- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Arabidopsis Hydroponics and Shoot Branching Assay
Published: Vol 2, Iss 19, Oct 5, 2012 DOI: 10.21769/BioProtoc.264 Views: 26868

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2183 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1886 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1427 Views
Abstract
This protocol establishes growth of Arabidopsis seedlings in a hydroponic medium that is suitable for the controlled addition or withdrawal of any number of nutrients, hormones or metabolites to the plants via the roots. In this case, the described protocol is used to assay sensitivity of shoot branching to exogenously supplied strigolactone, typically the artificial strigolactone GR24. Plants deficient in strigolactone synthesis or response will typically exhibit an increased number of axillary branches compared to wild type. Strigolactone synthesis mutants should respond to exogenous GR24 with reduced numbers and length of axillary shoots, while the phenotype of response mutants will not be rescued.
We present here a more detailed protocol extended from that described in Waters et al. (2012).
Materials and Reagents
- Rock wool/mineral wool (see http://en.wikipedia.org/wiki/Rock_wool; available from hydroponics suppliers)
- GR24, 10 mM in 100% acetone, stored in -20 °C freezer (limited availability; can be purchased from Chiralix: http://www.chiralix.com/rightclick.cfm?id=61574)
- Acetone
- MilliQ water
- Ca(NO3)2
- KNO3
- NH4NO3
- MgSO4.7H2O
- KH2PO4
- KCl
- H3BO3
- MnCl2.4H2O
- ZnSO4.7 H2O
- CuSO4.5 H2O
- CoCl2.6 H2O
- (NH4)6Mo7O24.4 H2O
- Fe-EDTA
- 2-(N-morpholino) ethanesulfonic acid, a buffering agent (MES)
- 0.5x Hoagland’s nutrient solution (pH 5.9) (see Recipes)
- Hoagland’s solutions (see Recipes)
Equipment
- Controlled environment growth chamber or room
- Sealed plastic box for growing plants, with transparent lid and with rack to hold 1.5 ml microcentrifuge tubes, e.g. I5100-43, Astral Scientific, NSW, Australia. Also available from:
http://www.bioexpress.com/divinity-cart/item/502100/GeneMate-Floater-Microtube-Rack/1.html
http://www.labsource.com/Catalog/Group.aspx?GroupID=1455 - Black 1.5 ml microcentrifuge tubes (opaque tubes minimizes light transmission and hence algal growth)
- Fine forceps
- Cork borer, 8 mm diameter, to produce cylinders of rock wool (see http://en.wikipedia.org/wiki/Cork_borer)

Figure 1. Three-week-old Arabidopsis Ler plants grown in hydroponics according to this protocol. The lid of the box has been removed in this photograph. Grid squares are 1 cm.
Procedure
- Plan how many plants you will need for the experiment. If you use the plastic box suggested above, you can comfortably fit 4 adult plants in a single pot (see Figure 1). If each pot counts as a replicate, and you want four biological replicates, then you will need 4 pots (16 plants) per genotype per treatment. You need at least as many microcentrifuge tubes as plants, plus some spares (10-20%) to allow for failures of seedlings to grow.
- Using scissors or a hot scalpel blade, cut off the cap and the bottom 5 mm (or so) from each microcentrifuge tube.
- Cut a slice of rock wool about 2 cm in height, saturate with tap water, and place in a suitable plastic dish.
- Using the cork borer, very gently punch out a cylinder of wet rock wool. This takes some practice. Push down with very little force, while rotating the puncher left and right to help it work its way down. Often the cylinder will be trapped within the borer; use a suitable implement to gently push it out from the top of the borer. It is important that the cylinders are not crushed, so that the seedling’s root can penetrate the matrix easily (see Figure 2A, B).
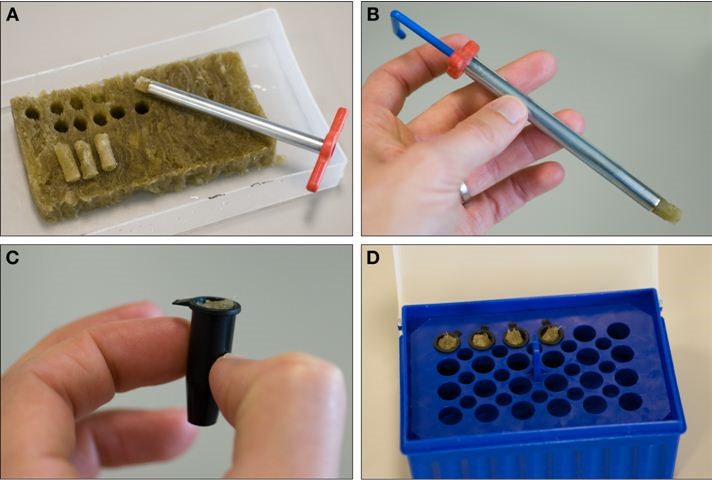
Figure 2. Setting up the rock wool plugs. A. Pushing gently and with a rotating motion, cut a series of cylinders from saturated rock wool using an 8 mm cork borer. When you reach the bottom, push firmly to cut the cylinder free. B. Use a plastic tool to gently push out the cylinder from the borer. C. Place each cylinder in a black microcentrifuge tube, such that the rock wool is level with the top of the tube. D. Place the tubes in the rack. You can fill every hole at this stage. - Make as many rock wool cylinders as are required for the number of microcentrifuge tubes prepared in step 2.
- Using forceps, place the cylinders in each tube, and gently press into place so that the cylinder is flush with the top of the tube. Again, do not compress the rock wool (see Figure 2C. It doesn’t matter if the rock wool sticks above the top edge of the tube by a few millimetres, but the closer to the bottom of the tube it is, the less solution you will need to keep it wet).
- Dilute some 0.5x Hoagland’s nutrient solution 1 in 2 (i.e. 0.25x), place sufficient in the base of the growth box to come up to the bottom of the rack, and fill the rack’s holes with the tubes containing rock wool. During early growth establishment, all of the positions in the rack can be filled with tubes. Later on, when the plants mature, the tubes will need to be split between multiple racks (see step 13). At this point the tubes and rockwool may be autoclaved, but this is optional.
- Wet the tip of some fine forceps, and then pick up about 5-10 Arabidopsis seed. Deposit the seed on the surface of the rock wool, and repeat for all tubes.
- Close the lid on the box and stratify the seed at 4 °C for three days.
- Transfer the box to a growth room with approx. 100-200 μmol photons m-2 sec-1 light intensity, 21 °C, long days (16 h light) for fast growth. Keep the lid closed.
- After about three days, lift the lid gradually over the next three days or so. We use a 1 ml pipette tip to prop the lid open by about 1 cm on the first day, moving it backwards gradually to open the lid further. This helps to protect the seedlings from shock to the drier atmosphere. Check that the nutrient solution is still in contact with the bottom of the tubes; if they dry out, the seedlings will die very quickly! Top up with 0.25x Hoagland’s solution if necessary.
- When the seedlings are seven days old, open the lid fully, and thin out the seedlings to 1 per tube, using fine forceps. At this point, replace the nutrient solution with 0.5x Hoagland’s, and commence the GR24 treatment by supplementing with the required volume of GR24 (5 μM final concentration) or acetone for untreated controls. It is suggested that you add the hormone/acetone to the solution in a bottle, mix well and then dispense the required volume into the boxes.
- After about 10-12 days, the roots should be coming out of the bottom of the tubes and floating in the medium. At this point, before the roots get too long, split the seedlings across as many boxes as required for your experiment, bearing in mind how much space each plant needs. Dispose of any excess plants (but keep the tubes for any future experiments).
- Allow the plants to grow, keeping an eye on the nutrient level. There is a trade-off between time spent looking after the plants, GR24 hormone consumed, and frequency of nutrient exchange. I suggest in the first two weeks of growth, changing the solution every 5 days or so. After three weeks, the plants consume more nutrients, so the solution will need exchanging every 3 to 4 days, depending on humidity. Stability of GR24 is another issue; it is prone to hydrolysis and a very approximate (and generous) guess would be that it has a half-life of 1 week under these conditions. Do not “top up” the solution; replace it fully with fresh solution plus GR24 as required.
- When the plants begin to flower, there is no longer any need to continue the GR24 treatment, as all rosette leaves will have been formed. At this point, count the number of rosette leaves, should you want to express numbers of branches per rosette leaf.
- When the primary inflorescence stops producing flowers, it is time to count the number of axillary branches and, if desired, the height of the main inflorescence stem. We normally count an axillary branch if it has grown more than 5 mm.
Expected Outcome
- For mutants that are deficient in the synthesis or levels of endogenous strigolactones, the number of axillary branches should be reduced relative to untreated controls. For strigolactone insensitive mutants, the number of branches should not be significantly affected. Wild type plants normally do not show a significant reduction in branch number, as it is normally low anyway (1-2 branches, depending on ecotype and growth conditions). In our hands, this method does not lead to complete rescue of the strigolactone-deficient mutant phenotype to wild type levels, but does noticeably reduce branching.
Recipes
- 0.5 x Hoagland’s nutrient solution (pH 5.9)
Modified from Heeg et al. (2008)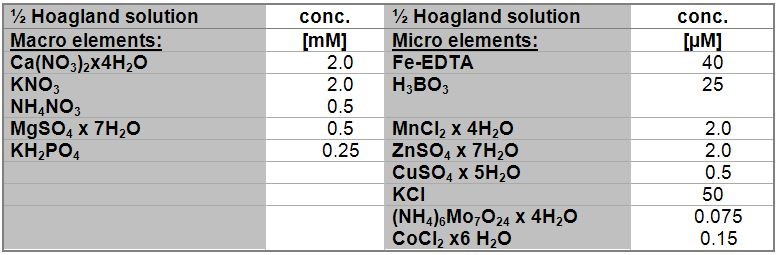
- Hoagland’s solutions
Use MilliQ water to prepare, store Solutions I and II at room temperature and all others at 4 °C.
Autoclave all solutions (EXCEPT Solution I) using a cycle with 15 min at 121 °C.
Stock solution I (500x): 1 M Ca(NO3)2.4H2O 236.2 g/L, filter-sterilise
Stock solution II (500x): 1 M KNO3 101.1 g/L, autoclave
Stock solution III (1,000x): 0.5 M NH4NO3 40.0 g/L, autoclave
Stock solution IV (500x): 0.25 M MgSO4.7H2O 61.6 g/L, autoclave
Stock solution V (1,000x): 0.25 M KH2PO4 34.0 g/L, autoclave
Stock solution VI (5,000x): 0.25 M KCl 18.6 g/L, autoclave
Micro element stock solution (2,000x):
0.05 M H3BO3 1.546 g
0.004 M MnCl2.4 H2O 0.396 g
0.004 M ZnSO4.7 H2O 0.575 g
0.001 M CuSO4.5 H2O 0.125 g
0.0003 M CoCl2.6 H2O 0.036 g
0.00015 M (NH4)6Mo7O24.4 H2O 0.093 g
Add 500 ml ddH2O, autoclave.
10 mM Fe-EDTA stock soln. (250x): 367.1 mg/100ml, autoclave.
Check pH if it is not dissolving and adjust pH to 8.0 using 5 N KOH or 1 N NaOH (if you want to get some Na+ ions into your solution).
For 10 L of Hoagland’s solution:
Dissolve 5 g MES in 8 L milliQ water and add, while mixing:
Stock solution I 20 ml
Stock solution II 20 ml
Stock solution III 10 ml
Stock solution IV 20 ml
Stock solution V 10 ml
Stock solution VI 2 ml
Micro elements 5 ml
Fe-EDTA 40 ml
Adjust the pH of the solution with 5 N KOH to pH 5.8-6.0. THIS IS CRITICAL: GR24 is increasingly unstable at low or high pH. Make up volume up to 10 L.
Acknowledgments
We are grateful to Dr Brent Kaiser (University of Adelaide, Australia) for providing us with the hydroponics method outlined in this protocol. This protocol was further developed from Hoagland and Arnon (1941). The water culture method for growing plants without soil was adapted from miscellaneous publications including Gibeaut et al. (1997) and Tocquin et al. (2003). The work was funded by grants from the Australian Research Council (LP0776252 (RJ) and DP1096717 (MW)).
References
- Gibeaut, D. M., Hulett, J., Cramer, G. R. and Seemann, J. R. (1997). Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115(2): 317-319.
- Heeg, C., Kruse, C., Jost, R., Gutensohn, M., Ruppert, T., Wirtz, M. and Hell, R. (2008). Analysis of the Arabidopsis O-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell 20(1): 168-185.
- Hoagland, D. R. and Arnon, D. I. (1941): The water culture method for growing plants without soil. Miscellaneous Publications 354: 347-461.
- Waters, M. T., Nelson, D. C., Scaffidi, A., Flematti, G. R., Sun, Y. K., Dixon, K. W. and Smith, S. M. (2012). Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139(7): 1285-1295.
- Tocquin, P., Corbesier, L., Havelange, A., Pieltain, A., Kurtem, E., Bernier, G. and Perilleux, C. (2003). A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol 3: 2.
Article Information
Copyright
© 2012 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Waters, M. T., Bussell, J. D. and Jost, R. (2012). Arabidopsis Hydroponics and Shoot Branching Assay. Bio-protocol 2(19): e264. DOI: 10.21769/BioProtoc.264.
Category
Plant Science > Plant physiology > Plant growth
Plant Science > Plant developmental biology > Morphogenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









