- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Instillation of Particulate Suspensions to the Lungs
Published: Vol 7, Iss 22, Nov 20, 2017 DOI: 10.21769/BioProtoc.2618 Views: 8488
Reviewed by: Ivan ZanoniAchille BroggiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2540 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Inhaled fine particulates are thought to cause chronic pulmonary inflammation through the deposition of particulates into the lungs. To investigate the effect of fine particulates on the lungs, instillation of suspension of particulates into the lungs is required. This protocol describes direct injection of suspensions of fine particulates into the airway. We also show examples of typical lung immune responses after particulate administration.
Keywords: ParticulatesBackground
Recently, many studies have demonstrated that particulate pollutants such as diesel exhaust particles, sand dusts and particulate matter 2.5 (PM 2.5), are involved in chronic pulmonary inflammation leading to lung cancer or allergic asthma. Epidemiological analysis revealed that increased particulate air pollution is related to increased asthma hospitalization. In general, upon inhalation, fine particles, such as PM 2.5, are known to reach deep into the lungs. Instillation of suspensions of particulates into the lungs has been widely used for understanding pulmonary inflammation induced by deposited particulates (Morimoto et al., 2016).
Materials and Reagents
- Pipet tip for gel loading (Vertex-GL 200 μl gel-loading tip) (SSIbio, catalog number: 4837-S0S )
- Parafilm
- 1 ml sterile syringe (without needle) (TERUMO, catalog number: SS-01T )
- Mice (C57BL/6, BALB/c etc.)
Note: For training, bigger mice (aged male mice) are better.
- Alhydrogel (InvivoGen, catalog number: vac-alu-250 ) as particulate for instillation
Note: Alhydrogel (alum) is suspended in dH2O. For instillation of alum into the lungs, buffer exchange is required. Centrifuge a suspension of alum in a microtube at 2,000 x g for 2 min. Discard supernatant (H2O) and add an equal volume of saline or PBS. Mix well and centrifuge again. Repeat this procedure five times to exchange H2O to saline or PBS. Finally, adjust the concentration of alum to 2 mg/ml in saline and use for instillation.
- Anesthetic (ketamine/xylazine mixture)
Note: 10 ml of Ketalar (Ketamine, 50 mg/ml, Daiichi Sankyo Co. Ltd., Tokyo, Japan) is mixed with 2.2 ml of Selactar (Xylazine, 20 mg/ml, Bayer HealthCare Ltd., Tokyo, Japan). Anesthetize mice with 50 to 75 μl of ketamine/xylazine mixture by s.c. injection into the back.
Equipment
- Ear pick earwax remover with light (Japan Smile Kids)
Note: Before use, silicone rubber at the tip of ear pick will be removed (Figure 1).
- Stainless steel micro spatula (Figure 1) (ASONE, catalog number: 9-891-02 )
Note: This is used as a tongue depressor, so small size is better.

Figure 1. Ear puck with light and spatula
- Platform for instillation into the lungs. As shown in Figure 2, stretch a string across the wooden (cork) board (approximate size: 20 x 30 cm)

Figure 2. Platform for instillation into the lungs
- Injector for instillation (Figure 3)
- FACS analyzer
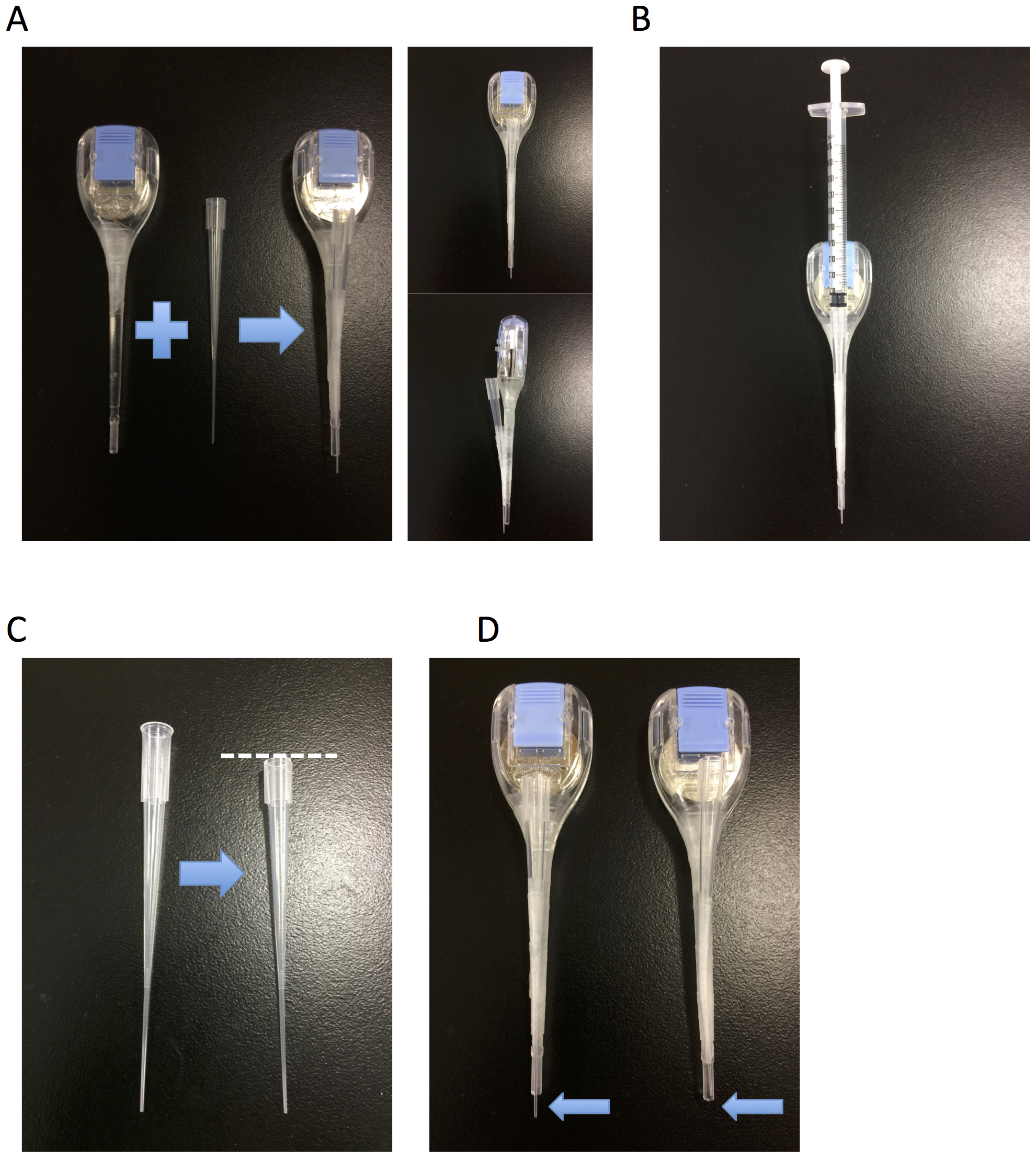
Figure 3. Injector for instillation. A. Fix a thin pipet tip to the ear pick by Parafilm. B. Attach a syringe to the pipet tip. C. If needed, cut the top of pipet tip to adjust for syringe insertion. D. Cut the edge of the pipet tip (arrow) for injection.
Procedure
- As shown in Figure 4A, stand up the platform for instillation on the bench.
- Anesthetize mice with 50 to 75 μl of ketamine/xylazine mixture by s.c. injection into the back.
- As shown in Figure 4B, hang anesthetized mice on the platform by their incisors.
- Take 50 μl of prepared alum suspension into an injector for instillation. In general, 50 μl of suspension (containing 50-100 μg of alum or 50-500 μg other particulates you want to inject, such as silica or nickel oxide) is the maximum volume for injection into the lungs.
- As shown in Figure 4C, use a spatula as a tongue depressor and insert injector into the throat, check the trachea (in order to find the trachea easily, put out mouse’s tongue using spatula and injector, first). It is difficult to check the trachea when young mice are used for the experiment. Because aged male mice are bigger, they are easier to find the trachea especially for training. Then, slowly drop suspensions of alum toward trachea. Dropped suspensions are aspirated into the lungs by breathing (aspiration method).
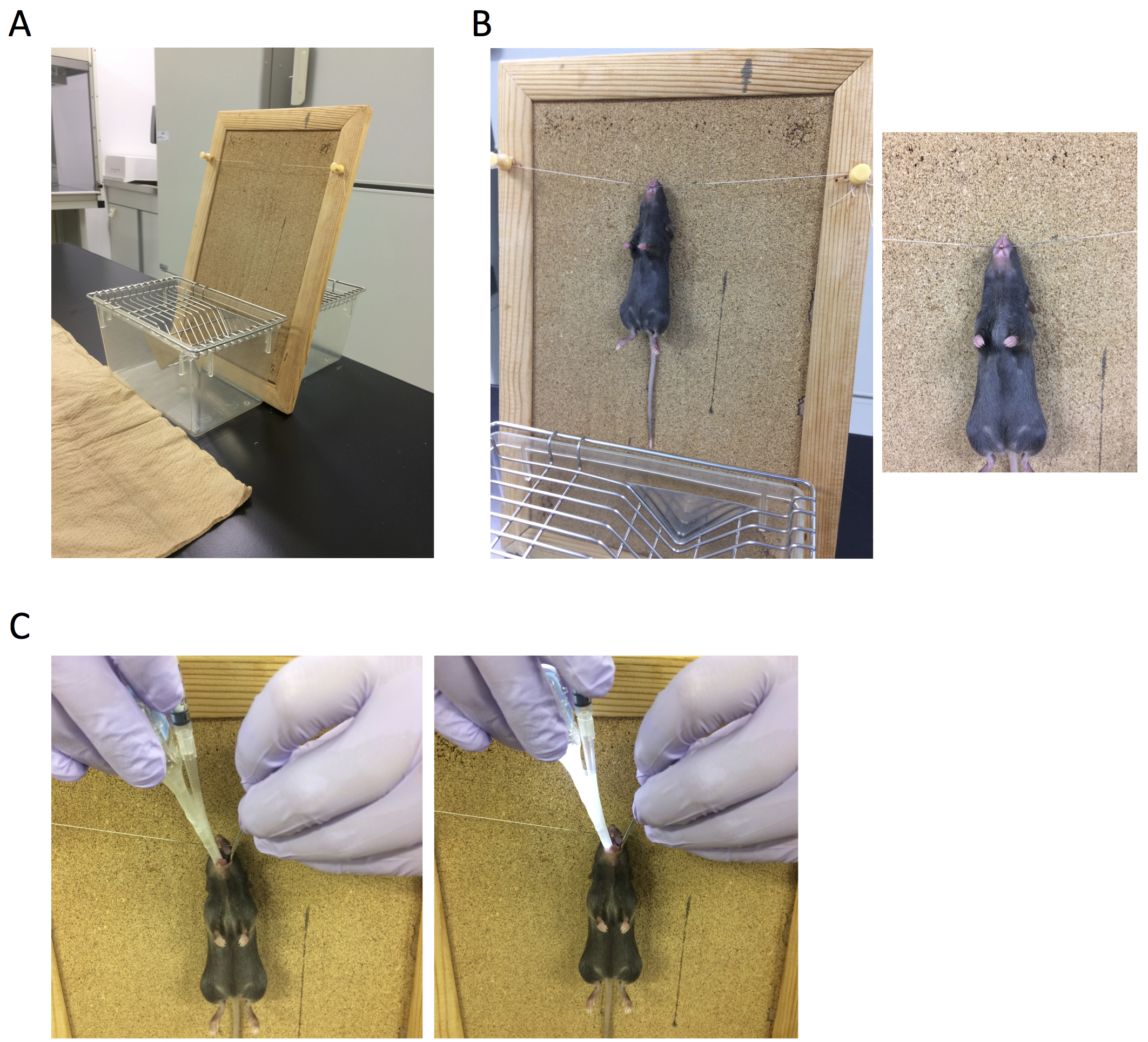
Figure 4. Administration into the lungs
- Intranasal administration (i.n.) is also effective for administration into the lung. As shown in Figure 5, hold anesthetized mice and drop 30 μl (15 μl in each nostril) of suspensions of particulates into the nose. Dropped suspensions are also aspirated by breathing. In general, it is known that i.n. administration of 30 μl of solution reaches the lung.
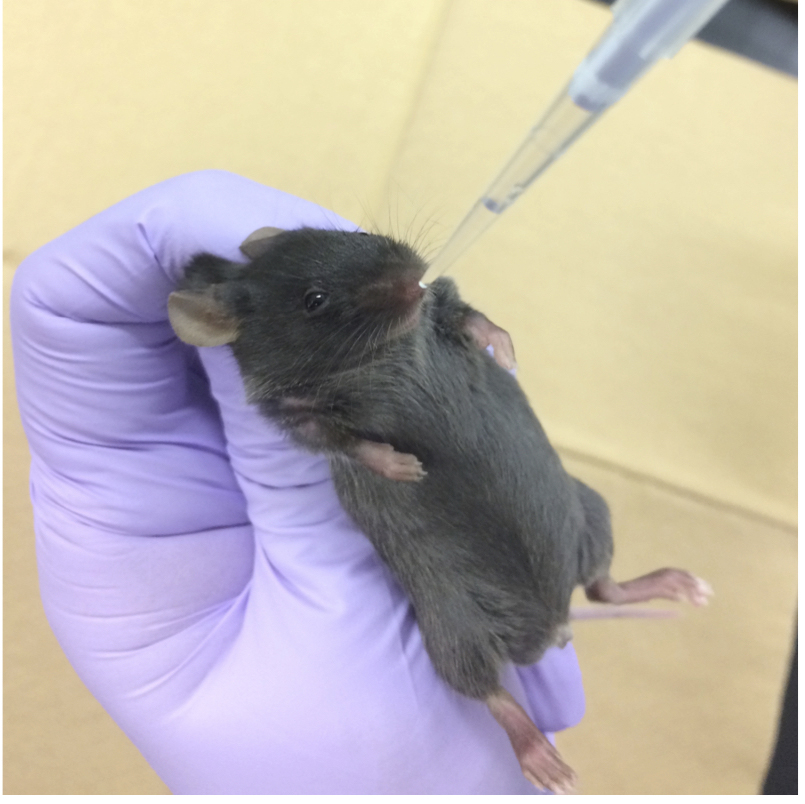
Figure 5. Intranasal administration
Data analysis
- As shown in Figure 6, we examined the efficacy of administration into the lungs between i.n. administration and the aspiration method, using black ink. Lungs were only partially stained by i.n. administration. On the other hand, lungs were more diffusely stained by the aspiration method, indicating that this method is more effective for administration into the lung.
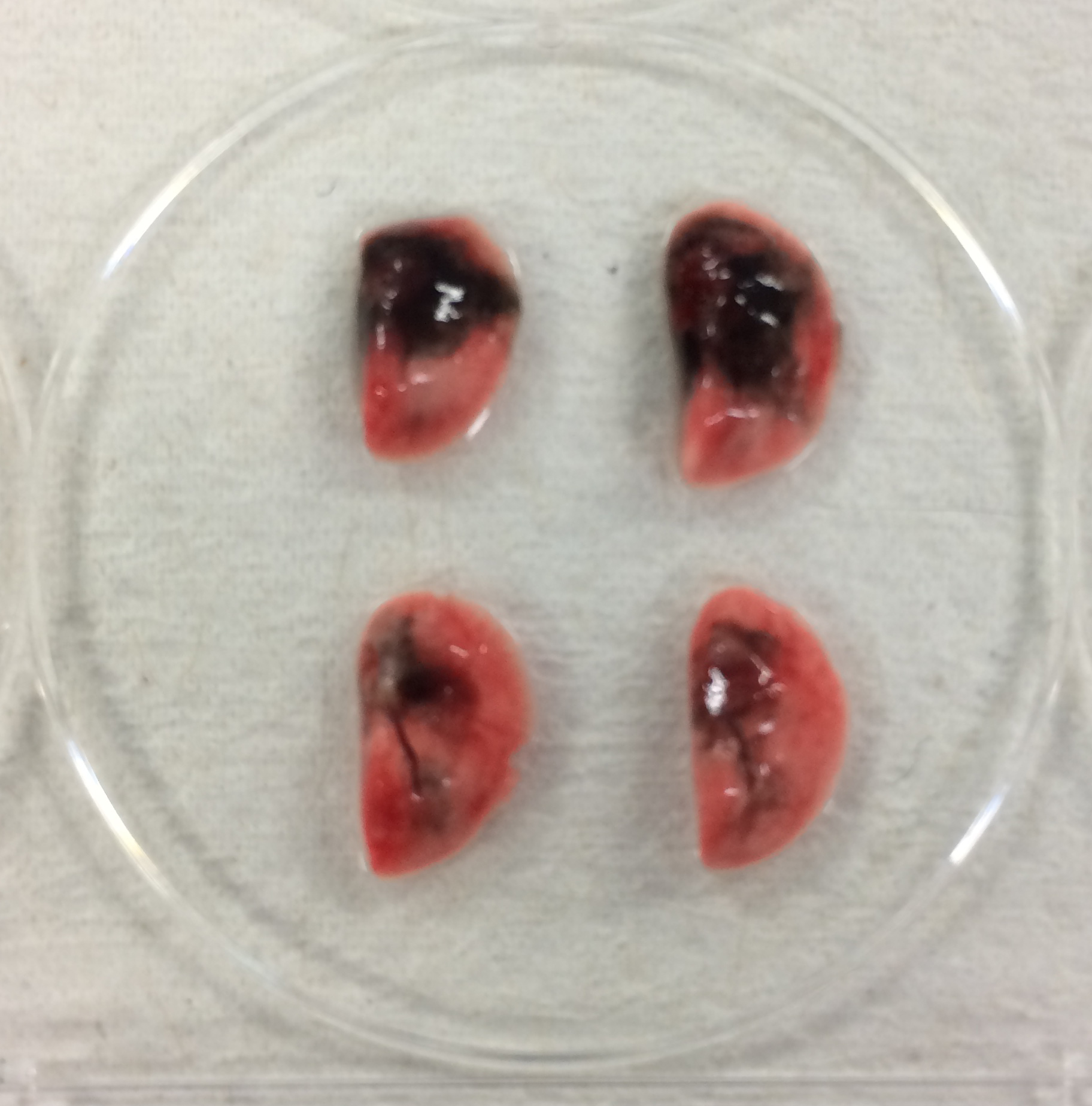
Figure 6. Comparison of efficacy of intranasal administration and aspiration method. Upper two samples are lungs from mice administered black ink by aspiration. Lower samples are those administrated by intranasal administration.
- A change in the number of alveolar macrophages 3 days after administration of alum into the lungs were observed. Alum is known to induce cell death by phagocytosis in macrophages. As shown in Figure 7, the percentage of alveolar macrophages (CD11c+ and siglec F+ cells) in bronchoalveolar-lavage fluid (BALF) were significantly decreased after the direct administration of alum into the lungs.
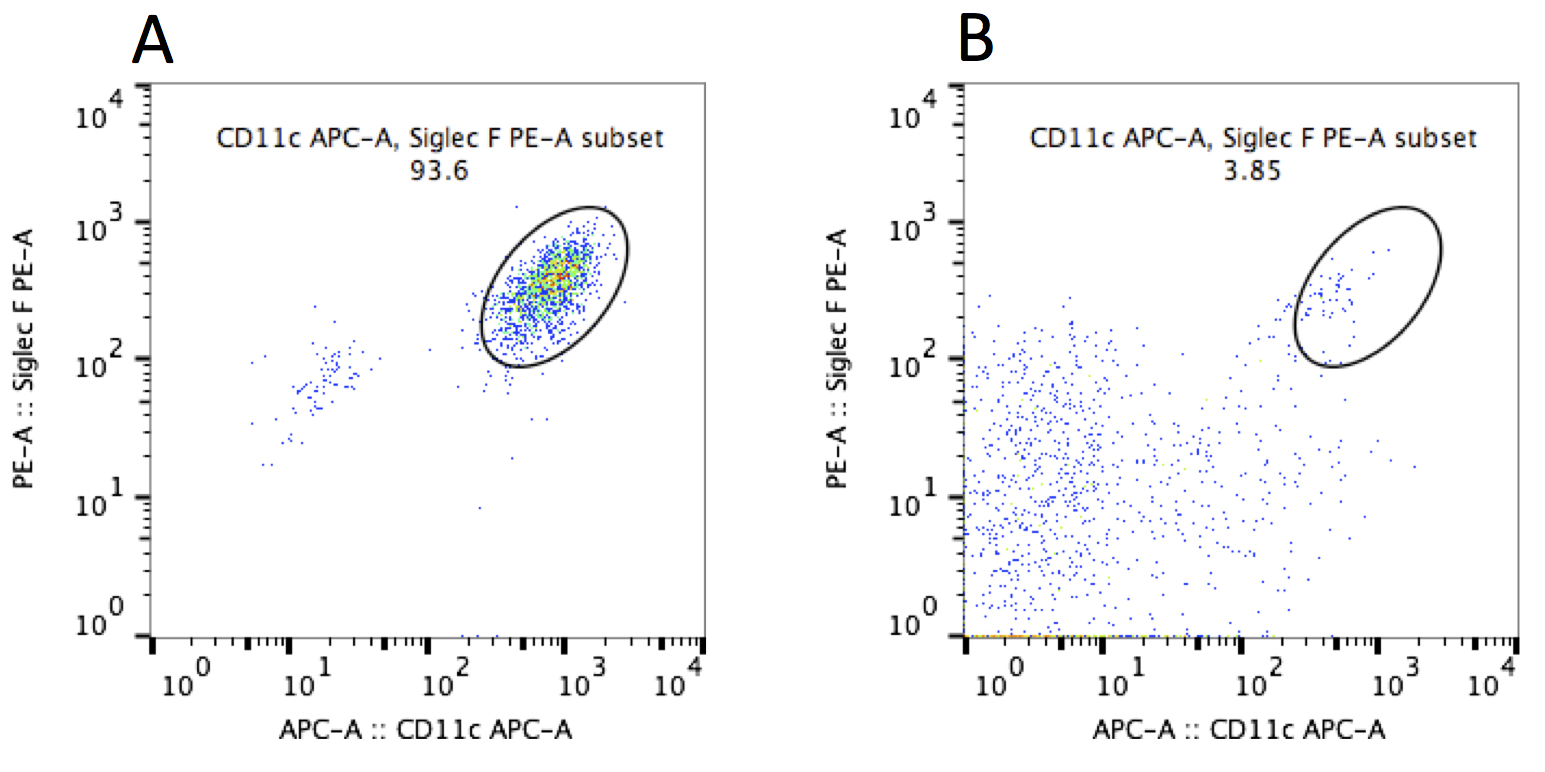
Figure 7. FACS analysis of BAL cells from saline- or alum-administered mice. BAL cells were collected and stained by anti-CD11c and anti-Siglec F antibodies (Leukocytes in BAL cells were gated as CD45+ cells). A. BAL cells from saline-administered mice. B. BAL cells from alum-administered mice. This data is representative of at least two independent experiments (n = 4-5).
Notes
- Similar experiments can be performed for other particulates such as crystalline silica and nickel oxide nanoparticles (Kuroda et al., 2016).
- Suspensions more than 50 μl volumes sometimes choke mice to death. If mice often die by suffocation after administration of 50 μl of suspensions, administration of 30 μl of solution would be safer than administration of 50 μl. However we recommend 50 μl volumes for administration of solutions into the lungs, because of less variation among samples.
Acknowledgments
We thank Dr. Patrick Lelliotte and Burcu Temizoz for helpful discussion. Authors have no conflict of interest to declare.
References
- Kuroda, E., Ozasa, K., Temizoz, B., Ohata, K., Koo, C. X., Kanuma, T., Kusakabe, T., Kobari, S., Horie, M., Morimoto, Y., Nakajima, S., Kabashima, K., Ziegler, S. F., Iwakura, Y., Ise, W., Kurosaki, T., Nagatake, T., Kunisawa, J., Takemura, N., Uematsu, S., Hayashi, M., Aoshi, T., Kobiyama, K., Coban, C. and Ishii, K. J. (2016). Inhaled fine particles induce alveolar macrophage death and interleukin-1α release to promote inducible bronchus-associated lymphoid tissue formation. Immunity 45(6): 1299-1310.
- Morimoto, Y., Izumi, H., Yoshiura, Y., Tomonaga, T., Lee, B. W., Okada, T., Oyabu, T., Myojo, T., Kawai, K., Yatera, K., Shimada, M., Kubo, M., Yamamoto, K., Kitajima, S., Kuroda, E., Horie, M., Kawaguchi, K. and Sasaki, T. (2016). Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicology 10(5): 607-618.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kuroda, E., Morimoto, Y. and Ishii, K. J. (2017). Instillation of Particulate Suspensions to the Lungs. Bio-protocol 7(22): e2618. DOI: 10.21769/BioProtoc.2618.
Category
Immunology > Animal model > Mouse
Cell Biology > Tissue analysis > Physiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link