- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Model of Immune Complex-mediated Vasculitis in Dorsal Skin and Assessment of the Neutrophil-mediated Tissue Damage
Published: Vol 7, Iss 24, Dec 20, 2017 DOI: 10.21769/BioProtoc.2660 Views: 8977
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3910 Views
Abstract
Neutrophils are the most abundant leukocytes in the blood. In the recent decades, their crucial roles in host defense, immune regulation and tissue damage have been studied in a deeper dimension. In this protocol, we described a mouse model of immune complex-mediated vasculitis in the dorsal skin induced by Arthus reaction, and the subsequent analysis of edema, hemorrhage and tissue damage due to neutrophil activation by means of Evans blue area analysis, histology, and immunofluorescence. This protocol could facilitate the investigation of cellular therapy strategy against over-activated neutrophil-mediated tissue damage.
Keywords: VasculitisBackground
Neutrophils constitute the largest, evolutionary conserved fraction of circulating leukocytes. They lead the first wave of host defense against infection or tissue damage. In vitro models of neutrophil-mediated cellular cytotoxicity are well established (Incani et al., 1981; Dallegri et al., 1984; Saffarzadeh et al., 2012). However, to dissect the complexity of neutrophil-mediated sterile tissue injury, in vivo models are indispensable.
Immune complex (IC)-mediated vasculitis is a disease initiated by the deposition of antigen-antibody complexes in blood vessels, which subsequently lead to complement activation, neutrophil recruitment and activation. The large amount of reactive oxygen species and proteases released from activated neutrophils damage the endothelial lining of the vessel wall and result in edema and hemorrhage (Sindrilaru et al., 2007; Goerge et al., 2008; Feld et al., 2012). The previously described IC-mediated vasculitis induced by Arthus reaction in mouse ears (Sindrilaru et al., 2007) is one of successful models to study the neutrophil-mediated tissue damage. However, with very thin layer of tissue, mouse ear is not suitable to study the effect of potential therapeutic agents in a large volume, for example, with cellular therapy strategy.
In this protocol, we describe a mouse model of IC-mediated vasculitis in dorsal skin, which enables to overcome the above-mentioned pitfall. Furthermore, we provide the details of subsequent analysis of neutrophil induced hemorrhage and tissue injury by Evans blue quantification, together with histological and immunofluorescence techniques. Our results indicated that this mouse model closely resembles the features of tissue damage in human vasculitis patients caused by over-activated neutrophils. This protocol has been applied successfully for our recent discoveries that mesenchymal stem cells suppress hemorrhage and tissue damage in immune-complex mediated vasculitis mediated by over-activated neutrophils (Jiang et al., 2016).
Materials and Reagents
- 1 ml syringe (Omnifix-F) (B. Braun Medical, catalog number: 9161406V-02 )
- Needle 26 G x ½” (B. Braun Medical, catalog number: 4665457-02 )
- 0.22 µm filter (Merck, catalog number: SLGV033RS )
- Cover glasses, 24 x 55 mm, thickness No. 1 (VWR, catalog number: 631-0146 )
- C57BL/6J mice at preferred age of 8-12 weeks, both male and female are suitable for this protocol (THE JACKSON LABORATORY, catalog number: 000664 )
- Anti-bovine albumin antibody produced in rabbit, whole antiserum (Sigma-Aldrich, catalog number: B1520 ) (aliquots store at -20 °C)
- Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- Formalin solution, neutral buffered, 10% (Sigma-Aldrich, catalog number: HT5014 )
- Xylene (Carl Roth, catalog number: CN80.1 )
- Ethanol (Carl Roth, catalog number: 9065.2 )
- Hematoxylin solution acid acc. to Mayer (Carl Roth, catalog number: T865 )
- Eosin Y solution 0.5% in water (Carl Roth, catalog number: X883 )
- Acetic acid (Carl Roth, catalog number: 3738.1 )
- Roti-histol (Carl Roth, catalog number: 6640 )
- Roti-histokitt (Carl Roth, catalog number: 6638 )
- Target retrieval solution, 10x (Agilent Technologies, DAKO, catalog number: S169984-2 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- Purified rabbit anti-human/mouse neutrophil elastase/NE antibody (Abcam, catalog number: ab68672 )
- Purified rat anti-mouse Ly6G antibody (Clone RB6-8C5) (Abcam, catalog number: ab25377 )
- Alexa Fluor 488-conjugated goat anti-rat IgG secondary antibody (Thermo Fisher Scientific, Invitrogen, catalog number: A-11006 )
- Alexa Fluor 488-conjugated donkey anti-rabbit IgG secondary antibody (Thermo Fisher Scientific, Invitrogen, catalog number: A-21206 )
- Purified mouse IgG2a κ isotype control (clone C1.18.4) (BD, BD Biosciences, catalog number: 550339 )
- Purified mouse anti-DNA/histone H1 antibody (Merck, catalog number: MAB3864 )
- Purified goat anti-human/mouse myeloperoxidase/MPO antibody (R&D Systems, catalog number: AF3667 )
- Alexa Fluor 555-conjugated goat anti-mouse IgG secondary antibody (Thermo Fisher Scientific, Invitrogen, catalog number: A-21422 )
- Alexa Fluor 555-conjugated donkey anti-goat IgG secondary antibody (Thermo Fisher Scientific, Invitrogen, catalog number: A-21432 )
- 4’,6-Diamidino-2-phenylindole, Dihydrochloride (DAPI) (Thermo Fisher Scientific, InvitrogenTM, catalog number: D1306 )
- Fluorescence mounting medium (Agilent Technologies, DAKO, catalog number: S302380-2 )
- Ketanest S 25 mg/ml (ketamine) (Pfizer, authorization number: 39945.00.00)
- Rompun 2% (xylazine) (Bayer HealthCare, drug identification number: 02169606 )
- Evans blue (Sigma-Aldrich, catalog number: E2129 )
- Rabbit serum (Sigma-Aldrich, catalog number: R9133 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Goat serum (Sigma-Aldrich, catalog number: G9023 )
- Fetal bovine serum (FBS) (Biochrom, catalog number: S 0615 )
- Anesthesia solution (see Recipe 1)
- Evans blue-BSA solution (see Recipe 2)
- Blocking buffer (see Recipe 3)
- Antibody diluent (see Recipe 4)
Equipment
- Balance
- Heating table (e.g., MEDAX, model: 13801 )
- Animal hair clipper (e.g., Aesculap Exacta Tierschermaschine, B. Braun Medical, Aesculap, catalog number: GT415 )
- Tissue processor (e.g., Leica Biosystems, model: Leica TP1020 )
- Digital camera (e.g., Panasonic, model: Lumix DMC-LX7 )
- Fluorescent microscope: Zeiss Axiophot microscope with an AxioCam digital color camera and AxioVision software v4.7 (Carl Zeiss)
- Steam cooker (e.g., Braun MultiGourmet Food Steamer, Braun, model: FS 20 )
- Slide staining trays (e.g., StainTrayTM Systems for 20 Slides with Base with Black Cover, Simport, catalog number: M920-2 )
Software
- ImageJ
- Graphpad Prism
- AxioVision (ZEISS)
Procedure
- Arthus reaction in dorsal skin
- Weigh the experimental C57BL/6J mice and intraperitoneally inject anesthesia solution (see Recipe 1) according to the body weight at 5 µl/g body weight (e.g., 150 µl for a 30 g mouse). The mice are expected to be anesthetized for at least 30 min. Mice are placed on a heating table (39 °C) during anesthesia to maintain the body temperature.
- Shave the hair on the dorsal skin by using an animal hair clipper.
- Optional: application of therapeutic agents and the respective controls.
Notes:- The method and duration of application of therapeutic agents need to be determined for individual treatment condition. For example, to study the effect of mesenchymal stem cells (MSCs) on IC-mediated vasculitis, 2.5 x 105 adipose tissue-derived MSCs in 100 µl PBS per injection were intradermally injected to both sides of the shaved dorsal skin of mice.
- Mice intradermally injected with 100 µl PBS served as controls. Injection of such volumes into mouse ears is not possible.
- Arthus reaction can be induced half an hour after injection of MSCs.
- The method and duration of application of therapeutic agents need to be determined for individual treatment condition. For example, to study the effect of mesenchymal stem cells (MSCs) on IC-mediated vasculitis, 2.5 x 105 adipose tissue-derived MSCs in 100 µl PBS per injection were intradermally injected to both sides of the shaved dorsal skin of mice.
- Intravenously (or intraperitoneally) inject 100 µl of Evans blue-BSA solution (see Recipe 2).
Note: Compared to i.v. injection, i.p. injection resulted in smaller Evans blue areas in the skin at the injection sites of anti-BSA antibody, but the difference between anti-BSA and rabbit serum control was clear (Figure S1). Therefore, i.p. injection of Evans blue-BSA solution can also be used for this procedure. - Intradermally inject 40 µl of rabbit anti-BSA antibody (undiluted whole antiserum, concentration varies in different lots, in our experiments ranged from 3-5 mg/ml) into the dorsal skin of interest (e.g., areas that had been treated testing therapeutic agent, such as with AT-MSCs, or respective control). In control mice, 40 µl of rabbit serum was injected intradermally.
- Four hours later, to quantify the extent of Evans blue-marked vascular leakage, sacrifice the mice, harvest skin specimen (Video 1) and immediately photograph digitally (Figure 1B).Video 1. Dissection of mouse skin after Arthus reaction. The Arthus reaction was elicited by i.p. injection of 100 µl PBS solution containing 1% Evans blue and 2% BSA. Afterward, 40 µl of anti-BSA antibody (left-side of back-skin) or 40 µl rabbit serum (right-side of back-skin) was intradermally injected. 4 h later, mice were sacrificed and the skin specimen was harvested and digitally photographed.
- Analyze the Evans blue areas in the skin, indicative of the extent of vessel damage due to BSA/anti-BSA complex formation, with ImageJ (details see Data analysis) (Figure 1).
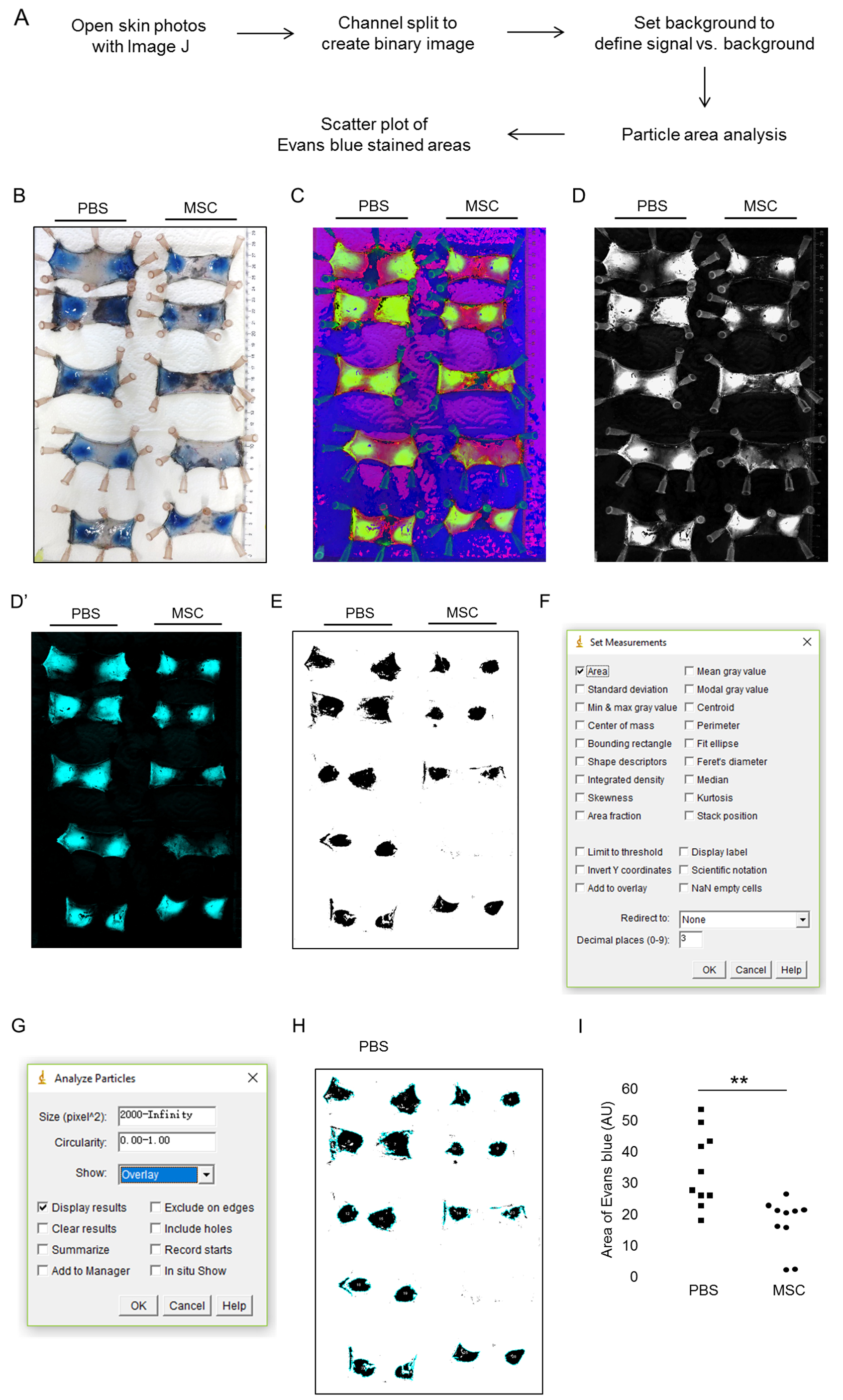
Figure 1. Induction of IC-mediated vasculitis in mouse dorsal skin and data analysis. A. Flow chart of the data analysis; B. 100 µl PBS or 2.5 x 105 adipose tissue-derived mesenchymal stem cells (MSCs) were intradermally injected to both sides of the shaved dorsal skin of mice. The Arthus reaction was elicited by i.v. injection of 100 µl PBS solution containing 1% Evans blue and 2% BSA. Afterward, 40 µl of anti-BSA antibody was intradermally injected to the area that had been injected with MSCs or PBS. 4 h later, mice were sacrificed and the skin specimen was harvested and digitally photographed. C. With ImageJ, the original photo in RGB format was converted to HSB format with plugin ‘Color Space Converter’. D. After splitting channels, the green channel was kept for Evans blue area analysis. D’. Alternatively, the RGB photo was converted to CYMK format with plugin ‘RGB to CYMK’ and the cyan channel was kept for analysis. E. A binary image was created with the Evans blue areas in black. F and G. Settings for measurement and particle analysis. H. Particle analysis indicating the Evans blue areas in skin. I. Results were presented as a scatter plot by GraphPad Prism. Intradermal injection of MSCs resulted in significantly reduced areas of Evans blue staining by 52.1% compared to PBS control (15.8 vs. 33.0 AU). **, P < 0.01 by Mann-Whitney tests; AU, arbitrary unit.
- Weigh the experimental C57BL/6J mice and intraperitoneally inject anesthesia solution (see Recipe 1) according to the body weight at 5 µl/g body weight (e.g., 150 µl for a 30 g mouse). The mice are expected to be anesthetized for at least 30 min. Mice are placed on a heating table (39 °C) during anesthesia to maintain the body temperature.
- Histology and immunofluorescence of neutrophils
Note: Histological examination with H&E staining and immunofluorescence staining of neutrophil activation markers are optional but are recommended, in order to confirm the observed tissue damage is indeed due to neutrophil recruitment and activation.- Arthus reaction without Evans blue
Follow the Procedure A. In Step A4, replace the Evans blue-BSA solution to sterile filtered 2% BSA in PBS. In Step A6, the harvested skin specimen was fixed in 10% formalin solution at 4 °C for overnight. Fixed tissues were processed with tissue processor for dehydration and clearing and embedded in paraffin blocks and sectioned into 5 µm sections using a microtome (the details of making formalin-fixed-paraffin-embedded [FFPE] sections can be referred to existing literature, e.g., Canene-Adams, 2013). - H&E staining (Figure 2)
- Heat paraffin sections at 50-60 °C for 30 min.
- Put sections in xylene for 10 min for 2 times.
- Hydrate sections in 100%, 95%, 80%, 70% ethanol for 3 min for 2 times for each.
- Incubate the sections in distilled water for 5 min.
- Incubate in Mayer’s hematoxylin for 10 min.
- Wash in cold tap water for 5 min.
- Rinse with distilled water (dip the slides in distilled water for several times).
- Incubate in Eosin Y (+ 1 drop of acetic acid/100 ml) for 2 min.
- Rinse with distilled water.
- Dehydrate the sections in 80%, 95%, 100% ethanol for 5 min each.
- Clear with Roti-histol.
- Mount with Roti-histokitt.
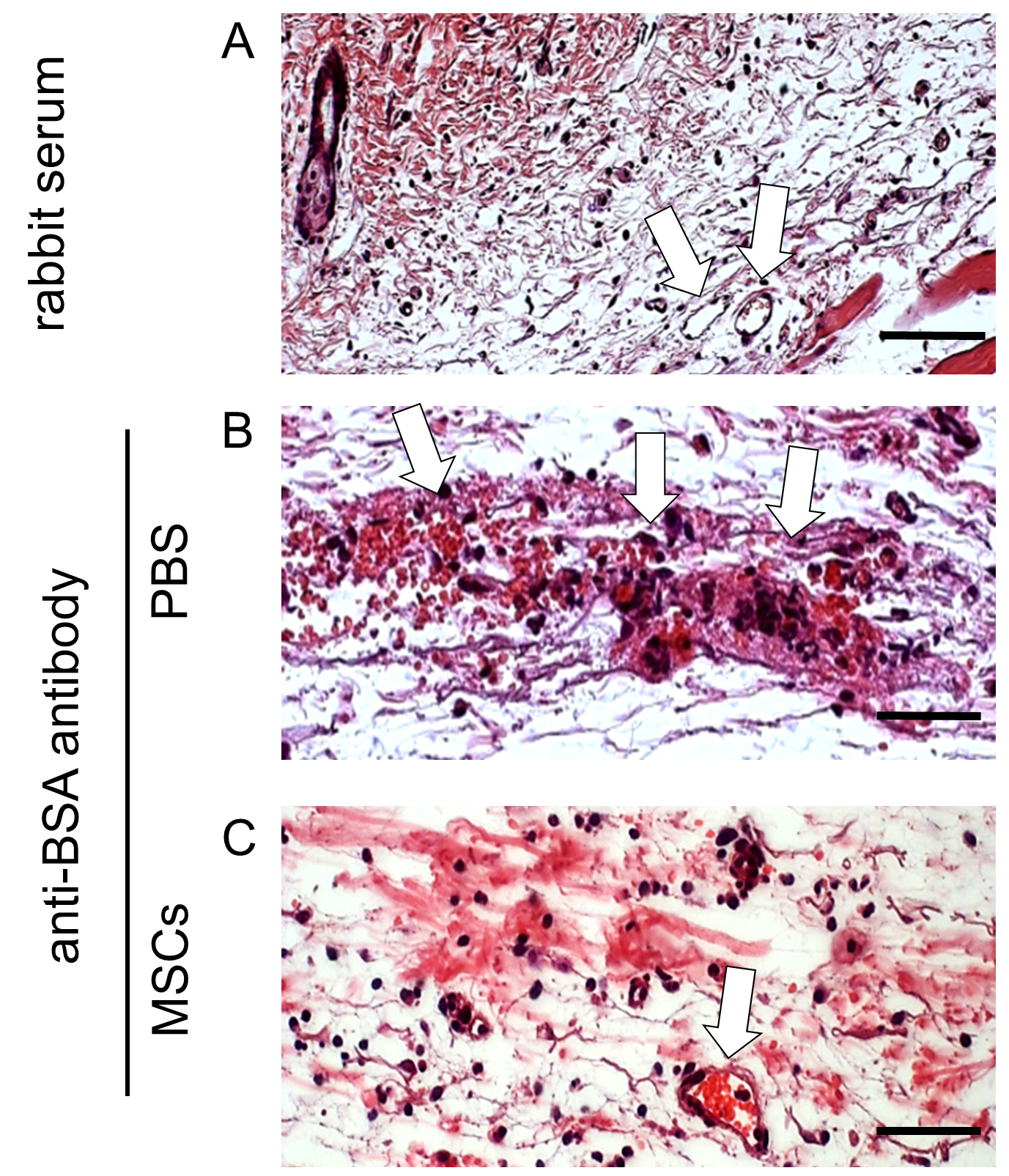
Figure 2. H&E staining of FFPE skin sections after induction of IC-mediated vasculitis. Mice were intradermally injected with PBS or MSCs and subjected to Arthus reaction by i.v. injection of 100 µl PBS solution containing 2% BSA. Afterwards, anti-BSA antibody was intradermally injected into the area that had been injected with MSCs or PBS. Mice received rabbit serum served as healthy controls. 4 h later, mice were sacrificed and the skin specimen was harvested and processed to 5 µm paraffin-embedded sections. Representative pictures of H&E staining of skin sections from 4 mice of each group are shown. White arrows indicate blood vessels. Scale bars = 50 µm. The dermis of the mice subjected to Arthus reaction with PBS injection showed severe damage of vessels surrounded by red blood cells and neutrophils (B). Pre-treatment of MSCs reduced vessel destruction (C), and histology was similar to skin specimen from healthy control mice (A).
- Heat paraffin sections at 50-60 °C for 30 min.
- Immunofluorescence staining of neutrophil marker (Ly6G), neutrophil extracellular traps (NETs), neutrophil elastase (NE) and myeloperoxidase (MPO)
- Follow Steps B2a-B2d.
- During Step B3a, prepare antigen retrieval buffer: 20 ml of 10x target retrieval solution + 180 ml distilled water, and heat the buffer in a steam cooker.
- Antigen retrieval: heat sections (in plastic chambers) in buffer for 15 min in a steam cooker.
- Cooling for 10 min, and put sections in PBS for 5 min.
- Permeabilization: put sections in 0.25% Triton X-100 for 10 min (0.5 ml Triton X-100 in 200 ml PBS).
- Wash with PBS for 3 times with each 5 min.
- Incubate the sections with blocking buffer (see Recipe 3) for 1 h at room temperature.
- Incubate the sections with the first primary antibody at 4 °C for overnight in a staining tray: anti-Ly6G (1:100 dilution in antibody diluent [see Recipe 4]) (Figures 3A-3C), or anti-NE (1:200 dilution in antibody diluent) (Figures 3D-3F).
- Wash in PBS 3 times, each time 5 min.
- Incubate the section with the first secondary antibody at room temperature for 1 h in a staining tray: AF488-conjugated goat anti-rat IgG (1:200 dilution in antibody diluent) (Figures 3A-3C), or AF488-conjugated donkey anti-rabbit IgG (1:200 dilution in antibody diluent) (Figures 3D-3F).
- Wash in PBS 3 times, each time 5 min. Avoid the direct light exposure.
- Incubate the section with the second primary antibody at room temperature for 2 h in a staining tray: anti-DNA/histone H1 antibody for NETs (1:100 dilution in antibody diluent) (Figures 3A-3C), or anti-MPO (1:50 dilution in antibody diluent) (Figures 3D-3F).
- Wash in PBS 3 times, each time 5 min.
- Incubate the section with the second secondary antibody at room temperature for 1 h in a staining tray: AF555-conjugated goat anti-mouse IgG (1:200 dilution in antibody diluent) (Figures 3A-3C), or AF555-conjugated donkey anti-goat IgG (1:200 dilution in antibody diluent) (Figures 3D-3F).
- Wash in PBS 3 times, each time 5 min. Avoid the direct light exposure.
- Incubate the section with DAPI solution (1:5,000 dilution in PBS) at room temperature for 3 min in a staining tray.
- Rinse the slides with PBS, and air dry. Avoid the direct light exposure.
- Mount cover slip with fluorescent mounting media. The slides can be stored at 4 °C.
- Fluorescence microscopy.
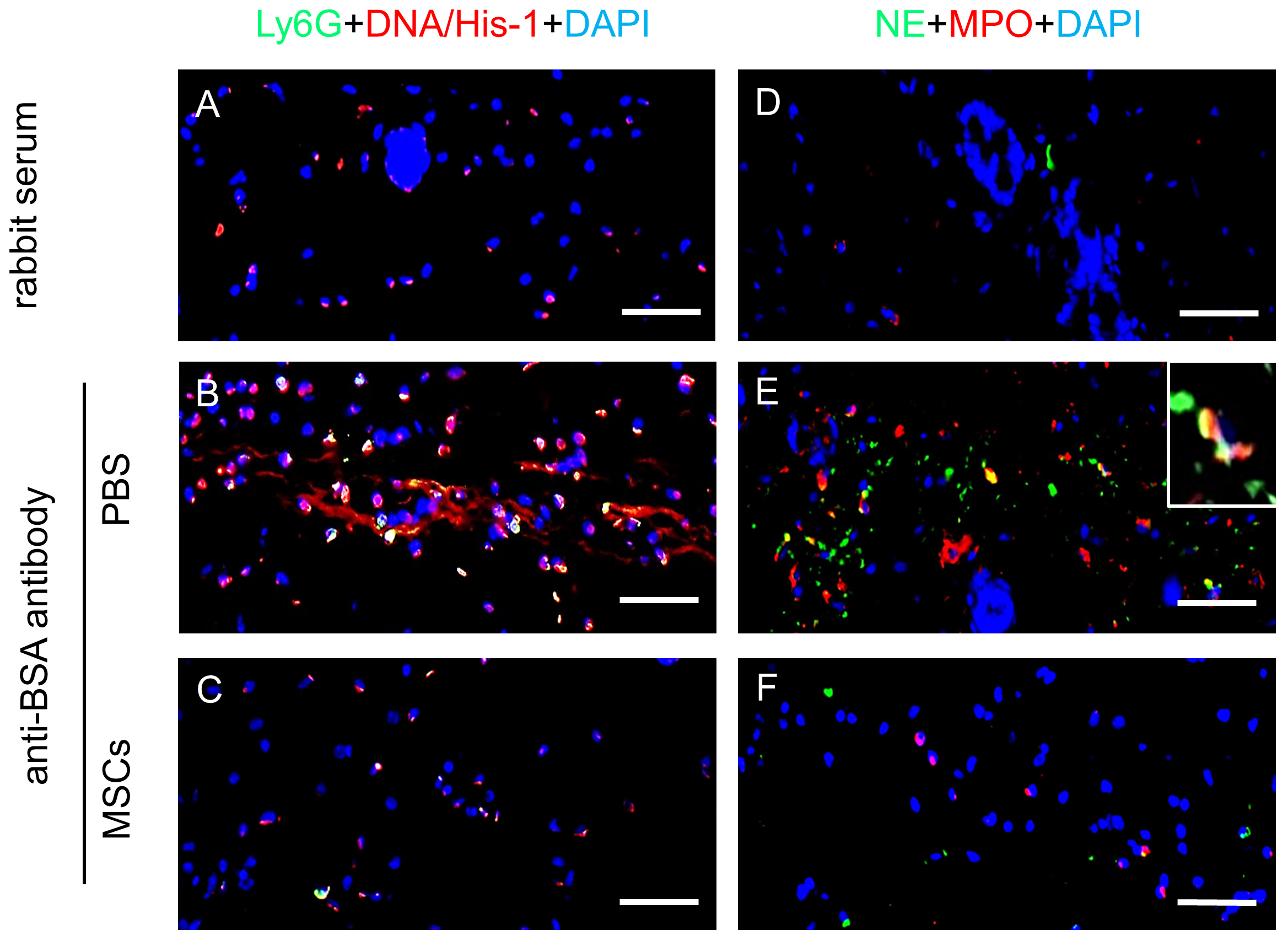
Figure 3. Immunofluorescence staining of neutrophil activation markers on FFPE skin sections after induction of IC-mediated vasculitis. Mice were intradermally injected with PBS or MSCs and subjected to Arthus reaction by i.v. injection of 100 µl PBS solution containing 2% BSA. Afterwards anti-BSA antibody was intradermally injected into the area that had been injected with MSCs or PBS. Mice received rabbit IgG served as healthy controls. 4 h later, mice were sacrificed and the skin specimen was harvested and processed to 5 µm paraffin-embedded sections. Representative pictures of skin sections from 4 mice of each group with immunostaining of murine neutrophil marker Ly6G (green) and NETs marker DNA/histone-1 (red) (A-C), and immunostaining of NE (green) and MPO (red) (D-F) are shown. Nuclei were counterstained with DAPI (blue) in immunostainings. Scale bars = 50 µm. The dermis of the mice subjected to Arthus reaction with PBS injection showed massive infiltration of neutrophils with NETs (B), NE and MPO in the tissue (E), which very much resemble the features of human vasculitis. In contrast, intradermal injection of MSCs significantly reduced NETs (C) and the numbers of NE+MPO+ neutrophils in the dermis (F), which were similar to skin specimen from healthy control mice (A and D).
- Follow Steps B2a-B2d.
- Arthus reaction without Evans blue
Data analysis
Analysis of areas of Evans blue in skin affected by Arthus reaction by ImageJ. The flow chart of the analysis is shown in Figure 1A.
- Download ImageJ plugin ‘Color Space Converter’ under the category ‘Color’:
https://imagej.nih.gov/ij/plugins/color-space-converter.html. Save ‘Color_Space_Converter.jar’ in the plugins folder or subfolder, e.g., Tools: ...\ImageJ\plugins\Tools - Open the original RGB photo. ‘Plugins-Tools-Color Space Converter’: Choose RGB to HSB, leave other settings alone, click ‘OK’ (Figure 1C).
- ‘Image-Color-Split channels’. Use the green channel for the area analysis (Figure 1D), close the other 2 channels (red and blue).
Note: Alternative Steps 1-3; alternative plugin to be used: ‘RGB to CYMK’.
Download the plug-in at https://imagej.nih.gov/ij/plugins/cmyk/index.html. After channel split, keep the cyan (C) channel (Figure 1D’) to convert to binary, close other channels (M, Y, K). - ‘Image-Adjust-Threshold’. Check ‘dark background’ and set the threshold to a fixed value for all the photos and apply. This creates a binary (black and white) image with the Evans blue areas in black (Figure 1E). Set the background value to have a balance in adequate intensity of Evans blue signal but minimized background.
- On the binary, ‘Analyze-set measurements’: check ‘Area’, leave other options alone, click ‘OK’ (Figure 1F).
- ‘Analyze-analyze particles’: Set ‘size (pixel^2)’: e.g., 2000-infinity. ‘Show’: choose ‘overlay’.
Check ‘Display results’ (Figures 1G and 1H) (the size value may need to adjust for smaller areas, depends on what is counted in the end, details see Notes section). You may need manually remove the data of non-Evans blue background areas. - Save the results in GraphPad Prism for analysis. This assay is recommended to be performed with at least 5 mice per treatment group. Data could be presented as a scatter plot (vertical) by GraphPad Prism. Mann-Whitney tests can be used for significance test (Figure 1I).
Notes
- Troubleshooting for ImageJ data analysis
- If you do not see a window with results opening, you forgot to check ‘Display results’ in ‘Analyze–analyze particles’.
- If you do not see which number of area corresponds to which area in the binary, you forgot to set ‘Show’ to ‘overlay’ in ‘Analyze–analyze particles’.
- If you get hundreds of very small areas, you should increase the minimum size of particles in ‘Analyze–analyze particles’.
- If one of your black areas is not analyzed, you should increase minimum size of particles in ‘Analyze–analyze particles’.
- If you do not see a window with results opening, you forgot to check ‘Display results’ in ‘Analyze–analyze particles’.
Recipes
- Anesthesia solution (ketamine and xylazine based)
Per 10 ml anesthesia solution contains:
4 ml of Ketanest S 25 mg/ml
0.5 ml of 2% rompun
5.5 ml of sterile PB - Evans blue-BSA solution (can be kept at 4 °C for a week)
1% Evans blue + 2% BSA in PBS, pass through a 0.22 µm filter - Blocking buffer (prepare fresh)
PBS containing 5% BSA + 5% goat serum in PBS - Antibody diluent (prepare fresh)
PBS containing 1% BSA
Acknowledgments
This work was supported in part by research grants from the Baden-Württemberg Stiftung (P-BWS-ASII/15), the European Commission (CASCADE HEALTH-FP7-223236) and the German Research Foundation (SFB1149) to K.S.-K., the Baustein Program from the Medical Faculty, University of Ulm (LSBN.0100) to D.J. Part of figures are adapted and modified from the study of Jiang et al., 2016. The authors indicate no potential conflicts of interest.
References
- Canene-Adams, K. (2013). Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry. Methods Enzymol 533: 225-233.
- Dallegri, F., Patrone, F., Frumento, G., Ballestrero, A. and Sacchetti, C. (1984). Neutrophil-mediated cellular cytotoxicity triggered by immobilized aggregated IgG: an in vitro model of cell injury during immune complex diseases. J Clin Immunol 4(6): 439-444.
- Feld, M., Goerge, T., Hillgruber, C., Steingraber, A. K., Fastrich, M., Shpacovitch, V. and Steinhoff, M. (2012). α-1-antitrypsin and IFN-γ reduce the severity of IC-mediated vasculitis by regulation of leukocyte recruitment in vivo. J Invest Dermatol 132(9): 2286-2295.
- Goerge, T., Ho-Tin-Noe, B., Carbo, C., Benarafa, C., Remold-O’Donnell, E., Zhao, B. Q., Cifuni, S. M. and Wagner, D. D. (2008). Inflammation induces hemorrhage in thrombocytopenia. Blood 111(10): 4958-4964.
- Incani, R. N. and McLaren, D. J. (1981). Neutrophil-mediated cytotoxicity to schistosomula of Schistosoma mansoni in vitro: studies on the kinetics of complement and/or antibody-dependent adherence and killing. Parasite Immunol 3(2): 107-126.
- Jiang, D., Muschhammer, J., Qi, Y., Kugler, A., de Vries, J. C., Saffarzadeh, M., Sindrilaru, A., Beken, S. V., Wlaschek, M., Kluth, M. A., Ganss, C., Frank, N. Y., Frank, M. H., Preissner, K. T. and Scharffetter-Kochanek, K. (2016). Suppression of neutrophil-mediated tissue damage-a novel skill of mesenchymal stem cells. Stem Cells 34(9): 2393-2406.
- Saffarzadeh, M., Juenemann, C., Queisser, M. A., Lochnit, G., Barreto, G., Galuska, S. P., Lohmeyer, J. and Preissner, K. T. (2012). Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7(2): e32366.
- Sindrilaru, A., Seeliger, S., Ehrchen, J. M., Peters, T., Roth, J., Scharffetter-Kochanek, K. and Sunderkotter, C. H. (2007). Site of blood vessel damage and relevance of CD18 in a murine model of immune complex-mediated vasculitis. J Invest Dermatol 127(2): 447-454.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jiang, D., de Vries, J. C., Muschhammer, J., Sindrilaru, A. and Scharffetter-Kochanek, K. (2017). Mouse Model of Immune Complex-mediated Vasculitis in Dorsal Skin and Assessment of the Neutrophil-mediated Tissue Damage. Bio-protocol 7(24): e2660. DOI: 10.21769/BioProtoc.2660.
Category
Immunology > Animal model > Mouse
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











