- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Detection of Intracellular Reduced (Catalytically Active) SHP-1 and Analyses of Catalytically Inactive SHP-1 after Oxidation by Pervanadate or H2O2
Published: Vol 8, Iss 1, Jan 5, 2018 DOI: 10.21769/BioProtoc.2684 Views: 7049
Reviewed by: Ivan ZanoniGuadalupe Ortiz-MuñozHenrique Borges da Silva

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2277 Views

Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
Ilyssa E. Ramos [...] James M. Cherry
Nov 20, 2025 2336 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 482 Views
Abstract
Oxidative inactivation of cysteine-dependent Protein Tyrosine Phosphatases (PTPs) by cellular reactive oxygen species (ROS) plays a critical role in regulating signal transduction in multiple cell types. The phosphatase activity of most PTPs depends upon a ‘signature’ cysteine residue within the catalytic domain that is maintained in the de-protonated state at physiological pH rendering it susceptible to ROS-mediated oxidation. Direct and indirect techniques for detection of PTP oxidation have been developed (Karisch and Neel, 2013). To detect catalytically active PTPs, cell lysates are treated with iodoacetyl-polyethylene glycol-biotin (IAP-biotin), which irreversibly binds to reduced (S-) cysteine thiols. Irreversible oxidation of SHP-1 after treatment of cells with pervanadate or H2O2 is detected with antibodies specific for the sulfonic acid (SO3H) form of the conserved active site cysteine of PTPs. In this protocol, we describe a method for the detection of the reduced (S-; active) or irreversibly oxidized (SO3H; inactive) form of the hematopoietic PTP SHP-1 in thymocytes, although this method is applicable to any cysteine-dependent PTP in any cell type.
Keywords: Reactive oxygen speciesBackground
Reactive oxygen species (ROS) are generated by cellular NADPH oxidases and mitochondria. Most Protein Tyrosine Phosphatases (PTPs) contain a conserved catalytic cysteine with a low dissociation constant (pKa) that is highly susceptible to oxidation by ROS (Rudyk and Eaton, 2014). ROS-inactivation of PTPs plays an important role in regulating tyrosine-kinase-mediated signaling responses in numerous cell types. PTPs are rapidly oxidized in cells treated with the ROS H2O2 or the PTP inhibitor pervanadate (Huyer et al., 1997; Choi et al., 2017). Iodoacetyl-polyethylene glycol-biotin (IAP-biotin) selectively and irreversibly reacts with de-protonated (S-) cysteine thiols (Rudyk and Eaton, 2014). To label basal reduced (active) cellular PTPs, IAP-biotin is added at the time of cell lysis.
PTP active site cysteines can be oxidized by ROS to the sulfenic acid form (-SOH), which can then be converted into either sulfenylamide (-SN-) or cysteine disulfide (S-S) forms. Sulfenic acid, sulfenylamide and disulfide oxidized PTPs can be ‘re-activated’ by treatment with thiol reducing agents such as dithiothreitol (DTT) which convert the catalytic cysteine to the active (SH) state. PTP oxidation can be detected indirectly by a three-step method (irreversible alkylation of reduced active site cysteines with an alkylating agent followed by reduction of reversibly oxidized (SOH) active site cysteines with a reducing agent, then finally labeling of the newly formed cysteine thiols) (Weibrecht et al., 2007). This method for direct detection of reversibly oxidized PTPs is not widely used due to the fact that the sulfenic acid state is labile and transient (Karisch and Neel, 2013). PTP catalytic cysteines can also be progressively irreversibly ‘hyper-oxidized’ to the sulfinic (-SO2H) followed by the sulfonic (-SO3H) forms by higher concentrations of ROS or prolonged exposure to ROS. The availability of monoclonal antibodies specific for the sulfonic acid (-SO3H) form of the conserved active site of PTPs has provided a straightforward way to detect oxidized PTPs following treatment of cells with oxidizing agents (Persson et al., 2004). The susceptibility of PTPs in different cell populations to oxidation can be assessed by treatment of cells with oxidizing agents (varying the concentration of oxidizing agent and/or treatment time) followed by blotting of proteins from cell lysates after separation by SDS-PAGE with anti-oxidized-PTP mAb. Here, we describe methods used to detect either reduced (S-; active) or irreversibly oxidized (SO3H; inactive) SHP-1 in thymocytes.
Materials and Reagents
- Consumables
- Push pins or syringe needles
- Pipette tips (Neptune Scientific, catalog numbers: BT10F , BT100 , BT1250 )
- 14 ml conical tubes (Corning, Falcon®, catalog number: 352059 )
- 1.6 ml microfuge tubes (Neptune Scientific, catalog number: 4445.S.X )
- 3MM paper (GE Healthcare, catalog number: 3030-861 )
- 6-well plate (Nest Biotechnology, catalog number: 703001 )
- Cell strainer, 70 μm (Southern Labware, catalog number: C4070 )
- PVDF membrane (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 88518 )
- Western blotting film (Next Day Science, catalog number: A8803 )
- Nitrocellulose membrane (GE Healthcare, catalog number: 10600002 )
- Push pins or syringe needles
- Biological reagents
- Female 6-8 week old C57BL/6 mouse (Taconic Biosciences, catalog number: B6NTac )
- Female 6-8 week old C57BL/6 mouse (Taconic Biosciences, catalog number: B6NTac )
- Chemical reagents
- Trypan blue solution, 0.4% (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- Serum-free medium (Corning, Mediatech, catalog number: 40-101-CV )
- Methanol (Sigma-Aldrich, catalog number: 32213 )
- 1 M HEPES pH 7.0 (Sigma-Aldrich, catalog number: H0887 )
- 1 M Tris-HCl pH 7.5 (Quality Biological, catalog number: 351-006-101 )
- 5 M NaCl (Quality Biological, catalog number: 351-036-101 )
- 10% SDS (Quality Biological, catalog number: 351-032-101 )
- Sodium deoxycholate (Sigma-Aldrich, catalog number: D6750 )
- Nonidet P40 (Sigma-Aldrich, Roche Diagnostics, catalog number: 11754599001 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Sodium fluoride (NaF) (Sigma-Aldrich, catalog number: S7920 )
- Phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626 )
- Iodoacetyl PEG-biotin (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 21334 )
- Diethylenetriaminepentaacetic acid (DTPA) (Sigma-Aldrich, catalog number: D6518 )
- Catalase (Sigma-Aldrich, catalog number: C30 )
- Protease inhibitor tablets (Roche Diagnostics, catalog number: 5056489001 )
- Anti-Oxidized PTP active site mAb (R&D Systems, catalog number: MAB2844 )
- Anti-SHP-1 antibody (Santa Cruz Biotechnology, catalog number: sc-287 )
- Goat anti-mouse IgG HRP (Santa Cruz Biotechnology, catalog number: sc-2302 )
- Mouse anti-rabbit IgG HRP (Santa Cruz Biotechnology, catalog number: sc-2357 )
- GammaBind G Sepharose (GE Healthcare, catalog number: 17088501 )
- SDS protein gel loading solution (Quality Biological, catalog number: 351-082-661 )
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M3148 )
- Tris-glycine transfer buffer (25x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: LC3675 )
- Tris-glycine SDS running buffer (10x) (Thermo Fisher Scientific, InvitrogenTM, catalog number: LC2675 )
- NovexTM Wedge well 10% Tris-glycine gel (Thermo Fisher Scientific, InvitrogenTM, catalog number: XP00100 )
- Non-fat dry milk (Santa Cruz Biotechnology, catalog number: sc-2325 )
- Streptavidin-HRP (GE Healthcare, catalog number: RPN1231-2ML )
- SuperSignalTM West Pico chemiluminescent substrate (Thermo Fisher Scientific, catalog number: 34080 )
- Tween 20 (Sigma-Aldrich, catalog number: P7949 )
- EGTA (Sigma-Aldrich, catalog number: 03777 )
- β-Glycerophosphate (Sigma-Aldrich, catalog number: G6501 )
- N-Ethylmaleimide (Sigma-Aldrich, catalog number: E3876 )
- H2O2 (3%) (Sigma-Aldrich, catalog number: 88597 )
- Sodium orthovanadate (Na3VO4) (Sigma-Aldrich, catalog number: 450243 )
- Iodoacetamide (Sigma-Aldrich, catalog number: I1149 )
- Oxidation lysis buffer (see Recipe 1)
- Oxidation wash buffer (see Recipe 2)
- TBST buffer (see Recipe 3)
- 1 mM pervanadate (see Recipe 4)
- Lysis buffer (see Recipe 5)
- Trypan blue solution, 0.4% (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
Equipment
- VANTAGE Strabismus Scissors (Steeles, model: V95-312 )
- Forceps (Polysciences, model: 5 Dumont INOX )
- Pipettes (Gilson, models: P10, P100, P1000; catalog numbers: F144802 , F123615 , F123602 )
- Clay Adams Nutator mixer (BD, model: 421105 )
- Mini tank transfer unit (GE Healthcare, catalog number: TE22 )
- X cell SureLockTM mini-cell (Thermo Fisher Scientific, InvitrogenTM, catalog number: EI0001 )
- Hemocytometer (Daigger Scientific, catalog number: EF16034F )
- CO2 incubator (Thermo Fisher Scientific, model: HeracellTM 150 )
- Platform shaker (Heidolph, model: Unimax 1010 , catalog number: 036130180)
- Centrifuge (Eppendorf, model: 5418 , catalog number: 5401000137)
- Chemical resistant vacuum pump (Southern Labware, model: Model 400 )
- Table top centrifuge for 14 ml tubes (Thermo Fisher Scientific, model: SorvallTM LegendTM XTR )
- Film developer (Kodak, model: KODAK X-OMAT 2000 Processor )
Software
- MultiGauge (Fujifilm software)
- GraphPad Prism (GraphPad software)
Procedure
- Isolation of thymocytes
- Euthanize mouse following approved IACUC protocol.
- Isolate thymus from mice using scissors and forceps (see Figure 1).
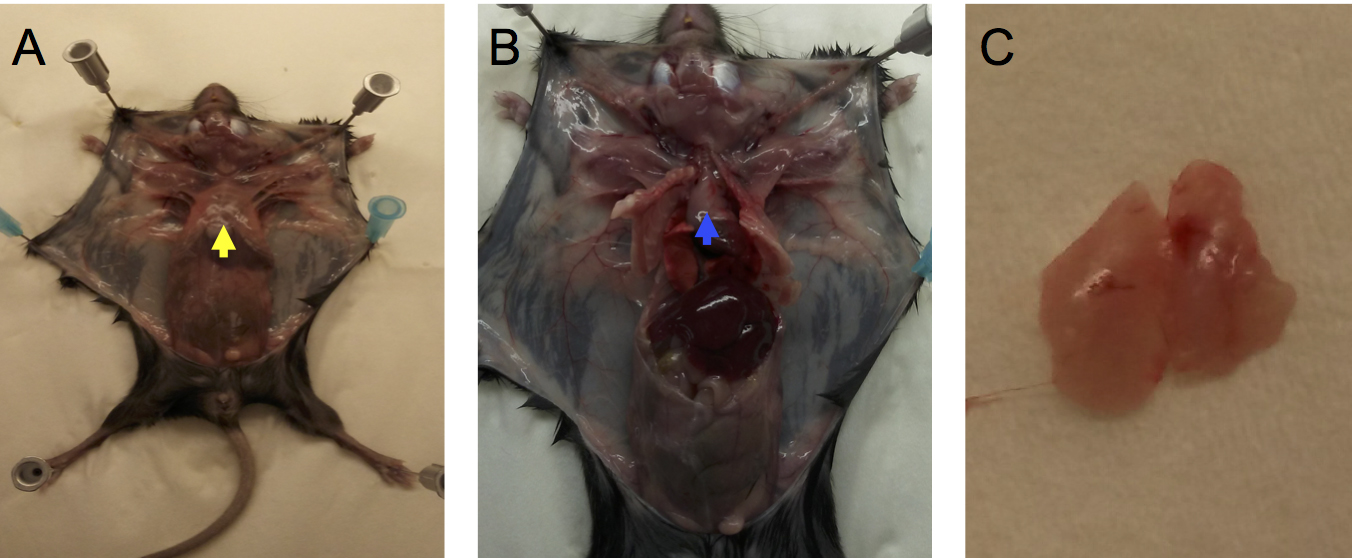
Figure 1. Removal of thymus from a euthanized mouse. Secure mouse limbs to cutting board with push pins or syringe needles. Carefully cut skin with scissors down the midline from the neck to the pelvis. Make short lateral cuts in the skin at the top and bottom of the midline cut and secure skin flaps to the cutting board. A. Grab xyphoid (yellow arrow) with forceps and gently lift, then cut sternum down the midline with scissors and fold back rib cage to expose thymus (B, blue arrow). Gently lift thymus with forceps in one hand and with forceps in other hand dislodge thymus from surrounding tissues by gentle teasing. C. Thymus (note 2 lobes) after removal from the mediastinum. - Transfer thymus to one well of a 6-well plate filled with 1 ml of degassed serum-free media (see Note 1) then cut thymus into several small sections with scissors.
- Gently compress thymus pieces in a cell strainer with forceps to release thymocytes from stroma.
- Add 9 ml of degassed serum-free media on ice.
- Filter the thymocyte suspension by pipetting (1,000 μl pipette) through a cell strainer into a 14 ml tube.
- Centrifuge for 1 min at 1,000 x g, 4 °C.
- Remove the supernatant and resuspend the pellet with 10 ml of degassed serum-free media on ice.
- Euthanize mouse following approved IACUC protocol.
- Detection of reduced (catalytically active) SHP-1
- Enumerate thymocytes using a hemocytometer and trypan blue solution. The expected number of cells per thymus is 0.5-1 x 108.
- Put 1 x 107 cells into a 14 ml conical tube and centrifuge for 1 min at 1,000 x g, 4 °C.
- Discard supernatant and resuspend the pellet in 0.5 ml of degassed oxidation lysis buffer (Recipe 1, Note 1) on ice. Transfer to a 1.6 ml microfuge tube. Degassed oxidation lysis buffer without IAP-biotin is used as a control.
- Vortex for 15 sec.
- Incubate the lysate for 20 min on ice.
- Centrifuge for 10 min at 12,000 x g, 4 °C.
- Transfer supernatant to a 1.6 ml microfuge tube and add 0.5 ml of degassed oxidation lysis buffer.
- Add 5 μl (1 μg) of anti-SHP-1 Ab and incubate overnight in a nutator at 4 °C.
- Add 20 μl of degassed oxidation wash buffer (Recipe 2) equilibrated GammaBind G-Sepharose and incubate in a nutator for 1 h at 4 °C.
- Add 1 ml of degassed oxidation buffer, pipet to disperse sepharose then centrifuge for 1 min at 1,000 x g. Remove buffer by aspiration. Repeat three times.
- Add 30 μl of SDS protein gel loading solution containing 5% 2-mercaptoethanol to pellet.
- Boil samples for 5 min and load onto one lane of 10% Tris-glycine SDS-PAGE gel.
- Run the gel for 3 h at 100 V then transfer to PVDF membrane by electro-blotting.
Note: PVDF membrane is soaked in 10 ml of methanol for 5 min and then in 15 ml of transfer buffer for 5 min before electro-blot transfer. - Block PVDF membrane with 5% non-fat dry milk in TBST buffer (Recipe 3) for 1 h at room temperature on a platform shaker.
- Add Streptavidin-HRP at a 1:5,000 dilution in a 5 ml of TBST buffer for 4 h at room temperature on a platform shaker.
- Wash 3 x 10 min in 15 ml of TBST buffer at room temperature.
- Add 2 ml of SuperSignalTM West Pico chemiluminescent developing solution and incubate for 5 min.
- Pour off the developing solution.
- In a darkroom, place X-ray films on the wrapped membrane.
- Expose for 1 min (see Note 2). Develop the film.
- Enumerate thymocytes using a hemocytometer and trypan blue solution. The expected number of cells per thymus is 0.5-1 x 108.
- Analyses of SHP-1 oxidation after stimulation with pervanadate or H2O2.
- Enumerate thymocytes as described above.
- Put 1 x 107 cells into a 14 ml conical tube and centrifuge for 1 min at 1,000 x g, 4 °C.
- Discard supernatant, add 0.1 ml of degassed serum-free media and gently resuspend cells by repeated pipetting.
- Add 0.1 ml of 2x pervanadate (Recipe 4) for 10 min or 0.1 ml 2x H2O2 (2-20 mM) for 5 min at room temperature.
Note: 3% (w/v) H2O2 is 880 mM. Optimal final concentrations of H2O2 and pervanadate will need to be established for each cell type and will depend on the extent of PTP oxidation desired. Initial experiments should test a range of concentrations. Suggested ranges: Pervanadate, 10-200 μM (final concentration); H2O2: 0.1-10 mM (final concentration). Cells are also treated with the same amount of vehicle solution without H2O2 or pervanadate as a negative control. - Stop by centrifugation for 1 min at 1,000 x g at room temperature.
- Discard supernatant and wash the pellet once with degassed 1x PBS.
- Resuspend with 0.5 ml of degassed lysis buffer (Recipe 5).
- Incubate the lysates for 20 min on ice.
- Centrifuge for 10 min at 12,000 x g, 4 °C.
- Take supernatant, transfer to a 1.6 ml microfuge tube and add 0.5 ml of degassed lysis buffer.
- Add 5 μl (1 μg) of anti-SHP-1 Ab and incubate in a nutator overnight at 4 °C.
- Add 20 μl of degassed lysis buffer-equilibrated GammaBind G-Sepharose and incubate in a nutator for 1 h at 4 °C.
- Wash by pelleting (centrifuge for 1min at 1,000 x g), aspiration, and resuspension with 1 ml of degassed lysis buffer three times.
- Add 30 μl of SDS protein gel loading solution containing 5% 2-mercaptoethanol to pellet.
- Boil samples for 5 min and load each sample into well of 10% Tris-glycine SDS-PAGE gel.
- Run the gel for 3 h at 100 V and transfer to nitrocellulose membrane by electroblotting.
- Block nitrocellulose membrane with 10 ml of 5% non-fat dry milk in TBST buffer for 1 h at room temperature on a platform shaker.
- Incubate with anti-oxidized PTP active site Ab in 5 ml of TBST buffer for 4 h at room temperature.
Pour off the antibody solution.
Note: Anti-oxidized PTP active site antibody detects the conserved PTP active site with the catalytic cysteine residue oxidized to a sulfonic acid form (Persson et al., 2004). - Wash twice with 10 ml TBST at room temperature.
- Add Goat anti-mouse IgG HRP at a 1:2,000 dilution in a 5 ml of TBST buffer for 1 h at room temperature.
- Pour off secondary antibody solution.
- Wash 3 x 10 min with 15 ml of TBST buffer at room temperature.
- Add 2 ml of SuperSignalTM West Pico chemiluminescent developing solution and incubate for 5 min.
- Pour off the developing solution.
- In a darkroom, put X-ray films on the wrapped membrane.
- Expose for 1 min (see Note 2). Develop the film.
- See Figure 2 and Figure 3 for representative results.
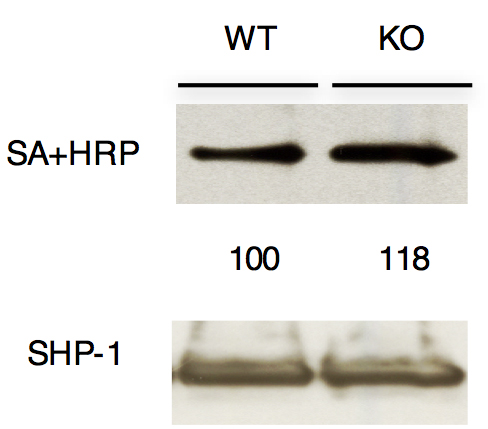
Figure 2. Analysis of reduced (active) SHP-1 in thymocytes. Immunoblot analysis of anti-SHP-1 immunoprecipitates from total thymocytes from wild type (Themis+/+; WT) or Themis-/- (KO) mice after labeling reduced active PTP active site cysteines with iodoacetyl-polyethylene glycol-biotin. The blot was probed with streptavidin-horseradish peroxidase (SA-HRP) to detect biotinylated proteins. The reduced SHP-1 was quantitated by densitometry. Values are normalized to total SHP-1 band density in each experiment.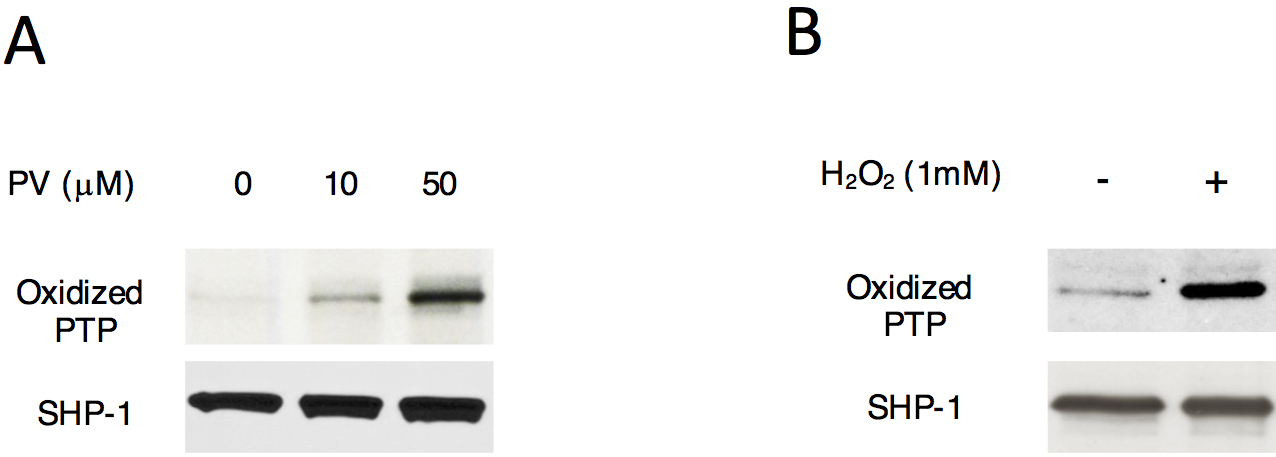
Figure 3. SHP-1 oxidation after stimulation with pervanadate or H2O2 treatment. Immunoblot analysis of active-site oxidation of SHP-1 in total thymocytes treated with various concentrations of pervanadate (PV) (A) or H2O2 (B). Proteins immunoprecipitated with anti-SHP1 were analyzed, and the blots were probed with antibody specific for the sulfonic acid (SO3H) form of the conserved active site cysteine of PTPs for the detection of irreversible oxidation of SHP1.
- Enumerate thymocytes as described above.
Data analysis
For quantitation of band density, we used MultiGauge (Fujifilm software). At least three, preferably 4-6 independent experiments were performed for statistical analysis. For statistical analysis, we used GraphPad Prism (GraphPad software). Typically, we provide a representative blot that was used for statistical analysis in the body of the paper and provide all additional blots used for statistical analysis in the supplemental section. Since band densities are normalized to control bands (e.g., total SHP-1 blots), all data should be usable and included unless there were technical problems with a particular gel or blot. Normalization to control total protein bands is considered essential especially if separate blots are included in the statistical analysis. We have also found that the marginal lanes on gels (first and last) can be problematic, so we try to avoid using those two lanes for critical samples. See Choi et al. (2017) for an example of published data from these experiments.
Notes
- All solutions and media should be degassed for 1 h with a membrane vacuum pump (37 L/min) and stored on ice.
- Exposure time will be different for each antibody.
Recipes
- Oxidation lysis buffer
50 mM Tris (pH 7.5)
100 mM NaCl
0.1% SDS
0.5% sodium deoxycholate
0.5% Nonidet P-40
0.5% Triton X-100
50 mM NaF
1 mM PMSF
0.4 mM Iodoacetyl PEG-biotin
100 μM DTPA
200 U/ml catalase
1 tablet of protease inhibitor per 50 ml - Oxidation wash buffer
50 mM Tris (pH 7.5)
100 mM NaCl
0.5% Nonidet P-40
0.5% Triton X-100
50 mM NaF - TBST buffer
20 mM Tris (pH 7.5)
135 mM NaCl
0.05% Tween 20 - 1 mM pervanadate
1 mM Na3VO4
5 mM H2O2
Note: Make fresh and incubate for 5 min at room temperature before use. - Lysis buffer
1% Nonidet P-40
10 mM Tris (pH 7.5)
150 mM NaCl
2 mM EGTA
50 mM β-glycerophosphate
2 mM Na3VO4
10 mM NaF
10 mM Iodoacetamide
10 mM NEM
1 tablet of protease inhibitor per 50 ml
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver, National Institute of Child Health and Human Development (1ZIAHD001803 to P.E.L.). The protocol has been adapted from Choi et al. (2017) Nature Immunology 18, 433-441. The authors have no conflicts of interests or competing interests relating to this publication.
References
- Choi, S., Warzecha, C., Zvezdova, E., Lee, J., Argenty, J., Lesourne, R., Aravind, L. and Love, P. E. (2017). THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat Immunol 18(4): 433-441.
- Huyer, G., Liu, S., Kelly, J., Moffat, J., Payette, P., Kennedy, B., Tsaprailis, G., Gresser, M. J. and Ramachandran, C. (1997). Mechanism of inhibition of protein tyrosine phosphatases by vanadate and pervanadate. J Biol Chem 272: 843-51.
- Karisch, R. and Neel, B. G. (2013). Methods to monitor classical protein-tyrosine phosphatase oxidation. FEBS J 280(2): 459-475.
- Persson, C., Sjoblom, T., Groen, A., Kappert, K., Engstrom, U., Hellman, U., Heldin, C. H., den Hertog, J. and Ostman, A. (2004). Preferential oxidation of the second phosphatase domain of receptor-like PTP-α revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci U S A 101(7): 1886-1891.
- Rudyk, O. and Eaton, P. (2014). Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol 2: 803-813.
- Weibrecht, I., Bohmer, S. A., Dagnell, M., Kappert, K., Ostman, A. and Bohmer, F. D. (2007). Oxidation sensitivity of the catalytic cysteine of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Radic Biol Med 43(1): 100-110.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Choi, S. and Love, P. E. (2018). Detection of Intracellular Reduced (Catalytically Active) SHP-1 and Analyses of Catalytically Inactive SHP-1 after Oxidation by Pervanadate or H2O2. Bio-protocol 8(1): e2684. DOI: 10.21769/BioProtoc.2684.
Category
Immunology > Immune cell function > General
Biochemistry > Protein > Activity
Cell Biology > Cell signaling > Intracellular Signaling
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










