- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation, Stimulation and Other Uses of Adult Rat Brain Synaptosomes
Published: Vol 7, Iss 24, Dec 20, 2017 DOI: 10.21769/BioProtoc.2664 Views: 7984
Reviewed by: Anca Flavia SavulescuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Single-Particle Tracking of AMPA Receptor-Containing Vesicles
Victor C. Wong [...] Erin K. O’Shea
Jun 5, 2025 2262 Views

Local Iontophoretic Application for Pharmacological Induction of Long-Term Synaptic Depression
Borys Olifirov [...] Pavel Belan
Jun 5, 2025 1898 Views

Ultrafast Isolation of Synaptic Terminals From Rat Brain for Cryo-Electron Tomography Analysis
Rong Sun and Qiangjun Zhou
Sep 5, 2025 3556 Views
Abstract
In this paper, our protocol for preparation of brain synaptosomes is described. Synaptosomes are a valuable model system for analysis of structural components of the synapse as well as for investigation of synaptic function. Synaptosomal preparations are necessary for understanding molecular changes at synapses where critical post-translational modifications of synaptic proteins may occur. Not only are synaptosomes rich in synaptic proteins, but they can be used for analyzing uptake of neurotransmitters into synaptic vesicles and for analysis of the involvement of neurotransmitter synthesis and release. Synaptosomes can be stimulated with increased calcium influx to release neurotransmitters. Synaptosomal preparations have been used in characterizing calcium dependent phosphorylation and activation of the GABA synthesizing enzyme GAD65 (L-glutamic acid decarboxylase with molecular weight of 65 kDa). By examining protein complexes on the membrane of synaptic vesicles obtained from synaptosomal preparations, it was possible to characterize the role of GAD65 in the coupled synthesis and vesicular uptake of GABA (γ-aminobutyric acid) culminating in GABA vesicular release, which contributes in an important way to fine-tuning of GABAergic neurotransmission.
Keywords: GAD65Background
Synaptosomal preparation methods were established in neuroscience research laboratories over 40 years ago and have been of great value in scientific innovations relating to neurotransmitter release with respect to elevated extracellular potassium as well as responses to increased intracellular calcium. In addition to elucidating the process of neurotransmitter release, synaptosomal preparations have been a valuable source of synaptic vesicles. Studies on synaptic vesicles have for example been used in the characterization of protein components that participate in coupled neurotransmitter synthesis and vesicular release. Synaptosomal preparations have also been very valuable as an intermediate in isolation of synaptic vesicles which are then analyzed in terms of protein complexes located on the vesicular membrane that include neurotransmitter synthesizing enzymes. Of key significance in this regard is the vesicular membrane GAD65 complex which includes CSP (Cystine-String Protein), VGAT (Vesicular GABA transporters) and CaMKII (calcium/calmodulin-dependent protein kinase II). Regulation of GAD65 activity has been further characterized in terms of GAD65 cleavage by calpain and activation of membrane bound GAD through phosphorylation as well as accelerated anchoring of GAD65 to SV by palmitoylation.
The basic methodology for vesicular preparation presented in this paper does not significantly differ from the originally established technology from the 1970s but the applications have been greatly extended over this time and therefore expanded to include analytical investigations on neurotransmitter synthesizing enzymes, on signaling complexes associated with these enzymes, on mechanisms of regulation of these enzymes and on functional outcomes of enzyme activation including vesicular neurotransmitter release in response to high extracellular potassium levels or to increased calcium influx.
Materials and Reagents
- Pipette tips (VWR, catalog number: 82028-570 )
- Centrifuge tubes (round bottom–50 ml) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 3118-0050 )
- 15 ml Falcon tubes (VWR, catalog number: 10026-076 )
- Glass tube (DWK Life Sciences, Kimble, catalog number: 885451-0022 )
- Scintillation vial (Fisher Scientific, catalog number: 03-337-20 )
- Experiments involving animals:
The use of animals and animal tissues in this protocol was approved by and in accordance with the requirements of Institutional Animal Care and Use Committee, Florida Atlantic University. The Public Health Service animal welfare assurance number is A3883-01. For all experiments, Adult 250-300 g Sprague-Dawley male rats (Harlan, IL, USA) were used. Two animals per cage were housed and maintained at 22 °C with an alternating 12-h light/dark cycle. The animals were allowed a minimum stabilization period of at least 3 days after which they were utilized for experimentation. - Scintillation cocktail (RPI, catalog number: 111175 )
- Sucrose (Fisher Scientific, catalog number: BP220-212 )
- HEPES-NaOH (RPI, catalog number: H2393 )
- Tris-HCl (Fisher Scientific, catalog number: BP152-1 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Fisher Scientific, catalog number: C79-500 )
- Disodium phosphate (Na2HPO4) (Sigma-Aldrich, catalog number: 255793-50G )
- Monopotassium phosphate (KH2PO4) (Fisher Scientific, catalog number: P286-1 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761 )
- Sodium chloride (NaCl) (VWR, catalog number: BDH0286 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: EDS-100G )
- Triton X-100 (Sigma-Aldrich, catalog number: X100-100ML )
- Mammalian Protease Inhibitor (MPI) (Sigma-Aldrich, catalog number: P8340 )
- Phosphatase inhibitor (PhI) (HaltTM) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 78426 )
- 2-Aminoethylisothio-uronium bromide (AET) (Sigma-Aldrich, catalog number: A5879 )
- Pyridoxal 5’ Phosphate (PLP) (Sigma-Aldrich, catalog number: P9255-25G )
- Dipotassium phosphate (K2HPO4) (Sigma-Aldrich, catalog number: P8281 )
- Pyruvate kinase (Sigma-Aldrich, catalog number: P9136-25KU )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911-500G )
- Phospho(enol) pyruvate (Sigma-Aldrich, catalog number: P7127-250MG )
- Adenosine 5’-triphosphate (ATP) (Sigma-Aldrich, catalog number: A26209-10G )
- Magnesium sulfate (MgSO4) (Fisher Scientific, catalog number: M65-500 )
- γ-Amino-n-Butyric acid (GABA) (Sigma-Aldrich, catalog number: A5835 )
- Glutamate (Glu) (Sigma-Aldrich, catalog number: G5889-100G )
- [3H] Glutamate (Amersham Biosciences, catalog number: TRK445 )
- [14C] GABA (PerkinElmer, catalog number: NEC290E250UC )
- Oxirane acrylic beads (Sigma-Aldrich, catalog number: O9754 )
- HEPES buffered sucrose solution (see Recipes)
- Krebs ringer phosphate (KRP) buffer (see Recipes)
- Brain lysis buffer (see Recipes)
- Standard GAD buffer (see Recipes)
- GPBS buffer (see Recipes)
- ATP mixture (see Recipes)
Equipment
- Pipettes (VWR, catalog number: 89079-976 )
- Guillotine (Stoelting, catalog number: 51330 )
- Con-torque homogenizer (Eberbach, model: E2355 , catalog number: 7265)
- Avanti J-25 centrifuge (Beckman Coulter, model: Avanti J-25 , catalog number: 363102)
- Optima L-90 K centrifuge (Beckman Coulter, model: Optima L-90K , catalog number: 365672)
- Tri-Carb 2900TR Liquid Scintillation Analyzer (PerkinElmer, model: Tri-Carb 2900TR )
Software
- ImageJ software (ImageJ)
- Prism software (GraphPad Software)
- SPSS software
Procedure
- Isolation of synaptosomes (Figure 1) (Wu et al., 1973; Wu, 1982)
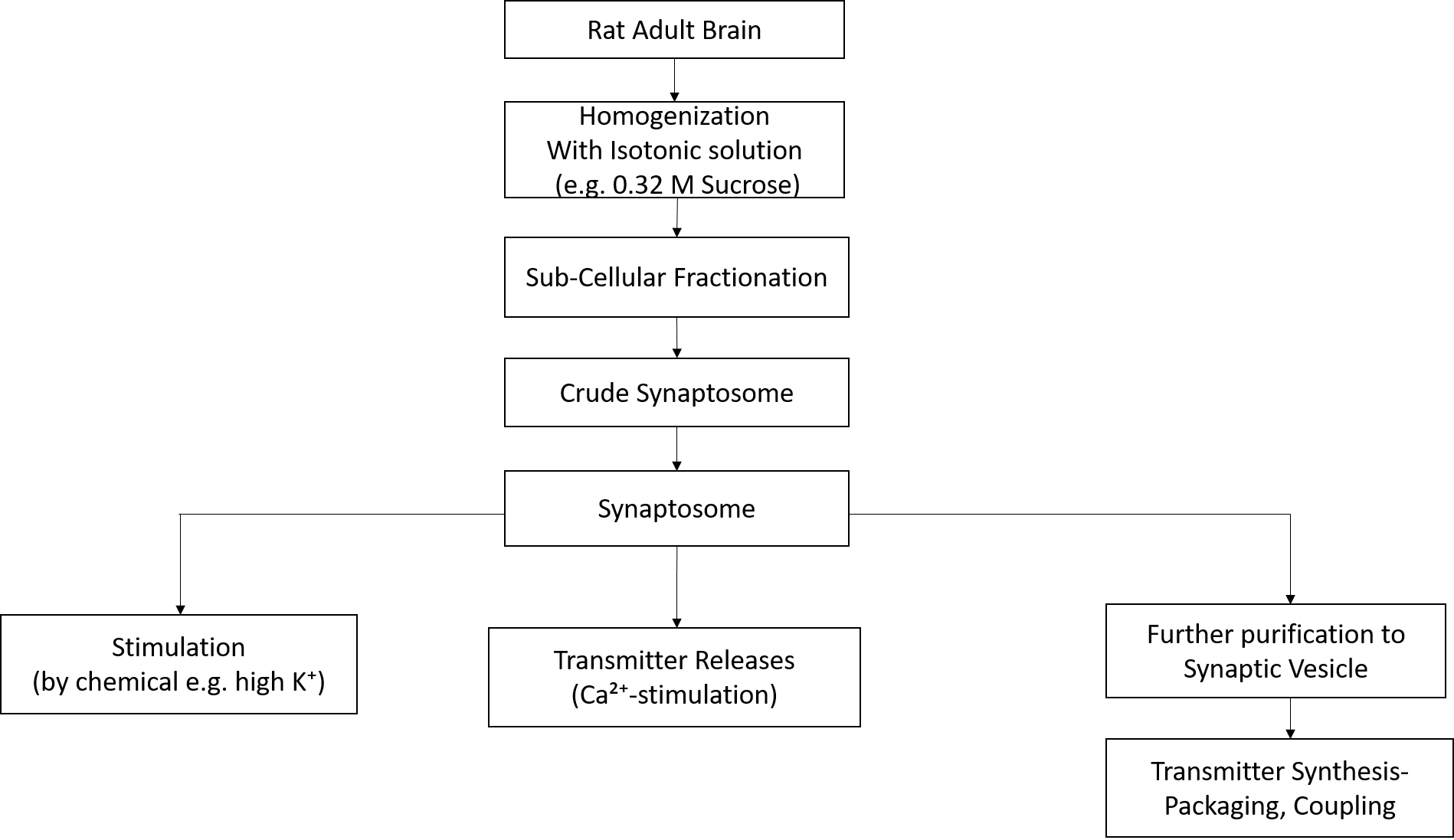
Figure 1. Schematic diagram for protocol- Add HEPES buffered sucrose solution (4 ml/g, see Recipe 1; use freshly prepared sucrose solution, and it should be cooled on ice to 4 °C before use) to freshly collected adult rat brain. Discard the cerebellum and meninges. A rat brain is approximately 4 g.
Note: All steps are performed on ice (4 °C) unless otherwise specified. - Homogenize thoroughly: Using con-torque homogenizer and glass tubes the mixture is subjected to vigorous manual homogenization (15 strokes) (Figure 1).
- Centrifuge at 1,000-1,500 x g for 10 min to remove the nuclear debris (use Avanti J-25 centrifuge and centrifuge tubes [round bottom]).
- The resulting supernatant (S1) is transferred to a new tube while the pellet (P1) is further homogenized with an equal volume of sucrose buffer (Recipe 1) and re-centrifuged under the same conditioned to yield supernatant (S1’) and pellet (P1’).
- The supernatants S1 and S1’ are combined and centrifuged at 20,000 x g for 30 min (Use Avanti J-25 centrifuge and centrifuge tubes [round bottom]).
- The resulting pellet (P2) is crude synaptosome fraction (Figure 1).
- Discard S2 and note the discarded volume. The pellet (P2) is washing by gently swirling it in fresh sucrose buffer whose volume is equivalent to the discarded supernatant (S2).
Note: Don’t pipette up and down, but only gently swirl the tube to resuspend P2. - The solution is then centrifuged again at 20,000 x g for 30 min to yield the wash crude synaptosomes (P2’). From one rat brain, it is about 1 ml. Use Avanti J-25 centrifuge and centrifuge tubes (round bottom).
Note: Store the P2’ at -80 °C if you don’t use it immediately; always keep it on ice if you carry on for further steps.
- Add HEPES buffered sucrose solution (4 ml/g, see Recipe 1; use freshly prepared sucrose solution, and it should be cooled on ice to 4 °C before use) to freshly collected adult rat brain. Discard the cerebellum and meninges. A rat brain is approximately 4 g.
Uses of isolated synaptosomes
Synaptosomal preparations can be used for investigation of neurotransmitter release (Figure 1) (neuronal depolarization) upon stimulation with high potassium as well for analysis of mechanisms of neurotransmitter release from a presynaptic neuron which can be calcium dependent or calcium independent (Wu et al., 1973; Wu, 1982; Bao et al., 1995). Furthermore, synaptic vesicles may be prepared from synaptosomes for analysis of GABA uptake into vesicles as well as analysis of the GAD complex interacting with the vesicular membrane (Jin et al., 2003; Wu et al., 2007; Buddhala et al., 2012). Modification of GAD function can be investigated in terms of regulation of GAD cleavage and regulation of GAD interaction with synaptic vesicles (Sha et al., 2005; Buddhala et al., 2012). Different uses of synaptosomes are outlined below.
- Synaptosomal stimulation with high K+
- Freshly prepared, washed crude synaptosomes(P2’) are re-suspended in 15 ml Falcon tubes in an equal volume of 2x stimulation buffer (freshly prepared cold Kreb’s ringer phosphate (KRP) buffer, see Recipe 2).
- Divide the synaptosomes into 5 equal fractions–Unstimulated, stimulated (Stim) 5’, Stim 10’, Stim 30’ and Stim 60’ (use 15 ml Falcone tubes).
- Aliquots of the synaptosomes in KRP buffer are stimulated by the addition of KCl at either 10, 50 or 100 millimolar and incubated for 37 °C at 45 min.
- A non-stimulated sample serves as the control (maintained at 4 °C or on ice).
- Further study of synaptosomal lysate for immuno-blotting or radioactive GAD activity assays, the synaptosomes in each aliquot are lysed in 1 ml of brain lysis buffer (see Recipe 3).
- The lysates are then rocked at 4 °C for 30 min and are centrifuged at 25,000 x g for 30 min (using Avanti J-25 centrifuge). After this step, the supernatant is collected and stored at -80 °C or else, for immediate preparation of synaptic vesicles, the supernatant is kept on ice or at 4 °C. The supernatant is collected and the protein concentrations are normalized to serve as the samples for subsequent steps.
- Freshly prepared, washed crude synaptosomes(P2’) are re-suspended in 15 ml Falcon tubes in an equal volume of 2x stimulation buffer (freshly prepared cold Kreb’s ringer phosphate (KRP) buffer, see Recipe 2).
- Release of neurotransmitters is calcium dependent (Wu et al., 1973; Fon and Edwards, 2001)
Neurotransmitter discharged from a presynaptic neuron in response to increased neural activity diffuses across the synaptic cleft and transduces the physiological signal by attaching to postsynaptic receptors.
The receptors therefore define the nature of the physiological signal. Classical neurotransmitters such as γ-aminobutyric acid (GABA), glutamate, and acetylcholine (ACh) activate ion channels and thus facilitate quick synaptic transmission. In comparison, neuromodulators, such as monoamines and peptides (as well as GABA, glutamate, and ACh), activate G-protein–coupled receptors, which stimulate second messengers and act on an extended time scale. The neurotransmitter cycle comprises transmitter biosynthesis, storage, reuptake, and degradation. The synaptic vesicle cycle includes targeting vesicles to the nerve terminal where docking, fusion, endocytosis, and recycling take place. High rates of exocytosis involve the well-organized packaging of neurotransmitter into SVs (vesicular neurotransmitter transporters) as well as fast recycling of SVs at the nerve terminal, either straight from the plasma membrane or through an endosomal intermediate. The local recycling comprises sorting of SV proteins from plasma membrane proteins, clathrin-mediated endocytosis, on top of docking of SVs at the plasma membrane. Regulated fusion follows in response to locally elevated Ca2+ arriving at the nerve terminal through voltage-gated Ca2+ channels, which cluster near the site of vesicle fusion. - Preparation of synaptic vesicles (Figure 1) (Jin et al., 2003)
- Synaptosomes (P2’) are rapidly osmolysed in 10 volumes of ice cold Nano pure water containing a 1:100 dilution of mammalian protease inhibitor (MPI) cocktail and phosphatase inhibitors (PhI) (use centrifuge tubes with round bottom).
- The diluted synaptosomal lysate is homogenized as described above and incubated on ice for 45 min.
- Centrifuge at 47,000 x g for 15 min (use Optima L-90 K centrifuge and centrifuge tubes (round bottom)) to remove large membrane fractions and mitochondria.
- Collect the supernatants (S3) without disturbing the pellet and centrifuge at 200,000 x g for 2 h (use Optima L-90 K centrifuge and centrifuge tubes (round bottom)).
- The pellet obtained in this way is crude synaptic vesicles (P4).
- Add 500 μl of standard GAD buffer (see Recipe 4) to each fraction to remove any contaminating diluting cytosolic fractions (S3) and tight pellet (P4) are washed three times. The pellets are to be used for the steps which are listed below in the details of GABA uptake assay.
- Synaptosomes (P2’) are rapidly osmolysed in 10 volumes of ice cold Nano pure water containing a 1:100 dilution of mammalian protease inhibitor (MPI) cocktail and phosphatase inhibitors (PhI) (use centrifuge tubes with round bottom).
- GABA uptake method (Jin et al., 2003)
- Crude SVs are purified as described above and mixed with anti-GAD65 IgG-coupled oxirane acrylic beads (Sigma O9754) for purification of GABAergic specific SVs. AntiGAD65 antibody -SV beads (2 mg of protein per ml) are incubated in 100 microliters total volume with 4 mg/ml of SV (final concentration: 2 mg/ml), 120 µg/ml pyruvate kinase (final concentration: 60 µg/ml in GPBS buffer (see Recipe 5)].
- Incubate SV mixture at 32 °C for 2 min.
- Add the equal volume of ATP mixture (the concentration of SV mixture with ATP mixture at 1:1 of ratio) (2 mg/ml; see Recipe 6) and pipette to combine.
- The combination is further incubated at 32 °C, and an aliquot of 30 µl of the reaction mixture is removed and vacuum-filtered through nitrocellulose membrane at 1-, 5-, 10-, 15-, and 20-min intervals.
- The membranes are washed two times with 5 ml of ice-cold GPBS buffer.
- After the membrane is air dried, put the membrane in scintillation vial, add scintillation cocktail, vortex the vial and leave it overnight.
- The radioactivity persisting on the membrane is calculated based on scintillation counter measurements (dpm). The count in Disintegrations Per Minute (dpm) is measured using equipment Tri-Carb 2900TR Liquid Scintillation Analyzer (PerkinElmer, MA, USA)
- For experiments involving the GAD inhibitor, hydrazine, or aminooxyacetate, the inhibitor is first preincubated with SV mixture for 10 min before the addition of ATP mixture. For uptake assays involving both [3H] Glu and [14C] GABA, the conditions are the same as explained above (Steps E1 to E4) except that both [3H] Glu and [14C] GABA are included in the reaction mixture.
- Crude SVs are purified as described above and mixed with anti-GAD65 IgG-coupled oxirane acrylic beads (Sigma O9754) for purification of GABAergic specific SVs. AntiGAD65 antibody -SV beads (2 mg of protein per ml) are incubated in 100 microliters total volume with 4 mg/ml of SV (final concentration: 2 mg/ml), 120 µg/ml pyruvate kinase (final concentration: 60 µg/ml in GPBS buffer (see Recipe 5)].
- Complex with GAD on the vesicular membrane (Jin et al., 2003; Buddhala et al., 2012; Modi et al., 2015)
GAD65 is attached to SVs by creating a protein complex (first with Heat Shock Cognate: HSC70) that was discovered through the attachment to proteins on SVs, e.g., CSP, VGAT, and (anti-calmodulin-dependent kinase II) CaMKII. This protein complex functions as a machine to verify that GABA biosynthesis and packaging into the SV is exactly coupled. This illustrates a functional and structural coupling of GABA synthesis, regulation, and packaging into SVs.
The physiological events initiated by neuronal stimulation through activation of SV-associated GAD and the subsequent packaging of GABA into the SV can be explained as below.
GABA is distributed by exocytosis after the onset of an action potential. The SV is recycled by a process involving clathrin-coated pits. The clathrin coat is then separated from the vesicles through interaction with HSC70. Vesicles are then returned to the resting state of SVs, where the proton gradient is returned by V-ATPase. GAD65 is stimulated through protein phosphorylation by a proton gradient dependent protein kinase. One of the candidates for the protein kinase is CaMKII. GABA that has been newly synthesized by GAD65 is then carried into SVs by VGAT. These refilled GABA-containing SVs are prepared to be released upon onset of a new action potential. SGAD is activated by calcineurin-mediated dephosphorylation and inhibited by protein kinase A-mediated protein phosphorylation. When GABA neurons are excited, the influx of Ca2+ into the terminal results in dephosphorylation and activation of SGAD/GAD67 (L-glutamic acid decarboxylase with molecular weight of 67 kDa). GABA synthesized by SGAD/GAD67 in the cytosol may also be transferred into SVs, although it represents a minor pathway. Cytosolic GABA may also be metabolized to create ATP through the GABA shunt pathway, which may be utilized to preserve the electrochemical proton gradient for GABA transport. GAD65 is mainly responsible for the synthesis of GABA to be utilized as a neurotransmitter, whereas GAD67 is employed for GABA to be used for other functions such as serving as a signaling molecule in growth, a source of increased cell viability, and a source of GABA released via nonvesicular mechanism. - Regulation of GABA synthesis through control of GAD activity (Bao et al., 1995; Sha et al., 2005; Wei and Wu, 2005; Sha et al., 2008; Buddhala et al., 2012; Modi et al., 2014; Chou et al., 2017)
The stimulation of the glutamic acid decarboxylase (GAD) enzymes GAD65 and GAD67 switches GABA neurotransmission at the pre-synaptic site. Transcription or mRNA splicing controls concentrations of GAD65 and GAD67. Post-translational modifications include proteolytic cleavage, phosphorylation and palmitoylation, that regulate the activities of these key enzymes (Wei et al., 2004). A truncated form of GAD65 (tGAD65) is more dynamic than full-length GAD65 (fGAD65) although, by comparison, truncated GAD67 (tGAD67) is less active than full-length GAD67 (fGAD67). The protein mu-calpain is responsible for cleaving of fGAD65 and fGAD67.
GABA neurotransmission is dependent on whether GAD is associated with synaptic vesicles (SV) and calpain carries out a critical function by producing the highly active tGAD65 causing in increased GABA synthesis and packaging uptake into SV.
It is known that reversible protein phosphorylation is a key mechanism responsible for the regulation of protein functions. Neurotransmitter systems regulated by phosphorylation of their synthesizing enzymes has also been demonstrated. Protein phosphorylation and dephosphorylation play a vital role in the regulation of GAD activity in the brain. Soluble GAD (sGAD) is inactivated upon phosphorylation, most likely by protein kinase A (PKA), and stimulated upon dephosphorylation by protein phosphatase 2B (PP2B, calcineurin). By the contrast, membrane-bound GAD (mGAD) is activated by phosphorylation and disabled by dephosphorylation. The kinase responsible for the phosphorylation of mGAD is not yet known. Although it is commonly assumed that GAD65 is in mGAD preparations and that GAD67 is principally present in sGAD preparation, GAD65 and GAD67 have been shown to exist in both soluble and membrane fractions of the brain preparations.
PKCε regulates GAD65 phosphorylation while GAD67 is inhibited through phosphorylation by PKA. Cysteine residues 455 and 446 in GAD67 and GAD65 alone are vital for full-length GAD regulation.
T91 was shown to be a key phosphorylation site for GAD67 and T95 in hGAD65 was shown to be phosphorylated by kinase C ε (PKCε) by analysis using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. Cooperation with the cofactor PLP at these respective targets controls the switch between PLP-bound active holoGAD and an unbound active apo GAD form. Temporary switching to the PLP bound active holoGAD is vital to GABA neurotransmission. Specific to GAD65 but not GAD67 is palmitoylation by (Huntingtin-interacting protein) HIP14 which accelerates GAD65 anchoring to SV and increases the input of vesicular GABA to neurotransmission.
Data analysis
Data for all experiments are analyzed with Prism or SPSS software. The statistical significance of the data is determined by t-tests or one-way analysis of variance (ANOVA). P values of less than 0.05 are considered significant. The data appears to be normally distributed with similar standard deviations and standard errors observed between and within experimental groups.
Recipes
Note: All the following solutions (Recipes 1-6) can be stored at 4 °C for up to 7 days.
- HEPES buffered sucrose solution
0.32 M sucrose
4 mM HEPES-NaOH
Adjust pH to 7.4 (10 ml/g of brain w/v)
Store at 4 °C - Kreb’s ringer phosphate (KRP) buffer
10 mM Tris-HCl
2.2 mM CaCl2
0.5 mM Na2HPO4
0.4 mM KH2PO4
4 mM NaHCO3
80 mM NaCl
Adjust pH to 7.5
Store at 4 °C - Brain lysis buffer
50 mM Tris-HCl
150 mM NaCl
2 mM EDTA
Adjust pH to 8
1% Triton X-100
1% MPI & PhI
Store at 4 °C - Standard GAD buffer
50 mM potassium phosphate
1 mM 2 amino ethylisothiouronium bromide (AET)
0.2 mM pyridoxal 5’ phosphate
Adjust pH to 7.2
Store at 4 °C - GPBS buffer
9.5 mM KH2PO4
40.5 mM K2HPO4
8 mM KCl
86.6 mM potassium gluconate
Adjust pH to 7.4
Store at 4 °C - ATP mixture
2 mM ATP
4.4 mM MgSO4
12 mM phospho(enol) pyruvate
50 µM GABA
2 mM Glu
110.1 µCi/µl [3H] Glu
Store at 4 °C
Acknowledgments
This work was funded by Grant 6JK08 from the Florida Department of Health and by a grant from the AEURA Trust. There are no conflicts of interest or competing interests.
References
- Bao, J., Cheung, W. Y. and Wu, J. Y. (1995). Brain L-glutamate decarboxylase. Inhibition by phosphorylation and activation by dephosphorylation. J Biol Chem 270(12): 6464-6467.
- Buddhala, C., Suarez, M., Modi, J., Prentice, H., Ma, Z., Tao, R. and Wu, J. Y. (2012). Calpain cleavage of brain glutamic acid decarboxylase 65 is pathological and impairs GABA neurotransmission. PLoS One 7(3): e33002.
- Chou, C. C., Modi, J. P., Wang, C. Y., Hsu, P. C., Lee, Y. H., Huang, K. F., Wang, A. H., Nan, C., Huang, X., Prentice, H., Wei, J. and Wu, J. Y. (2017). Activation of brain L-glutamate decarboxylase 65 isoform (GAD65) by phosphorylation at threonine 95 (T95). Mol Neurobiol 54(2): 866-873.
- Fon, E. A. and Edwards, R. H. (2001). Molecular mechanisms of neurotransmitter release. Muscle Nerve 24(5): 581-601.
- Jin, H., Wu, H., Osterhaus, G., Wei, J., Davis, K., Sha, D., Floor, E., Hsu, C. C., Kopke, R. D. and Wu, J. Y. (2003). Demonstration of functional coupling between γ-aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A 100(7): 4293-4298.
- Modi, J. P., Prentice, H. and Wu, J. Y. (2014). Regulation of GABA Synthesis and Transport. Nova Science Publishers ISBN: 978-1-63321-838-3.
- Modi, J. P., Prentice, H. and Wu, J. Y. (2015). Regulation of GABA Neurotransmission by glutamic acid decarboxylase (GAD). Curr Pharm Des 21(34): 4939-4942.
- Sha, D., Jin, Y., Wu, H., Wei, J., Lin, C. H., Lee, Y. H., Buddhala, C., Kuchay, S., Chishti, A. H. and Wu, J. Y. (2008). Role of μ-calpain in proteolytic cleavage of brain L-glutamic acid decarboxylase. Brain Res 1207: 9-18.
- Sha, D., Wei, J., Wu, H., Jin, Y. and Wu, J. Y. (2005). Molecular cloning, expression, purification, and characterization of shorter forms of human glutamic decarboxylase 67 in an E. coli expression system. Brain Res Mol Brain Res 136(1-2): 255-261.
- Wei, J., Davis, K. M., Wu, H. and Wu, J. Y. (2004). Protein phosphorylation of human brain glutamic acid decarboxylase (GAD)65 and GAD67 and its physiological implications. Biochemistry 43(20): 6182-6189.
- Wei, J. and Wu, J. Y. (2005). Structural and functional analysis of cysteine residues in human glutamate decarboxylase 65 (GAD65) and GAD67. J Neurochem 93(3): 624-633.
- Wu, H., Jin, Y., Buddhala, C., Osterhaus, G., Cohen, E., Jin, H., Wei, J., Davis, K., Obata, K. and Wu, J. Y. (2007). Role of glutamate decarboxylase (GAD) isoform, GAD65, in GABA synthesis and transport into synaptic vesicles-Evidence from GAD65-knockout mice studies. Brain Res 1154: 80-83.
- Wu, J. Y. (1982). Purification and characterization of cysteic acid and cysteine sulfinic acid decarboxylase and L-glutamate decarboxylase from bovine brain. Proc Natl Acad Sci U S A 79(14): 4270-4274.
- Wu, J. Y., Matsuda, T. and Roberts, E. (1973). Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem 248(9): 3029-3034.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Modi, J., Prentice, H. and Wu, J. (2017). Preparation, Stimulation and Other Uses of Adult Rat Brain Synaptosomes. Bio-protocol 7(24): e2664. DOI: 10.21769/BioProtoc.2664.
Category
Neuroscience > Cellular mechanisms > Synaptic physiology
Neuroscience > Cellular mechanisms > Protein isolation
Cell Biology > Cell signaling > Synaptic transmision
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








