- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Live Imaging of Axonal Transport in the Motor Neurons of Drosophila Larvae
Published: Vol 7, Iss 23, Dec 5, 2017 DOI: 10.21769/BioProtoc.2631 Views: 9880
Reviewed by: Jihyun KimAdler R. DillmanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Monitoring of Nucleotide Repeat Expansions Using Southern Blotting in Drosophila Models of C9orf72 Motor Neuron Disease and Frontotemporal Dementia
Joanne L. Sharpe [...] Ryan J. H. West
May 20, 2022 2372 Views

In situ Dephosphorylation Assay with Recombinant Nil Phosphatase
Nilay Nandi [...] Helmut Krämer
Sep 20, 2022 2157 Views

Live Imaging and Analysis of Meiotic Cytokinesis in Drosophila Testes
Govind Kunduri and Jairaj K. Acharya
Jan 20, 2024 3104 Views
Abstract
Axonal transport, which is composed of microtubules, motor proteins and a variety of types of cargo, is a prominent feature of neurons. Monitoring these molecular dynamics is important to understand the biological processes of neurons as well as neurodegenerative disorders that are associated with axonal dysfunction. Here, we describe a protocol for monitoring the axonal transport of motor neurons in Drosophila larvae using inverted fluorescence microscopy.
Keywords: Axonal transportBackground
Axons are a unique neuronal structure, by which neurons transmit electric and chemical signals to neighboring neurons, muscles and other tissues. Axonal function is maintained by the circular flow or shuttling of vesicles, organelles and materials (Liu et al., 2012; Wong et al., 2012; Alami et al., 2014). Axonal dysfunction is thought to be an early sign of neurodegeneration and a cause of neurodegenerative diseases such as Alzheimer’s, Parkinson’s and motor neuron diseases in humans (Hirokawa et al., 2010; Millecamps and Julien, 2013). However, it is difficult to monitor the process of axonal degeneration in these neurodegenerative diseases in both humans and mammalian models. The Drosophila model is a powerful tool for studying neurodegenerative diseases at the molecular-genetic level and has provided important evidence for therapeutic approaches through the live imaging of neurons in vivo (Shiba-Fukushima et al., 2014; Hosaka et al., 2017). Upright fluorescence microscopy techniques are generally used to analyze the live imaging of tissues and organ cultures. However, inverted fluorescence microscopy has seen an increase in demand due to its broad utility and extensibility. Here, we introduce our protocol to analyze the axonal transport of motor neurons in the third-instar larvae of Drosophila using inverted fluorescence microscopy.
Materials and Reagents
- 35-mm culture dishes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 153066 )
- Single sided plastic tape (0.2 mm in thickness) (3M, catalog number: 35 )
- Eight insect pins (Fine Science Tools, catalog number: 26002-10 , Minutien pins, tip diameter 0.0125 mm, rod diameter 0.1 mm, stainless steel)
Note: The heads of 6 pins are shortened to approximately 3 mm in length for dissection (Figures 1 and 4); the other 2 are not modified and are used for the observation tank (Figures 1 and 3).
Figure 1. Preparation of pins - Silicone (Shin-Etsu Silicone, KE-106 and CAT-RG)
- Cover glasses (24 x 40 mm and 18 x 18 mm, 0.12-0.17 mm in thickness, Matsunami Glass, catalog numbers: C024401 and C218181 )
- Drosophila melanogaster-harboring gene mutations or transgenes (most strains are available from public stock centers, which can be accessed through the website below: http://flybase.org/wiki/FlyBase:Stocks)
Note: We utilized the following lines in Figure 6 and our previous study (Hosaka et al., 2017): UAS-preproANF-EMD; D42-GAL4 (Bloomington Drosophila Stock Center, catalog numbers: 7001 and 8816). This line expresses preproANF-EMD, which visualizes the dense core vesicles in motor neurons with green fluorescence. - Sodium chloride (NaCl) (NACALAI TESQUE, catalog number: 31319-45 )
- Potassium chloride (KCl) (Wako Pure Chemical Industries, catalog number: 163-03545 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Wako Pure Chemical Industries, catalog number: 135-00165 )
- Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S6014 )
- Sucrose (Wako Pure Chemical Industries, catalog number: 196-00015 )
- Trehalose (Sigma-Aldrich, catalog number: T0167 )
- HEPES (Sigma-Aldrich, catalog number: H4034 )
- Calcium chloride dihydrate (CaCl2·2H2O) (NACALAI TESQUE, catalog number: 06731-05 )
- Modified HL-3 solution (Stewart et al., 1994) (see Recipes)
Equipment
- Tweezers (Fine Science Tools, catalog number: 11251-30 , Dumont #5 forceps dumoxel standard tip)
- Micro-scissors (World Precision Instruments, catalog number: 501778 , SuperFine vannas scissors, 3 mm straight blades)
- Incubator (Panasonic Healthcare, catalog number: MIR-262 )
- Inverted fluorescence microscopy (Leica Microsystems, model: Leica TCS SP5 )
Software
- ImageJ software (National Institutes of Health) for image analysis
Procedure
- Preparation of a dissection dish (Figure 2)
Mix two kinds of silicone solutions (3.6 ml KE-106 and 0.4 ml CAT-RG) in a 35-mm culture dish (or its lid) and incubate at 37 °C for 2 days to congeal silicone. This dissection dish may be used several times.
Figure 2. Dissection dish - Preparation of an observation tank (Figure 3)
- Put 2 pieces of plastic tape (20 x 25 mm) on a cutting board and cut out a square (10 x 10 mm) in the center of these tapes.
- Place the newly cut piece of tape on a cover slip (24 x 40 mm).
- Turn over the remaining piece of tape from step 2a.
- Put the tips of two insect pins on the adhesive surface of the tape and align the two pins at so that they are 2 mm apart (Figure 3).
- Put the tape with insect pins from step 2d on the tape attached to the cover glass as shown in Figure 3.
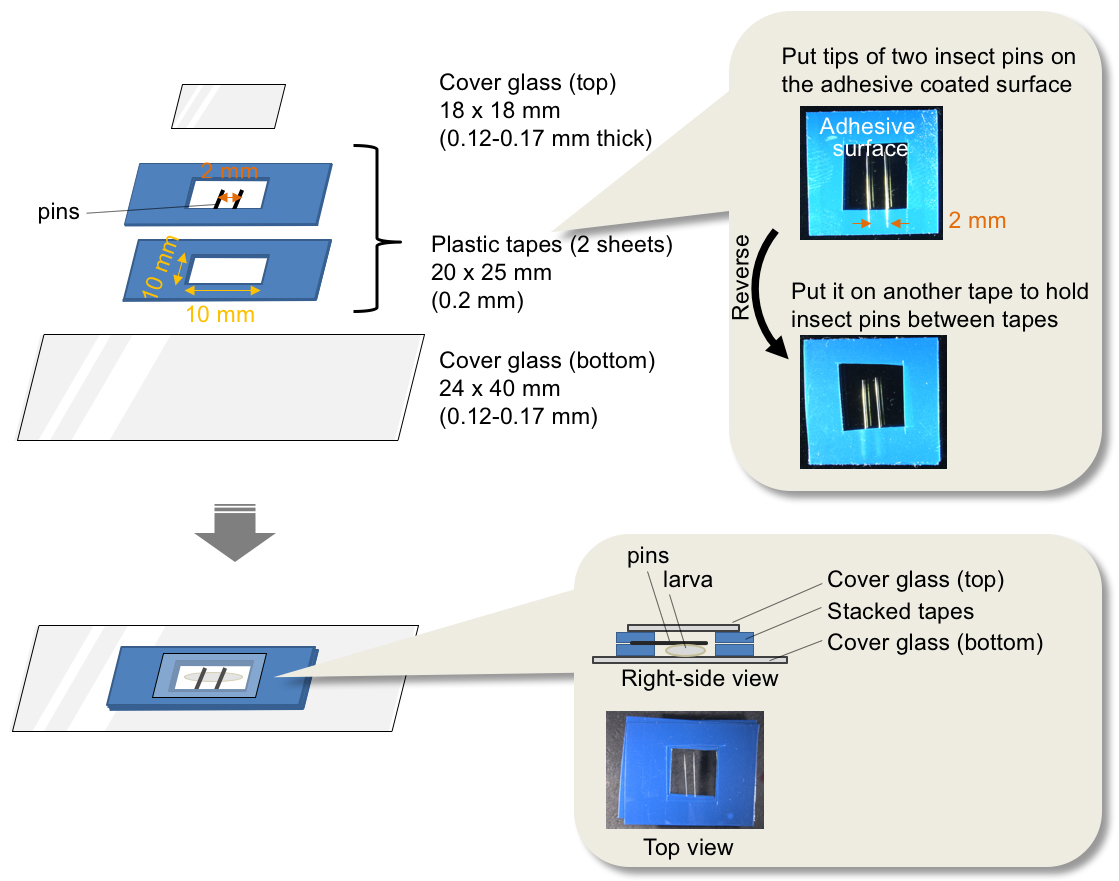
Figure 3. Assembly of an observation tank
- Put 2 pieces of plastic tape (20 x 25 mm) on a cutting board and cut out a square (10 x 10 mm) in the center of these tapes.
- Dissection of a larva (Figure 4)
Prepare a fillet of larva on the dissection dish according to the following steps (larval dissection protocol Brent et al., 2009) is slightly modified. We use HL-3 warmed to room temperature):- Pick up a third-instar larva and wash the body surface in HL-3 solution (see Recipes). Third-instar larvae (three days after hatching at 25 °C) stop feeding behavior and climb on wall of a vial, which is referred to as wandering behavior.
- Transfer the larva into 1 ml of HL-3 solution on the dissection dish.
- Hold both the front and tail edges of the larva using short insect pins (red arrowheads in Figure 4A), keeping its dorsal side upward so that the tracheae (white arrows in Figure 4A) can be seen through the body wall.
- Cut the body wall along the dorsal midline from the tail side (yellow dashed line in Figure 4B) to construct a fillet, holding the four corners of the body wall with short pins (red arrowheads in Figures 4C and 4D).
- Carefully remove the gut, fat bodies and salivary glands without injuring the ventral ganglion (orange arrows in Figures 4E and 4F), axons or body muscles. Fluorescence will be observed within the ventral ganglion (yellow dashed circles in Figures 4G and 4H) and axons (white arrowheads in Figures 4G and 4H) when using flies that express fluorescence markers in motor neurons. The green fluorescence signals in Figures 4F and 4H are derived from preproANF-EMD.
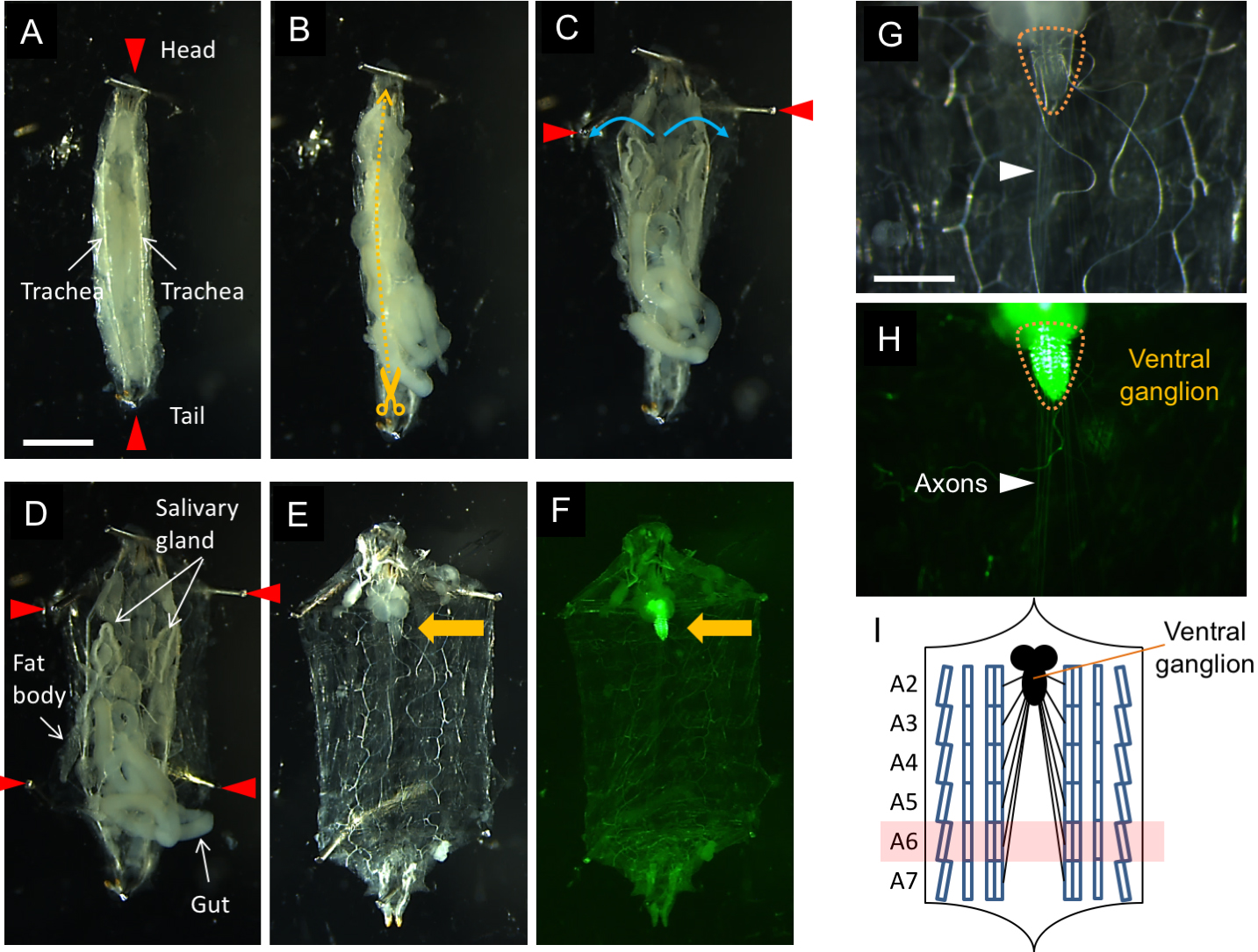 Figure 4. Dissection procedure for a larval fillet on a dissection dish. Scale bars = 1 mm for (A-F) and 5 µm for (G, H).
Figure 4. Dissection procedure for a larval fillet on a dissection dish. Scale bars = 1 mm for (A-F) and 5 µm for (G, H).
- Pick up a third-instar larva and wash the body surface in HL-3 solution (see Recipes). Third-instar larvae (three days after hatching at 25 °C) stop feeding behavior and climb on wall of a vial, which is referred to as wandering behavior.
- Placement of a larva in observation tank (Figure 5)
- Carefully remove the short pins from the larva and transfer the larva to the observation tank filled with HL-3.
- Extend the head and tail of the larva carefully and secure them with pins (epidermis side up, Figure 5).
Rinse by changing the HL-3 solution twice and put a cover glass (18 x 18 mm) to protect the larva against desiccation.
Note: There is no need to hold the cover glass with nail polish. An organic solvent in nail polish could affect the neuronal activity.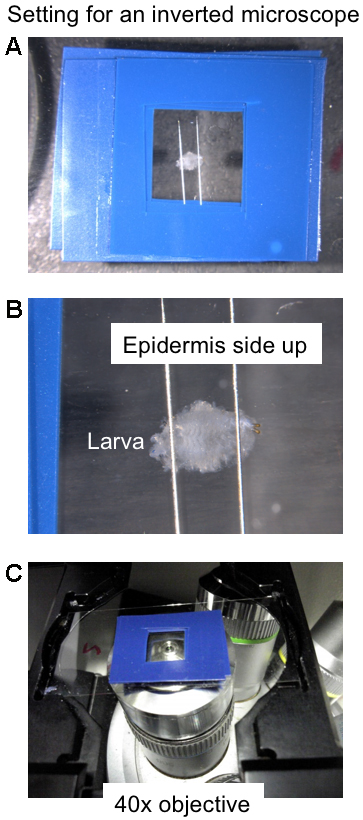
Figure 5. Setting for an inverted microscope. A. Top view of an observation tank where a larval fillet is retained. B. Enlarged top view of a larval fillet retained with two pins in an observation tank. C. Larval motor neuron axons are observed from below using a 40x objective.
- Carefully remove the short pins from the larva and transfer the larva to the observation tank filled with HL-3.
- Imaging
- Locate a motor neuron axon (Figure 6A) under an inverted fluorescence microscope. A laser-scanning microscope equipped with a high sensitivity detector is preferable.
- Capture live images every 0.1 sec for 30 sec under a 40x objective lens (Videos 1 and 2). To ensure that healthy neurons are imaged, it is important to complete the dissection within 5 min and subsequent observation within 5 min.
Note: We usually use motor neuron axons projecting to the A6 muscles (Figure 4I). See also Figure 1A in Matzat et al., 2015. Choosing specific axons and observation of the same axonal region may be important to evaluate transport property.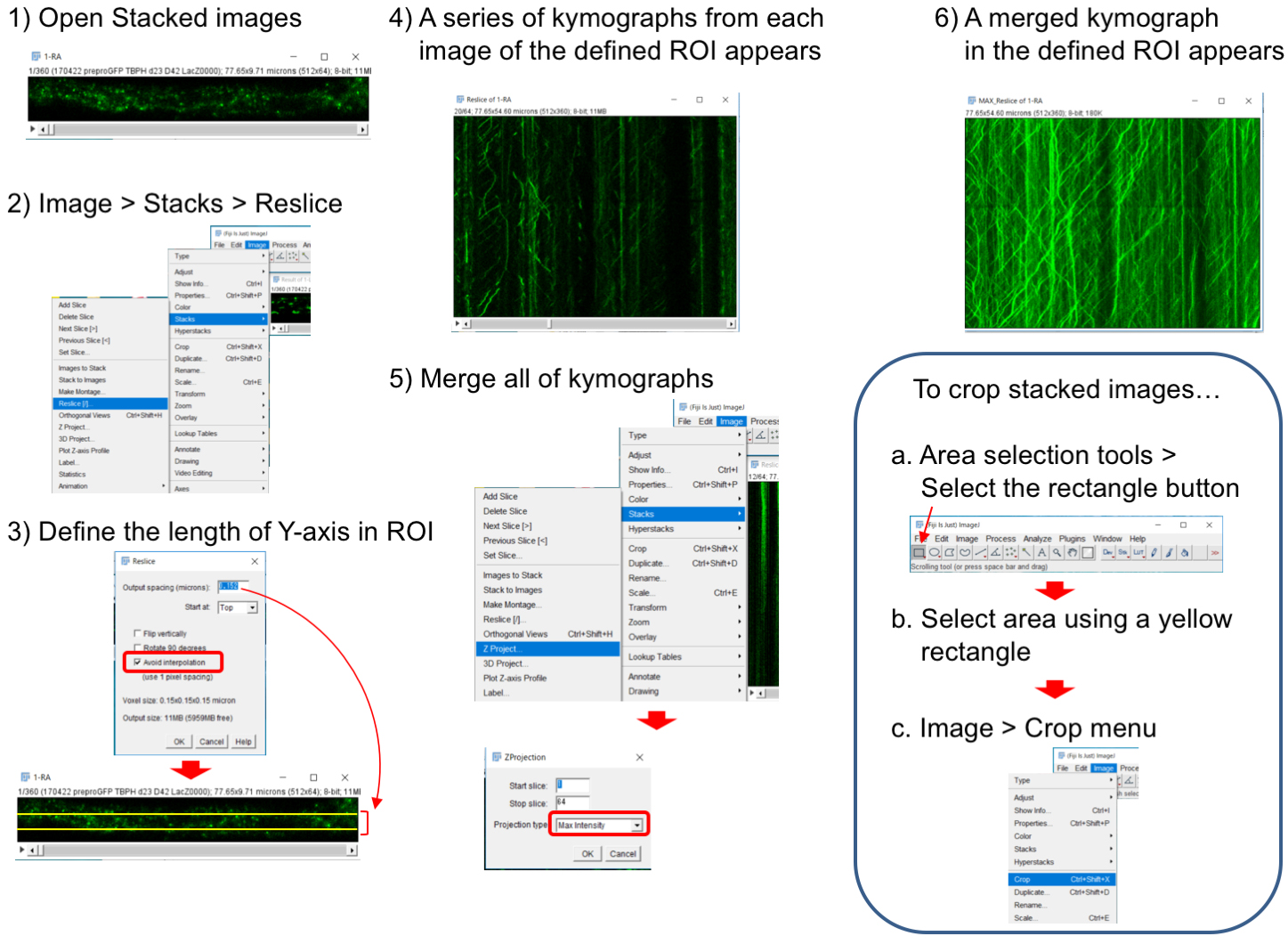
Figure 6. Generation of a kymograph from stacked images. 1) Open stacked images. To crop images, see an explanation in the box. 2) Select ‘Image > Stacks > Reslice’ menu. 3) Input an appropriate value in ‘Output spacing’ and check ‘Avoid interpolation’. ‘Output spacing’ defines the size of Y-axis ROI and we used 0.152. 4) Resultant resliced images represent kymographs of non-interpolated X-axis ROI (each pixel-line from top to bottom). 5) ‘Image > Stacks > Z-Projection’ menu merges all of stacked kymographs. Select ‘Max Intensity’ tab in the Z-Projection window. 6) Then, a merged kymograph appears.Video 1. A representative movie of axonal flow of the dense core vesicles in TBPH+/- flies. The genotype used is UAS-preproANF-EMD/+; TBPHΔ23/+; D42-Gal4/+.Video 2. A representative movie of axonal flow of the dense core vesicles in TBPH+/- flies expressing p150Glued. The genotype used is UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150Glued-HA; D42-Gal4/+.
- Locate a motor neuron axon (Figure 6A) under an inverted fluorescence microscope. A laser-scanning microscope equipped with a high sensitivity detector is preferable.
Data analysis
- Construct a kymograph using ImageJ software by using the ‘Image > Stacks > Reslice’ menu (Figure 6). The Reslice menu makes kymographs from each line ROI (X-axis ROI), which is set automatically on the stacked images. The resultant kymographs consist of stacked data for each line ROI. ‘Output spacing’ and ‘Avoid interpolation’ are parameters to define the size of Y-axis ROI. Because one line ROI cannot track vesicles snaking through the axon, appropriate setting of ‘Output spacing’ is required. ‘Z-Projection’ image from all stacked kymographs represents vesicle movements in the defined ROI during all observation time.
Note: To select a whole axon area, use Area selection tools as shown in the box of Figure 6. - ‘Image Calculator > Operation: Subtract’ menu can remove stationary vesicles from the kymograph (Figure 7).
- The kymographs obtained through the procedures in Figure 6 and Figure 7 indicate the overexpression of a dynein motor component p150Glued in TBPH+/- larvae inhibits axonal transport of dense core vesicles in both directions (Figure 8). Detailed data processing analysis and replicates including applied statistical tests could be found in the original manuscript, see Hosaka et al., 2017.
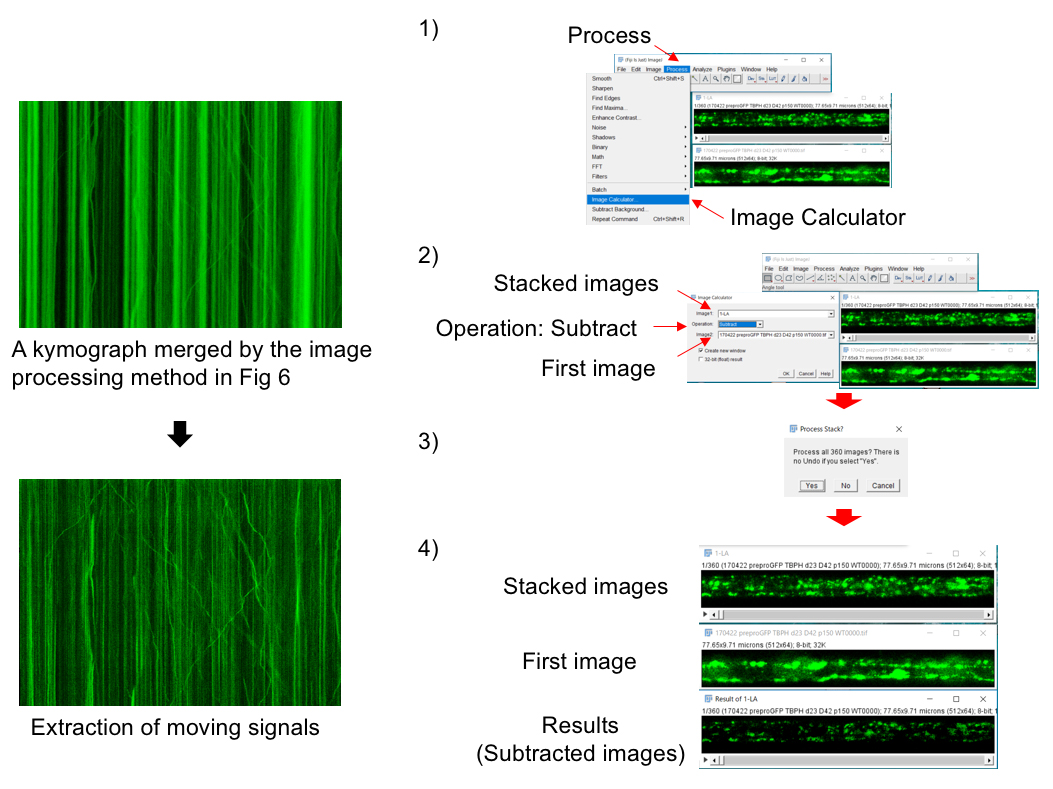
Figure 7. Image processing to extract moving vesicles. 1) Open stacked images and its first image. Select ‘Process > Image Calculator’ menu. Refer to the stacked images as Image1 and its first image as Image2. Select ‘Subtract’ tab in the ‘Image calculator’ window. 3) Signals in the stacked images are automatically subtracted from those in its first image. 4) The resultant images represent moving signals.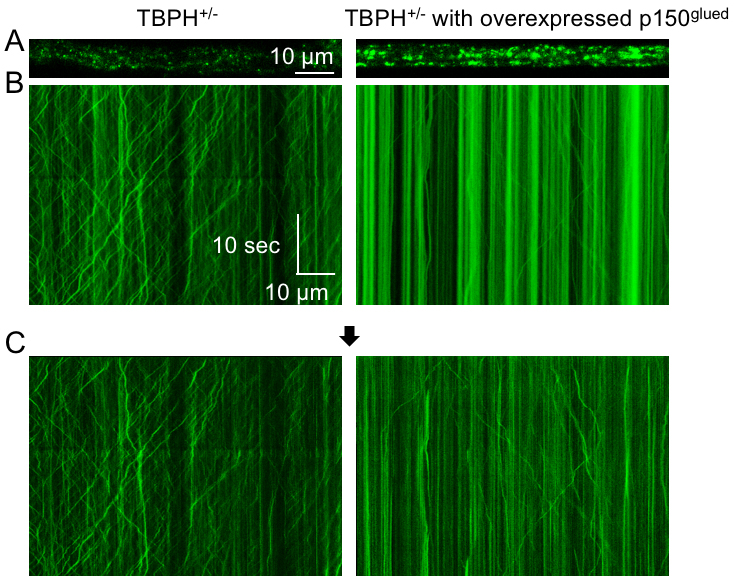
Figure 8. Typical imaging results of axonal transport. A. The dense core vesicles (green) in motor neuron axons of the indicated genotypes. B. Kymographs show motility of the dense core vesicles. The X-axis represents the position of the dense core vesicle, and the Y-axis is time (moving from top to bottom). Vertical lines correspond to stationary dense core vesicles, and diagonal lines are moving dense core vesicles. Anterograde movements have positive slopes, whereas retrograde movements have negative slopes. These kymographs were obtained by the methods in Figure 6. C. Resultant images after subtraction extract moving dense core vesicles as described in Figure 7.
Recipes
- Modified HL-3 solution (Stewart et al., 1994) containing the following:
70 mM NaCl
5 mM KCl
20 mM MgCl2
10 mM NaHCO3
115 mM sucrose
5 mM trehalose
5 mM HEPES (pH 7.2)
2 mM CaCl2 (mixed gently to avoid precipitation)
Note: The HL-3 solution should be stored at 4 °C and can be used within two weeks.
Acknowledgments
This study was funded by the Grant-in-Aid for Scientific Research (C, 16K09679 [T.I.]; B, 17H04049 [Y.I.]) from MEXT in Japan; additionally, partial support was obtained by a grant from Otsuka Pharmaceuticals (N.H. and Y.I.). This protocol is adapted from Hosaka et al., 2017. The authors declare no conflict of interest.
References
- Alami, N. H., Smith, R. B., Carrasco, M. A., Williams, L. A., Winborn, C. S., Han, S. S. W., Kiskinis, E., Winborn, B., Freibaum, B. D., Kanagaraj, A., Clare, A. J., Badders, N. M., Bilican, B., Chaum, E., Chandran, S., Shaw, C. E., Eggan, K. C., Maniatis, T. and Taylor, J. P. (2014). Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81(3): 536-543.
- Brent, J. R., Werner, K. M. and McCabe, B. D. (2009). Drosophila larval NMJ dissection. J Vis Exp 24: 1107.
- Hirokawa, N., Niwa, S. and Tanaka, Y. (2010). Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68(4): 610-638.
- Hosaka, Y., Inoshita, T., Shiba-Fukushima, K., Cui, C., Arano, T., Imai, Y. and Hattori, N. (2017). Reduced TDP-43 expression improves neuronal activities in a Drosophila model of Perry syndrome. EBioMedicine 21: 218-227.
- Liu, S., Sawada, T., Lee, S., Yu, W., Silverio, G., Alapatt, P., Millan, I., Shen, A., Saxton, W., Kanao, T., Takahashi, R., Hattori, N., Imai, Y. and Lu, B. (2012). Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet 8(3): e1002537.
- Matzat, T., Sieglitz, F., Kottmeier, R., Babatz, F., Engelen, D. and Klämbt, C. (2015). Axonal wrapping in the Drosophila PNS is controlled by glia-derived neuregulin homolog Vein. Development 142(7): 1336-1345.
- Millecamps, S. and Julien, J. P. (2013). Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 14(3): 161-176.
- Shiba-Fukushima, K., Inoshita, T., Hattori, N. and Imai, Y. (2014). PINK1-mediated phosphorylation of Parkin boosts Parkin activity in Drosophila. PLoS Genet 10(6): e1004391.
- Stewart, B. A., Atwood, H. L., Renger, J. J., Wang, J. and Wu, C. F. (1994). Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 175(2): 179-191.
- Wong, M. Y., Zhou, C., Shakiryanova, D., Lloyd, T. E., Deitcher, D. L. and Levitan, E. S. (2012). Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell 148(5): 1029-1038.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Inoshita, T., Hattori, N. and Imai, Y. (2017). Live Imaging of Axonal Transport in the Motor Neurons of Drosophila Larvae. Bio-protocol 7(23): e2631. DOI: 10.21769/BioProtoc.2631.
Category
Neuroscience > Nervous system disorders > Animal model
Neuroscience > Cellular mechanisms > Intracellular signalling
Cell Biology > Cell imaging > Live-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link


 2.jpg)








