-parametric substitute for one-way Analysis of Variance (ANOVA). Both tests require the number of animals per rank (in this case per second). When comparing more than two groups, corrections for multiple comparisons have to be considered. This should be done by performing an appropriate post-hoc test, typically Dunn’s method. The Kruskal-Wallis Test and Dunn’s post-hoc comparison can be performed with most commonly available statistics programs (e.g., Origin Pro 9.0, SigmaPlot, Prism, R or similar). The effect size and statistical power cannot be very easily computed in this case. However, based on the literature data and our own experience we recommend using n > 60 animals/genotype to ensure sufficient power to statistically assess biologically meaningful differences.
- Expected results (Figure 6)
Silencing C4da neurons using specific expression of Tetanus Toxin light chain (ppk-Gal4; UAS-TnT, Video 1) results in strongly increased latencies of the escape response compared to the control groups (ppkGal4 or UAS-TnT alone, Video 2).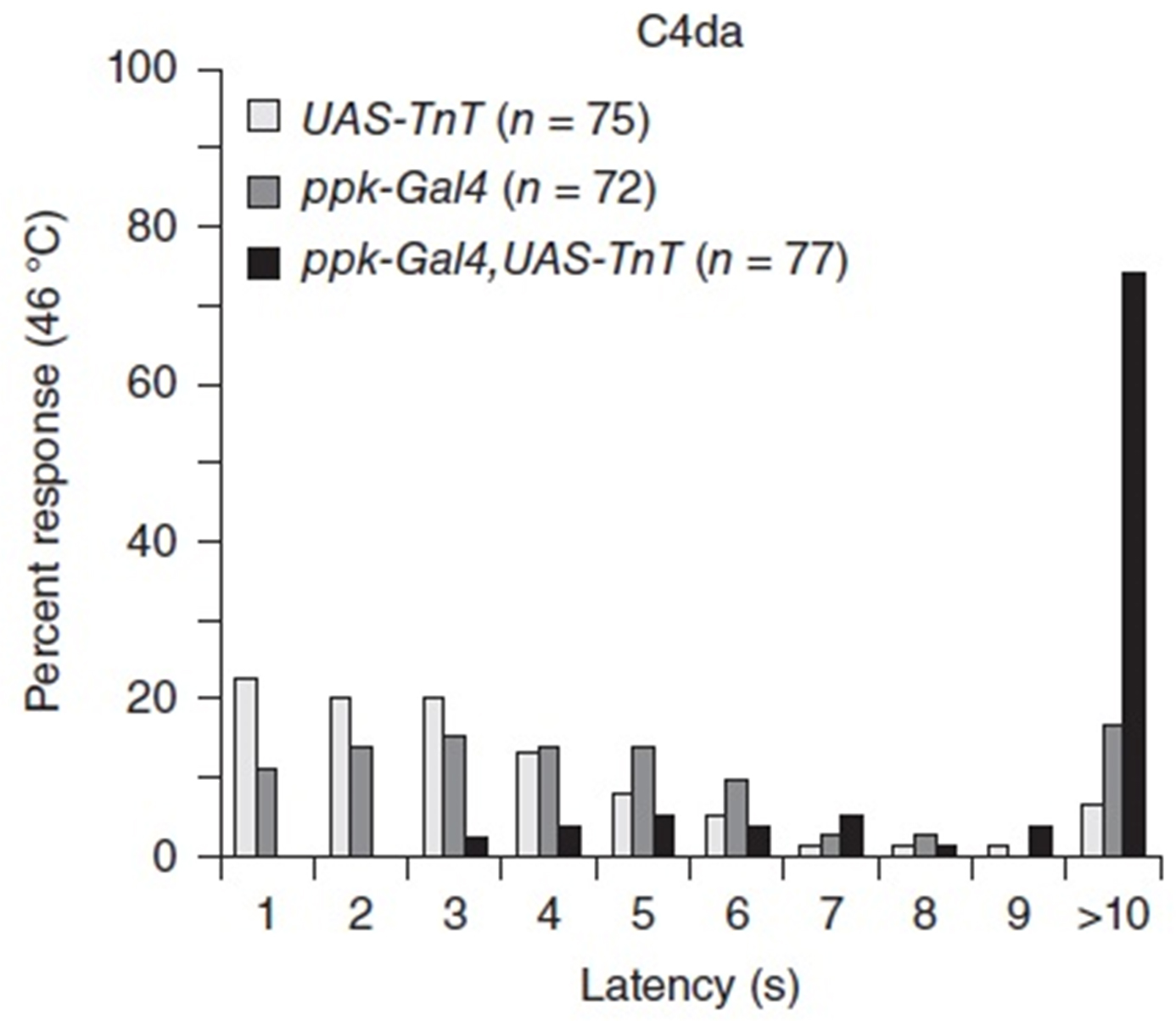
Figure 6. Typical experimental result of silencing C4da neuron function using Tetanus toxin light chain (TnT). Latencies of the rolling response are less than 10 sec for most control animals (ppk-Gal4 or UAS-TnT), while C4da neuron silencing (ppk-Gal4, UAS-TnT) results in over 70% non-responding larvae. Comparison of all three genotypes was performed using a Kruskal-Wallis Test with Dunn’s post-hoc comparison (P < 0.001 for ppk-Gal4, UAS-TnT compared to each control).Video 1. Hot probe assay with control animalsVideo 2. Hot probe assay with animals where C4da neurons were silenced (ppk-Gal4, UAS-TnT) - Block and circuit diagrams of custom-built temperature controller using a diode-based hot probe for temperature delivery and measurement, including custom programming (BASCOM) for the temperature controller (see supplementary material).
Notes
The animals will try to avoid contact and accelerate even before eventually showing a nociceptive rolling response. Take some practice to maintain contact of the hot probe with the body wall for 10 sec. Practice using a non-heated probe to ensure that no noxious mechanical stimulus is applied during the procedure. Then practice with control animals until the manual procedure is sufficiently reliable. Exclude animals from analysis where hot probe contact was not fully maintained until the first roll or up to 10 sec.
Recipes
- Agar plates
Note: For thermo-nociception assays, use 2% agar plates.
Dissolve Kobe agar I in dH2O and fill Petri dishes (Ø 10 cm) with a defined volume of 12 ml - Fly food
Use the following ingredients for 1 L of standard fly food: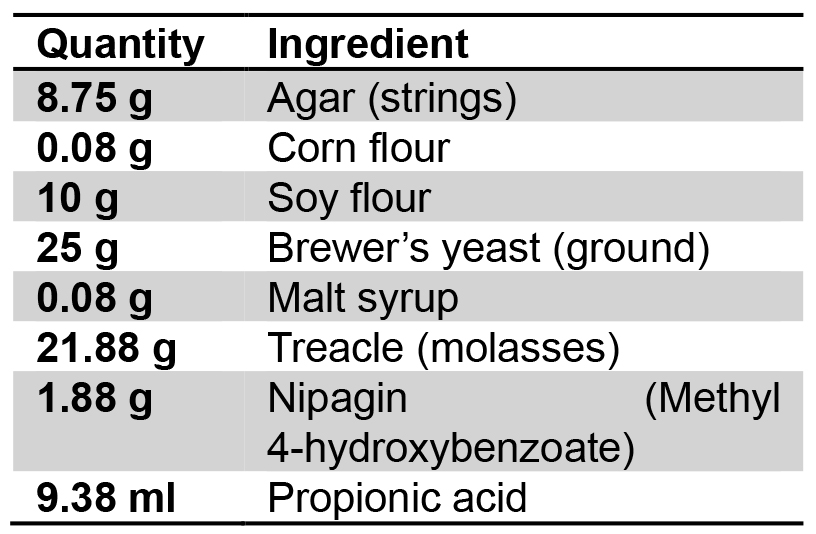
Dissolved in 1 L dH2O
Acknowledgments
This work was supported by the Landesförschungsförderung LFF-FV27 (to P.S.) and the Deutsche Forschungsgemeinschaft priority program SPP1926 (project SO1337/2-1 to P.S.). The authors would like to acknowledge the previous work and effort of several groups developing and applying thermo-nociceptive assays in Drosophila, in particular the labs of D. Tracey, M. Galko, C. Montell and G. Neely. The authors declare that no conflicts of interest or competing interests exist.
References
- Babcock, D. T., Landry, C. and Galko, M. J. (2009). Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol 19(10): 799-806.
- Babcock, D. T., Shi, S., Jo, J., Shaw, M., Gutstein, H. B. and Galko, M. J. (2011). Hedgehog signaling regulates nociceptive sensitization. Curr Biol 21(18): 1525-1533.
- Chattopadhyay, A., Gilstrap, A. V. and Galko, M. J. (2012). Local and global methods of assessing thermal nociception in Drosophila larvae. J Vis Exp (63): e3837.
- Honjo, K., Mauthner, S. E., Wang, Y., Skene, J. H. P. and Tracey, W. D., Jr. (2016). Nociceptor-enriched genes required for normal thermal nociception. Cell Rep 16(2): 295-303.
- Hu, C., Petersen, M., Hoyer, N., Spitzweck, B., Tenedini, F., Wang, D., Gruschka, A., Burchardt, L. S., Szpotowicz, E., Schweizer, M., Guntur, A. R., Yang, C. H. and Soba, P. (2017). Sensory integration and neuromodulatory feedback facilitate Drosophila mechanonociceptive behavior. Nat Neurosci 20(8): 1085-1095.
- Hwang, R. Y., Zhong, L., Xu, Y., Johnson, T., Zhang, F., Deisseroth, K. and Tracey, W. D. (2007). Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17(24): 2105-2116.
- Im, S. H., Takle, K., Jo, J., Babcock, D. T., Ma, Z., Xiang, Y. and Galko, M. J. (2015). Tachykinin acts upstream of autocrine Hedgehog signaling during nociceptive sensitization in Drosophila. Elife 4: e10735.
- Luo, J., Shen, W. L. and Montell, C. (2017). TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat Neurosci 20(1): 34-41.
- Neely, G. G., Hess, A., Costigan, M., Keene, A. C., Goulas, S., Langeslag, M., Griffin, R. S., Belfer, I., Dai, F., Smith, S. B., Diatchenko, L., Gupta, V., Xia, C. P., Amann, S., Kreitz, S., Heindl-Erdmann, C., Wolz, S., Ly, C. V., Arora, S., Sarangi, R., Dan, D., Novatchkova, M., Rosenzweig, M., Gibson, D. G., Truong, D., Schramek, D., Zoranovic, T., Cronin, S. J., Angjeli, B., Brune, K., Dietzl, G., Maixner, W., Meixner, A., Thomas, W., Pospisilik, J. A., Alenius, M., Kress, M., Subramaniam, S., Garrity, P. A., Bellen, H. J., Woolf, C. J. and Penninger, J. M. (2010). A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell 143(4): 628-638.
- Neely, G. G., Keene, A. C., Duchek, P., Chang, E. C., Wang, Q. P., Aksoy, Y. A., Rosenzweig, M., Costigan, M., Woolf, C. J., Garrity, P. A. and Penninger, J. M. (2011). TrpA1 regulates thermal nociception in Drosophila. PLoS One 6(8): e24343.
- Terada, S., Matsubara, D., Onodera, K., Matsuzaki, M., Uemura, T. and Usui, T. (2016). Neuronal processing of noxious thermal stimuli mediated by dendritic Ca2+ influx in Drosophila somatosensory neurons. Elife 5.
- Tracey, W. D., Jr., Wilson, R. I., Laurent, G. and Benzer, S. (2003). Painless, a Drosophila gene essential for nociception. Cell 113(2): 261-273.
- Zhong, L., Bellemer, A., Yan, H., Ken, H., Jessica, R., Hwang, R. Y., Pitt, G. S. and Tracey, W. D. (2012). Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep 1(1): 43-55.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Petersen, M., Tenedini, F. M., Hoyer, N., Kutschera, F. and Soba, P. (2018). Assaying Thermo-nociceptive Behavior in Drosophila Larvae. Bio-protocol 8(4): e2737. DOI: 10.21769/BioProtoc.2737.
Category
Neuroscience > Behavioral neuroscience > Sensorimotor response
Neuroscience > Sensory and motor systems > Animal model
Molecular Biology > DNA > Mutagenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











