- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Establishment of Mesenchymal Stem Cells from Wharton’s Jelly of Human Umbilical Cord
Published: Vol 8, Iss 4, Feb 20, 2018 DOI: 10.21769/BioProtoc.2735 Views: 19551
Reviewed by: Vasiliki KoliarakiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1538 Views

A Protocol to Induce Brown and Beige Adipocyte Differentiation From Murine and Human Adipose-Derived SVF
Rohit Raj Yadav [...] Narendra Verma
Dec 5, 2025 1648 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1466 Views
Abstract
Mesenchymal stem cells (MSCs) are currently considered as ‘medicinal signaling cells’ and a promising resource in regard to cell-based regenerative therapy. Umbilical cord is a human term perinatal tissue which is easily attainable, and a promising source of stem cells with no associated ethical concerns. MSCs have been isolated from different regions of the umbilical cord and Wharton’s jelly (WJ) is the gelatinous matrix that surrounds and provides protection to the umbilical cord blood vessels. Being more primitive, MSCs from human umbilical cord exhibit greater proliferative capacity and immunosuppressive ability as compared to adult stem cells which gives them a therapeutic advantage. To meet the requirements for cell therapy, it is important to generate MSCs at a clinical scale by following steps which are not time consuming or labor intensive. Here we present a simple, efficient protocol for isolation of MSCs from WJ of human umbilical cord by explant culture method which is reproducible and also, cost effective.
Keywords: Umbilical cordBackground
Mesenchymal stem cells (MSCs) have a remarkable clinical potential to treat a wide range of debilitating diseases, mainly due to their unique immunomodulatory role and regenerative capacity (Caplan and Sorrell, 2015). They exist in many tissues (Hass et al., 2011) and have been observed to be perivascular in vivo (Caplan and Correa, 2011). The niche of the source or the source itself, could lead to important functional differences between the various MSC types (Kwon et al., 2016). Though bone marrow is the most well studied and best characterized source of MSCs, there are certain limitations associated with it (Liu et al., 2016). An appealing and convenient alternative choice of MSC source is the fetus-derived umbilical cord, which is discarded after birth and provides an easily accessible and non-controversial source of stem cells for therapy (El Omar et al., 2014). Umbilical cord MSC-based trials are still at early phase, though no cell rejection, tumor formation or long-term adverse effects have been reported from them (Zhang et al., 2017). Moreover, they have been successfully used in experimental animal disease models. For convincingly establishing safety and therapeutic efficacy of umbilical cord-MSCs, followed by their use in clinical applications, a vast number of MSCs need to be generated for transplantations (Bartmann et al., 2007).
MSCs have been isolated from different compartments of the umbilical cord and Wharton’s jelly (WJ), is the connective tissue surrounding the umbilical cord vessels (Troyer and Weiss, 2008). Being a primitive stromal cell population, WJ-MSCs offer the advantage of faster proliferation rate and reduced immunogenicity as compared to adult tissue derived MSCs (Liu et al., 2016). Hence, successful isolation of robustly proliferating healthy MSCs from WJ of human umbilical cord, which retain all the basic MSC properties, assumes importance. Here we describe step-by-step an explant method of isolation procedure followed by establishment of MSC culture from WJ of human umbilical cord. This method of isolation eliminates the use of any enzymatic treatment making it more economical, and getting rid of batch-to-batch variations and endotoxin contaminations likely to be associated with enzymatic preparations. The isolated WJ-MSCs expressed MSC-characteristic surface antigens and were positive for the expression of CD73, CD90 and CD105 and negative for CD34.
Materials and Reagents
- 15 and 50 ml centrifuge tubes (Corning, catalog numbers: 430791 and 430829 respectively)
- 1.5 ml microcentrifuge tube (Corning, Axygen®, catalog number: MCT-150-C )
- 90 mm Petri dish sterile (Tarsons, catalog number: 460090 )
- 35 mm cell culture dish (Corning, Falcon®, catalog number: 353001 )
- 1 ml blunt-end pipette tips
- Corning® 1.2 ml External Threaded Polypropylene Cryogenic Vial, Self-Standing with Conical Bottom (Corning, catalog number: 430658 )
- FACS tubes (Corning, Falcon®, catalog number: 352054 )
- Millex-GP Syringe Filter Unit, 0.22 µm, polyether sulfone, 33 mm, gamma sterilized (Merck, catalog number: SLGP033RS )
- 20 ml and 5 ml syringes*
- Sterile Scalpel blade No. 20 (HiMedia Laboratories, catalog number: LA771 )
- 0.5-10 µl microtips (Corning, Axygen®, catalog number: T-300 )
- 20-200 µl pipette tips (Corning, Axygen®, catalog number: T-200-C )
- 100-1000 µl pipette tips (Corning, Axygen®, catalog number: T-1000-C )
- Biohazards disposable waste bags*
- Plastic beaker*
- 0.9% w/v saline*
- Antibiotic-Antimycotic (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15240096 )
- Isopropanol*
- Autoclaved distilled water
- Absolute ethanol (Merck, catalog number: 1.00983.0511 )
- Sodium hypochlorite solution (Merck, catalog number: 1.93607.1021 )
- Dulbecco’s phosphate-buffered saline (DPBS), No calcium, no magnesium (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- TrypLETM Express Enzyme (1x), no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 12604013 )
- Trypan blue solution 0.4% (Sigma-Aldrich, catalog number: T8154 )
- Flow cytometry antibodies (Table 1)
Table 1. Flow cytometry antibodiesS. No. Antigen (CD marker) Antibody Manufacturer Dilution 1. CD73 Anti-Human CD73 PE BD, BD PharmingenTM,
catalog number: 5502571.5 µl in 50 µl of cell suspension 2. CD90 Anti-Human CD90 PE BD, BD PharmingenTM,
catalog number: 5555961.5 µl in 50 µl of cell suspension 3. CD105 Anti-Human CD105-PE R&D Systems,
catalog number: FAB10971P1 µl in 50 µl of cell suspension 4. CD34 Anti-Human CD34 BD, BD PharmingenTM,
catalog number: 5507611 µl in 50 µl of cell suspension S. No. Isotype antibody Manufacturer Dilution 1. IgG1, kappa BD, BD PharmingenTM,
catalog number: 5506171µl of 1:16 dilution in 50 µl of cell suspension (for CD73 and CD34) 1.5 µl in 50 µl cell suspension for CD90 2. IgG1 R&D Systems,
catalog number: IC002P1µl of 1:16 dilution in 50 µl cell suspension - KnockOutTM D-MEM high glucose no glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 10829018 )
Note: DMEM-F12 or DMEM, high glucose could also be used in place of KnockOutTM D-MEM (Nekanti et al., 2010). - L-Glutamine (200 mM) (Thermo Fisher Scientific, GibcoTM, catalog number: 25030149 )
- Fetal bovine serum (FBS) mesenchymal stem cell qualified (Thermo Fisher Scientific, GibcoTM, catalog number: 12662029 )
Note: The source, grade and lot number of FBS play a critical role in MSC culture establishment. - Penicillin-Streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140148 )
- Phosphate buffered saline tablets (Sigma-Aldrich, catalog number: P4417 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog numbers: D2650 )
- Sheath fluid ( BD Biosciences, BD FACS FlowTM, catalog number: 342003 )
- MSC isolation media (see Recipes)
- MSC growth media (see Recipes)
- 1x sterile phosphate buffer saline, pH 7.4 (see Recipes)
- MSC freezing mixture (see Recipes)
*Note: This item can be ordered from any standard company.
Equipment
- Laminar flow hood* (EuroClone, model: S@feflowTM 0.9 , catalog number: LD80000)
- Surgical tools including scalpel holder, forceps (large and small), pointed scissors
- Incubator (BINDER, model: C 150 CO2 Incubator, catalog number: 9040-0078 )
- Centrifuge 5810 R (Eppendorf, model: 5810 R , catalog number: 5811000320)
- Haemocytometer depth 0.1 mm* (Rohem Instruments Private limited, http://www.sciencecorner.co.in/Rohem.php)
- Mr. FrostyTM Freezing Container (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 5100-0001 )
- -20 °C freezer* (Celfrost, model: BSF 150 )
- -86 °C Upright Ultra-Low Temperature Freezer (Revco value, Thermo Fisher Scientific, Thermo ScientificTM, model: ULT-1386-3 )
- Liquid nitrogen tank (Thermo Fisher Scientific, Thermo ScientificTM, model: Model 8031 )
- Flow cytometer (BD Biosciences, BD FACSCaliburTM)
- Inverted microscope* (Nikon Instruments, model: Eclipse TS100 )
- Refrigerator* (SAMSUNG, model: RT31 , 2007)
- 2-20 μl single channel variable pipette* (Eppendorf, model: Research® plus , catalog number: 3120000038)
- 20-200 μl single channel variable pipette* (Eppendorf, model: Research® plus , catalog number: 3120000054)
- 100-1,000 μl single channel variable pipette* (Eppendorf, model: Research® plus , catalog number: 3120000062)
- Autoclave*(Tuttnauer, model: 3850 ML )
*Note: This item can be ordered from any standard company.
Software
- BD Cell Quest pro
Procedure
- Isolation of WJ-MSCs
To isolate mesenchymal stem cells from Wharton’s jelly of human umbilical cord, use the explants culture method.- Collect fresh human umbilical cord after full-term birth (normal or cesarean) with informed consent (Figure 1) and transport it to the lab in an empty sterile 50 ml centrifuge tube on ice.
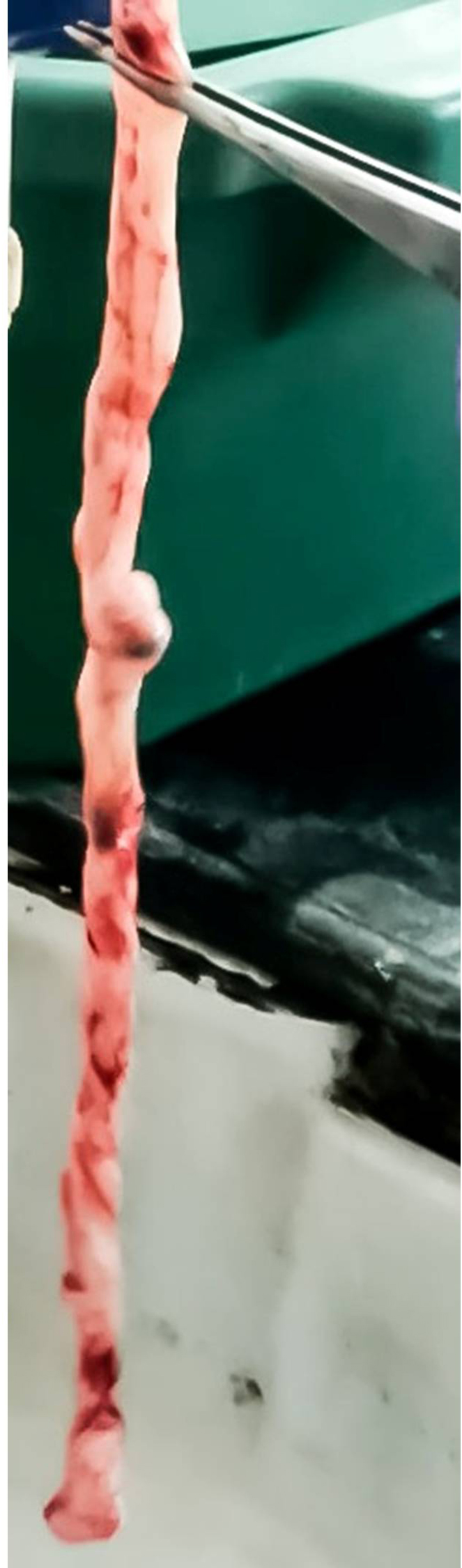
Figure 1. Fresh umbilical cord after collection - Rinse off the blood and blood clots using normal saline. Cut the cord into 2-3 cm pieces (Figure 2) and incubate the pieces in normal saline containing 1x antibiotic-antimycotic (100 U/ml of penicillin, 100 μg/ml of streptomycin and 250 ng/ml of amphotericin B) at 4 °C for about 2 h.
Note: This incubation step in saline containing antibiotic-antimycotic is carried out mainly to disinfect the umbilical cord tissue.
Figure 2. The umbilical cord cut into 2-3 cm long pieces - After 2 h, inside the laminar flow tissue culture hood, rinse the cord pieces three times with sterile PBS (Video 1).Video 1. Rinsing umbilical cord pieces with 70% ethanol and PBS
- Next disinfect the cord pieces briefly by rinsing with 70% ethanol for 30 sec. Wash thoroughly three more times with PBS to remove any traces of ethanol (Video 1).
- Transfer one cord piece into an empty 10 cm cell culture dish, while the tube containing the other pieces can be stored on ice. Using forceps try to straighten the twists, if any, in the cord piece and using a scalpel fixed to a scalpel holder make a longitudinal slit along the length (Video 2).Video 2. Removal of artery from umbilical cord piece
- There are two arteries and one vein inside the cord. Cut open flaps of tissue to expose an artery and then trace the artery using scissors and forceps and remove it. The underlying perivascular Wharton’s jelly tissue can now be neatly excised (Video 2).
- Chop the excised tissue into smaller 3-5 mm pieces with the help of the scalpel and place in an empty 35 mm tissue culture dish using a fine forceps (Video 3).Video 3. Plating small explants of WJ portion of human umbilical cord
- Allow the cord pieces to attach for about 5 min with air drying. Next add 2 ml of warm fresh MSC isolation medium carefully, drop-wise and gently, taking care not to dislodge the tissue pieces (Video 3).
- Place about 10-15 explants in each 35 mm tissue culture dish and use about four to five 35 mm tissue culture dishes per umbilical cord sample.
- Place the tissue culture dishes containing the cord explants in a CO2 incubator maintaining 37 °C and 5% CO2.
Note: Discard the remaining tissue pieces in a biohazard waste bag. All the liquid waste should be collected in a beaker containing sodium hypochlorite solution and discarded appropriately. - After 48 h, give the first medium change with 2 ml of warm fresh MSC isolation medium. Following this, media changes are given with 2 ml of fresh MSC isolation medium every 72 h.
- After about 7-10 days, once enough cells have come out from the explants (Figure 3), remove the cord pieces using 1 ml blunt-end tips and add 2 ml of warm fresh MSC isolation medium.

Figure 3. Phase contrast image of MSCs emerging from an umbilical cord tissue piece explant
- Collect fresh human umbilical cord after full-term birth (normal or cesarean) with informed consent (Figure 1) and transport it to the lab in an empty sterile 50 ml centrifuge tube on ice.
- Establishment of MSC culture
- After 24-48 h of cord piece removal and depending on confluency, carefully aspirate medium and wash cells twice with DPBS.
Note: As the cells are growing out in patches from the explants at this stage, within each patch cells should be ~50-80% confluent. - Add 250 µl of TrypLE to each 35 mm tissue culture dish and let the cells detach for 4-6 min at 37 °C.
Note: Trypsin can be used as a substitute for TrypLE. TrypLE is an animal origin-free recombinant enzyme which is gentle on stem cells, thus preserving well cell’s surface proteins. - Add 1 ml of warm fresh MSC growth medium to the cells and transfer them to a 15 ml centrifuge tube and centrifuge at room temperature for 2 min at 500 x g.
- Aspirate medium, add 500 µl of warm fresh MSC growth medium to the cell pellet and suspend the pellet. Mix a small aliquot of cell suspension with an equal volume of trypan blue, and count the live (non-blue) cells using a hemocytometer.
- As a next step, seed WJ-MSCs at a density of 5,000 cells/cm2 in 2 ml of warm fresh MSC growth medium in a 35 mm tissue culture dish or any appropriate cell culture vessel.
- Culture them for 72 h or till cells are 70-80% confluent. No medium change required in between.
- Detach cells with TrypLE, count and seed for the next passage, repeating Steps B2-B6.
- After 24-48 h of cord piece removal and depending on confluency, carefully aspirate medium and wash cells twice with DPBS.
- Freezing and reviving of WJ-MSCs
- Once the WJ-MSCs are 70-80% confluent, detach with 250 µl TrypLE as per Step B2.
- Add 1 ml of warm fresh MSC growth medium to the cells and transfer them to a 15 ml centrifuge tube. Take an aliquot for counting and centrifuge the remaining cells at 500 x g for 2 min at room temperature.
- Aspirate supernatant and suspend the cells directly in freezing medium at ~2 x 106 cells/ml.
- Aliquot 150-200 µl cell suspension into cryogenic vials and transfer the cryogenic vials to a Mr. FrostyTM freezing container containing isopropanol.
- Place the Mr. FrostyTM freezing container at -80 °C freezer for ~24 h before transferring the cryogenic vials to a liquid nitrogen tank for long-term storage.
- For revival, remove a cryogenic vial with frozen WJ-MSCs from the liquid nitrogen tank and immediately transfer it to a beaker containing water at 37 °C.
- Once the cell suspension has thawed, inside the laminar flow hood add 1 ml of warm fresh MSC growth medium to the cell suspension in the cryogenic vial and transfer the contents to a 15 ml centrifuge tube containing 3-4 ml of additional warm fresh MSC growth medium.
- Centrifuge the cells at room temperature at 500 x g for 2-3 min to wash off the DMSO.
- Aspirate the supernatant and resuspend the cell pellet in 0.5 ml of fresh warm MSC growth medium. Mix well by pipetting, count the live cells and plate up to 2-3 x 105 cells on a 35 mm tissue culture plate in 2 ml of warm fresh MSC growth medium.
Note: During thawing and revival of MSCs, cells are plated at a higher density in order to maximize recovery. - Transfer the dish to a CO2 incubator and replace medium with 2 ml of warm fresh MSC growth medium the next day.
- After 48 h of reviving and plating, detach cells with TrypLE and seed for the next passage.
- Once the WJ-MSCs are 70-80% confluent, detach with 250 µl TrypLE as per Step B2.
- Immunophenotyping by flow cytometry
- Take WJ-MSCs at passage 4-5. Detach them with TrypLE and count the cell number as per Steps B2-B4.
- Wash the harvested cells 1-2 times with ice cold PBS by centrifuging at 600 x g for 2 min.
- Discard the supernatant and suspend cells in PBS at 2 x 106 cells/ml.
- Distribute 1 x 105 cells or 50 µl of cell suspension in each of the pre-labeled 5 ml FACS tubes.
- Add the PE or FITC labeled antibodies as per Table 1 and incubate on ice for 45-60 min. Mouse isotype antibodies can serve as controls (Figure 4).
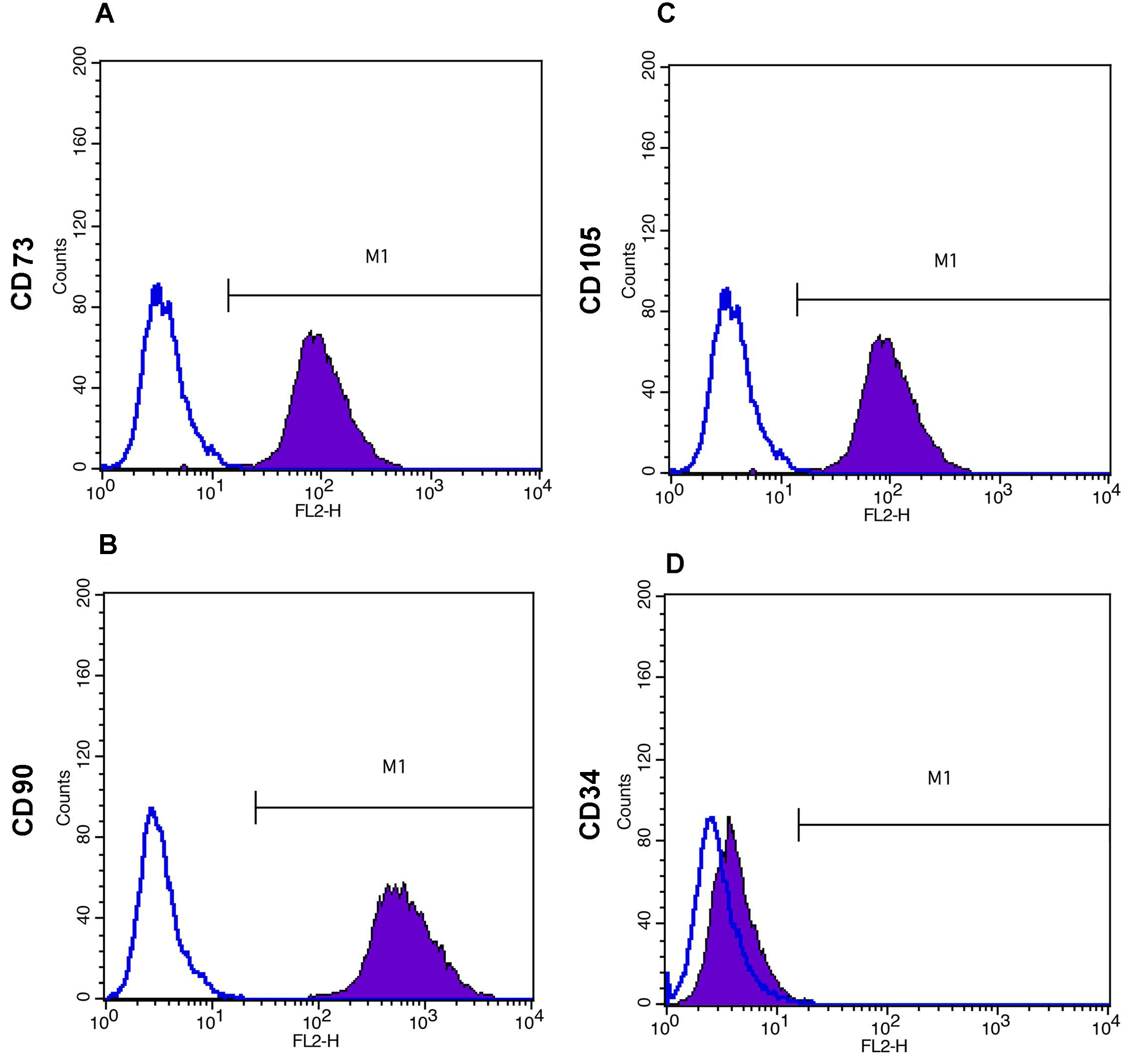
Figure 4. Immunophenotyping of WJ-MSCs as demonstrated by flow cytometry analysis. Open histograms represent background signal while shaded histograms indicate positive reactivity with the indicated antibodies. WJ-MSCs are positive for CD73 (A), CD90 (B) and CD 105 (C), and negative for CD34 (D).
- Take WJ-MSCs at passage 4-5. Detach them with TrypLE and count the cell number as per Steps B2-B4.
Data analysis
WJ-MSCs, isolated from human umbilical cord, are analysed for surface marker expression profile at passage 4-6. At least 10,000 events are acquired on BD FACSCalibur flow cytometer and results are analyzed using BD Cell Quest Pro software. Cultured MSCs are known to be strongly positive for the expression of CD73, CD90 and CD105 and negative for the expression of CD34, CD45, HLADR etc. All analyses are standardized against control cells incubated with PE-conjugated mouse IgG isotype antibody. Representative data from one WJ-MSC culture at passage 5 is presented in this protocol. However, similar results have been obtained from multiple independent WJ-MSC cultures (Himal et al., 2017).
Notes
- For MSC isolations, cords are usually collected from healthy donors.
- After collection, umbilical cords should be transported to the laboratory and processed as soon as possible, preferably within one hour.
- Depending on cord sample, WJ-MSCs might take 6-14 days to come out from the cord pieces.
- Pre-warm medium to 37 °C and add only the warm medium to the cells.
- All plasticwares like microcentrifuge tubes and pipet tips, and surgical instruments were sterilized by autoclaving before use in cell culture.
- Cell concentration can be calculated using a hemocytometer as, the total number of live (non-blue) cells in the four corner squares/4 x dilution factor x 10,000 = No. cells/ml.
Recipes
- MSC isolation medium
DMEM KO
2 mM glutamine
1x antibiotic-antimycotic
10% MSC FBS
Sterilize by filtration through a 0.22 μm syringe filter
Store at 4 °C, use within 2 weeks - MSC growth medium
DMEM KO
2 mM glutamine
1x penicillin-streptomycin
10% FBS
Sterilize by filtration through a 0.22 μm syringe filter
Store at 4 °C, use within 2 weeks - 1x sterile phosphate buffered saline (PBS), pH 7.4
Dissolve two tablets of PBS in 400 ml of distilled water
Sterilize by autoclaving
Store at 4 °C. Use within 1 month - MSC freezing mixture
90% FBS
10% DMSO
Sterilize by filtration through a 0.22 μm syringe filter
Store at 4 °C. Use within 2 weeks
Acknowledgments
This work has been financially supported by IISER-Kolkata and SERB, DST, India. We thank CSIR, India for the fellowship of Mr. Umesh Goyal. We thank Mr. Pritam Saha and Mr. Tamal Ghosh for their assistance with cell culture facility maintenance and flow cytometry data analysis, respectively. And we are thankful to Dr. Jayanta Chatterjee, Aastha, Kalyani, for generously providing us with human umbilical cord samples. This protocol has been adapted from our previous protocol (Venugopal et al., 2011). The authors declare no potential conflicts of interest.
References
- Bartmann, C., Rohde, E., Schallmoser, K., Purstner, P., Lanzer, G., Linkesch, W. and Strunk, D. (2007). Two steps to functional mesenchymal stromal cells for clinical application. Transfusion 47(8): 1426-1435.
- Caplan, A. I. and Correa, D. (2011). The MSC: an injury drugstore. Cell Stem Cell 9(1): 11-15.
- Caplan, A. I. and Sorrell, J. M. (2015). The MSC curtain that stops the immune system. Immunol Lett 168(2): 136-139.
- El Omar, R., Beroud, J., Stoltz, J. F., Menu, P., Velot, E. and Decot, V. (2014). Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev 20(5): 523-544.
- Hass, R., Kasper, C., Bohm, S. and Jacobs, R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9: 12.
- Himal I., Goyal, U. and Ta, M. (2017). Evaluating wharton’s jelly-derived mesenchymal stem cell’s survival, migration, and expression of wound repair markers under conditions of ischemia-like stress. Stem Cells Int 2017: 5259849.
- Kwon, A., Kim, Y., Kim, M., Kim, J., Choi, H., Jekarl, D. W., Lee, S., Kim, J. M., Shin, J. C. and Park, I. Y. (2016). Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep 6: 23544.
- Liu, C. B., Huang, H., Sun, P., Ma, S. Z., Liu, A. H., Xue, J., Fu, J. H., Liang, Y. Q., Liu, B., Wu, D. Y., Lu, S. H. and Zhang, X. Z. (2016). Human umbilical cord-derived mesenchymal stromal cells improve left ventricular function, perfusion, and remodeling in a porcine model of chronic myocardial ischemia. Stem Cells Transl Med 5(8): 1004-1013.
- Nekanti, U., Rao, V. B., Bahirvani, A. G., Jan, M., Totey, S. and Ta, M. (2010). Long-term expansion and pluripotent marker array analysis of Wharton's jelly-derived mesenchymal stem cells. Stem Cells Dev 19(1): 117-130.
- Troyer, D. L. and Weiss, M. L. (2008). Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3): 591-599.
- Venugopal, P., Balasubramanian, S., Majumdar, A. S. and Ta, M. (2011). Isolation, characterization, and gene expression analysis of Wharton's jelly-derived mesenchymal stem cells under xeno-free culture conditions. Stem Cells Cloning 4: 39-50.
- Zhang, Y. C., Liu, W., Fu, B. S., Wang, G. Y., Li, H. B., Yi, H. M., Jiang, N., Wang, G., Zhang, J., Yi, S. H., Li, H., Zhang, Q., Yang, Y. and Chen, G. H. (2017). Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy 19(2): 194-199.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Goyal, U., Jaiswal, C. and TA, M. (2018). Isolation and Establishment of Mesenchymal Stem Cells from Wharton’s Jelly of Human Umbilical Cord. Bio-protocol 8(4): e2735. DOI: 10.21769/BioProtoc.2735.
Category
Stem Cell > Adult stem cell > Mesenchymal stem cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











