- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Rodent Brain Vessels
Published: Vol 7, Iss 17, Sep 5, 2017 DOI: 10.21769/BioProtoc.2535 Views: 9403
Reviewed by: Pasquale PellegriniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3758 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2627 Views
Abstract
The prevalence of neurodegenerative diseases is increasing worldwide. Cerebrovascular disorders and/or conditions known to affect brain vasculature, such as diabetes, are well-known risk factors for neurodegenerative diseases. Thus, the evaluation of the brain vasculature is of great importance to better understand the mechanisms underlying brain damage. We established a protocol for the isolation of brain vessels from rodents. This is a simple, non-enzymatic isolation protocol that allows us to perform comparative studies in different animal models of disease, helping understand the impact of several pathological conditions on brain vasculature and how those alterations predispose to neurodegenerative conditions.
Keywords: Blood-brain barrierBackground
The brain is highly dependent on a constant supply of oxygen and nutrients that arrive through a vast network of blood vessels. The blood-brain barrier (BBB), mainly composed of microvascular endothelial cells that line cerebral microvessels along with periendothelial structures, which include pericytes, astrocytes and a basement membrane (Saraiva et al., 2016; Librizzi et al., 2017), guarantees the control of an homeostatic environment, necessary to maintain the health of brain cells. Thus, the study of how certain pathologies that can interfere with the integrity of cerebrovasculature is of great importance. Indeed, strong evidence from clinical, imaging, epidemiological and neuropathological studies confirmed over the past two decades that the presence of cerebrovascular disease has a pivotal role in Alzheimer disease (AD) and other dementias associated with aging (Chui et al., 2006; Schneider et al., 2007; Gorelick et al., 2011; Wharton et al., 2011; Yarchoan et al., 2012; Bennett et al., 2013; DeCarli, 2013; Toledo et al., 2013; Yates et al., 2014). Besides the low number of papers dedicated to the study of isolated brain vessels, the isolation protocols used in those studies present some inconsistencies rendering difficult the comparison and interpretation of the published observations. With this protocol, we intend to offer a standardized procedure to help researchers working in this field. This protocol was adapted from a previous protocol described by McNeill et al. (1999) and used in our laboratory to isolate total (arterial and venous) brain vessels from rodents (Figures 1 and 4) (Carvalho et al., 2010; Carvalho et al., 2013; Plácido et al., 2017).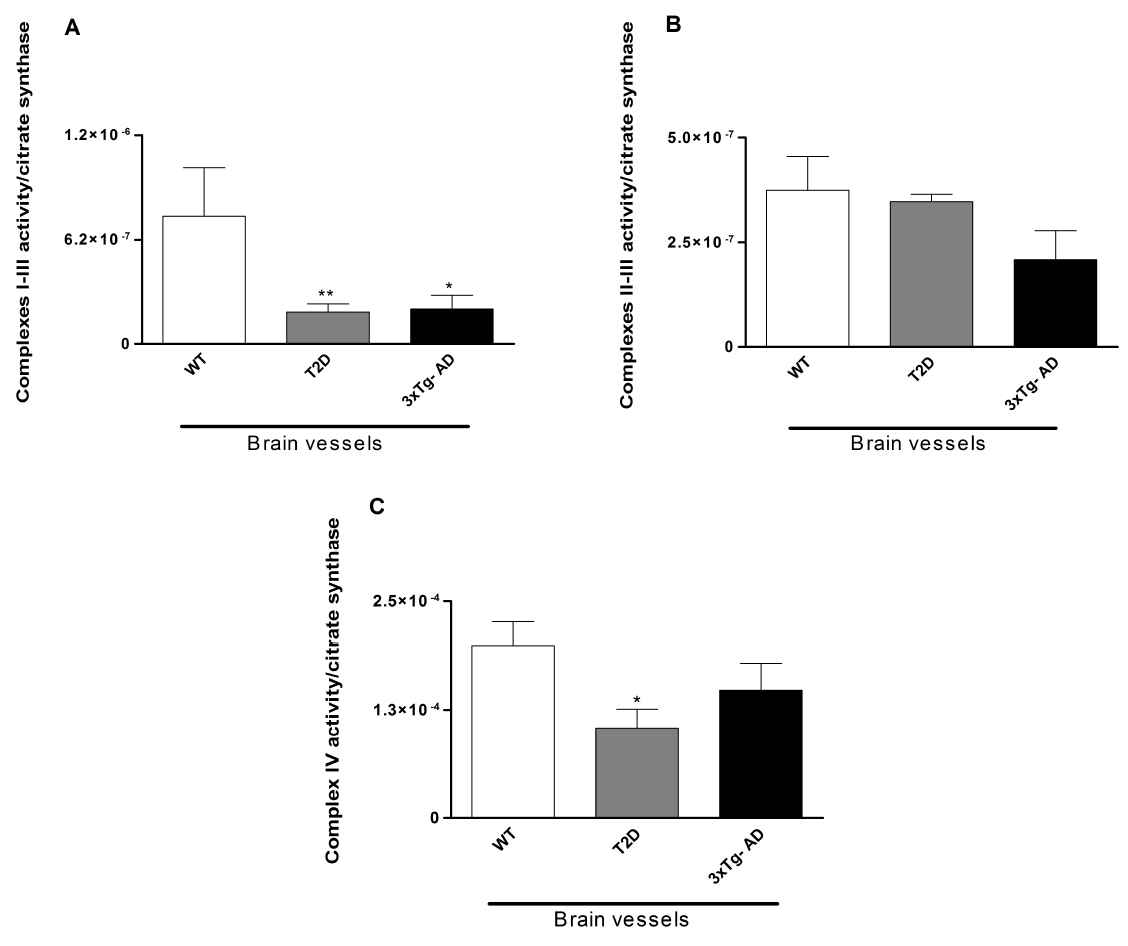
Figure 1. Evaluation of the activity of the mitochondrial enzymatic complexes of mice brain vessels. Mitochondrial complexes I-III (A), II-III (B) and IV (C) were determined in vessels isolated from the brains of 11-month-old male wild type (WT; C57BL6/129S), type 2 diabetes-like mice (WT mice exposed to 20% sucrose solution during 7 months) and triple transgenic mice for Alzheimer disease (3xTg-AD, B6;129-Psen1 Tg(APPSwe,tauP301L)1Lfa/Mmjax). A significant decrease in the activity of mitochondrial complexes I-III was observed in brain vessels isolated from 3xTg-AD and type 2 diabetes-like mice. Also, a significant decrease in the activity of complex IV was observed in brain vessels isolated from 3xTg-AD mice. Data shown represent mean ± SEM from 6-8 pools of n = 3. Statistical significance: *P < 0.05; **P < 0.01 when compared with WT mice. Statistical significance was determined using the paired Student’s t-test and Kruskal-Wallis test for multiple comparisons, followed by the posthoc Dunn test (GraphPad Prism 5). These graphs have been previously published in Journal of Alzheimer’s Disease (DOI: 10.3233/JAD-130005) with permission from IOS Press.
Materials and Reagents
- 50 ml Oak Ridge polysulfone centrifuge tubes w/screw caps (Thermo Fisher Scientific, catalog number: 3115-0050 )
- Eppendorf microtubes 1.5 ml (VWR, catalog number: 700-5239 )
- PIPETMAN TIPS Diamond–ECOPACKTM D1000 (Gilson, catalog number: F161670 )
- PIPETMAN TIPS Diamond–ECOPACKTM D200 (Gilson, Catalog number: F161930 )
- PIPETMAN TIPS Diamond–ECOPACKTM D10 (Gilson, catalog number: F161630 )
- Stainless steel surgical blades (Swann Morton, catalog number: 0308 )
- Rodent brain
Note: This protocol has only been tested with brains from male young and mature (3- and 12-month-old) Wistar rats and wild type, type 2 diabetes-like and triple transgenic for Alzheimer disease (3xTg-AD) mice (11-month-old). Nevertheless, we believe that this protocol can be applied to different strains, ages and sex, though the amount of obtained sample can be a limiting factor. - Ice
- Distilled water
- Isoflurane (Lab. Vitória, Portugal)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S7907 )
- Dextran from Leuconostoc mesenteroides (Sigma-Aldrich, catalog number: 31398 )
- Phosphate buffer (0.01 M) (see Recipes)
- Dextran (16%) (see Recipes)
Equipment
- 50 ml FisherbrandTM reusable glass low-form Griffin beakers (Fisher Scientific, catalog number: FB10050 )
- Bone cutting forceps (Aesculap, catalog number: FO611R )
- Centrifuge (Refrigerated Centrifuge) (Sigma Laborzentrifugen, model: SIGMA 3-16K )
- Curved fine tip forceps (Aesculap, catalog number: FB401R )
- Heidolph mechanical overhead stirrers, Brinkmann (Heidolph Instruments, model: RZR 1 )
- Laboratory bottles, narrow mouth, with screw cap (VWR, catalog number: 215-1594 )
- Micropipette PIPETMAN L (Light) type P200L (Gilson, catalog number: FA10005 )
- Micropipette PIPETMAN L (Light) type P1000L (Gilson, catalog number: FA10006 )
- Nickel/SS Lab spatulas with 1.63” Flat Rounded Ends (Cole-Parmer, catalog number: EW-06369-05 )
- Petri dish with cover 60 x 15 mm (Corning, catalog number: 70165-60 )
- Potter-Elvehjem with PTFE pestle and glass tube (DWK Life Sciences, Kimble®, catalog number: 886000-0023 )
- Precision scale (Mettler-Toledo International, model: AE240 )
- Precision balance PLE-N (KERN, model: PLE-N )
- Scalpel handle (Aesculap, catalog number: BB084R stainless)
- Soft hair brush, 3 mm dia. (CONTROLS, catalog number: 86-D1672 )
- Swing-out rotor (Sigma Laborzentrifugen, catalog number: 11133 )
Procedure
All the isolation protocol steps must be performed at 4 °C (always maintain solutions, homogenates and pellets on ice).
- Euthanize rodents by cervical displacement (mice) and decapitation (rats and mice). All procedures are approved by the Federation of Laboratory Animal Science Associations (FELASA).
- Perform a midline incision, posterior to anterior, along the scalp to reveal the skull (Video 1).Video 1. Brain removal, dissection and homogenization
- Cut the cranium carefully from the neck to the nose through the lateral cranial sutures. Two additional cuts can be performed, in the occipital hole to the ears direction, to facilitate the access to the brain (Figure 2A, Video 1).
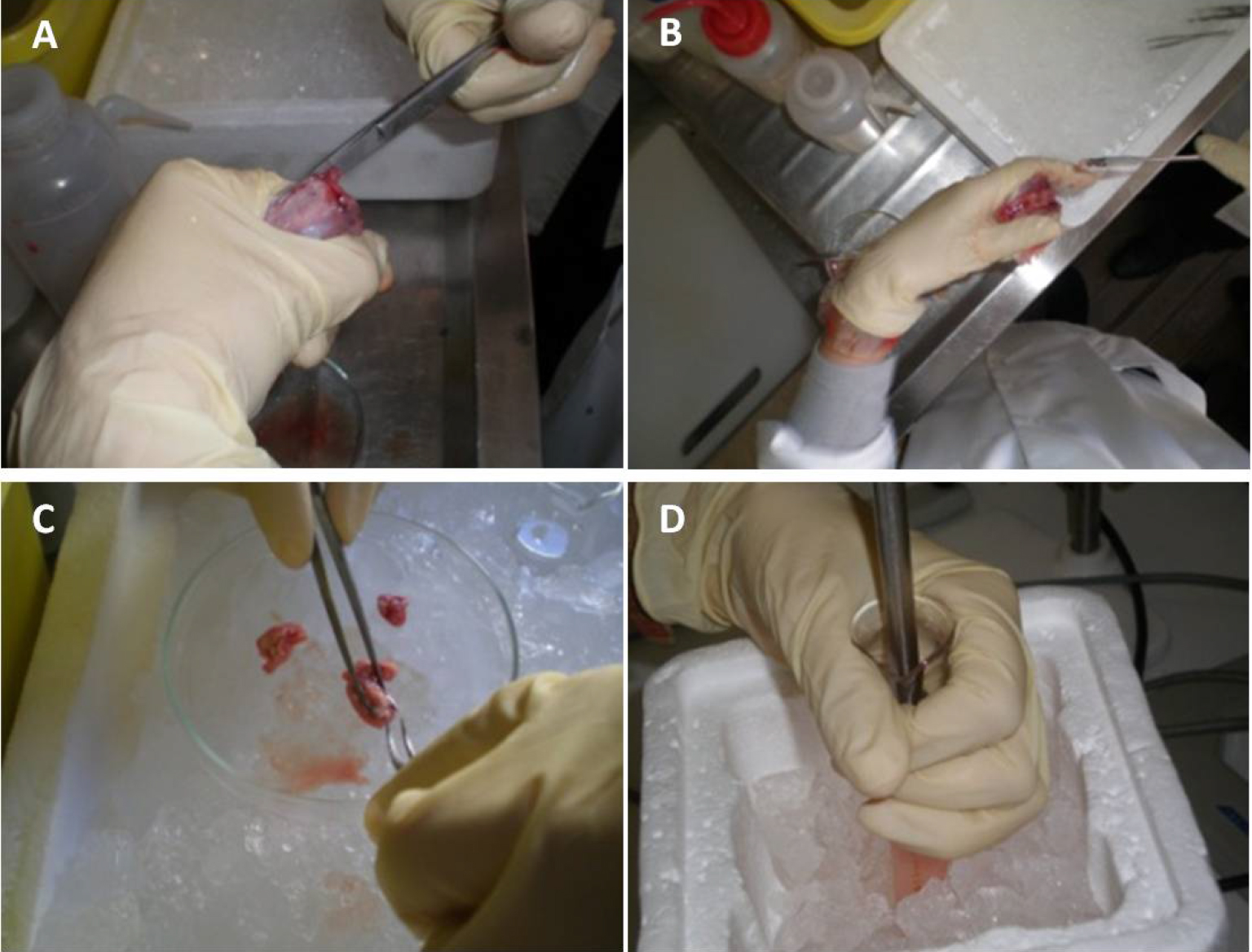
Figure 2. Illustrative images of brain dissection and homogenization. Cut the cranium carefully from the neck to the nose through the lateral cranial sutures (A). Remove the intact brain from the cranial box using a small spatula (B). Then, remove the cerebellum, olfactory bulbs and white matter using fine dissection forceps and keep the brain cortices (C). Finally, homogenize brain tissue, at 4 °C, in 10 ml PBS using a Potter-Elvehjem with PTFE pestle and glass tube, at 800 rpm/min (D). - Remove the intact brain from the cranial box using a small spatula (Figure 2B, Video 1).
- Remove the cerebellum, olfactory bulbs and white matter using fine dissection forceps; keep the brain cortices (Figure 2C, Video 1, see Note 1).
- Perform a quick wash (approximately 30 sec) in non-sterile PBS (Video 1; see Recipes).
- Homogenize fresh brain tissue, at 4 °C, in 10 ml PBS using a Potter-Elvehjem with PTFE pestle and glass tube, at 800 rpm/min (Figure 3, Video 1).
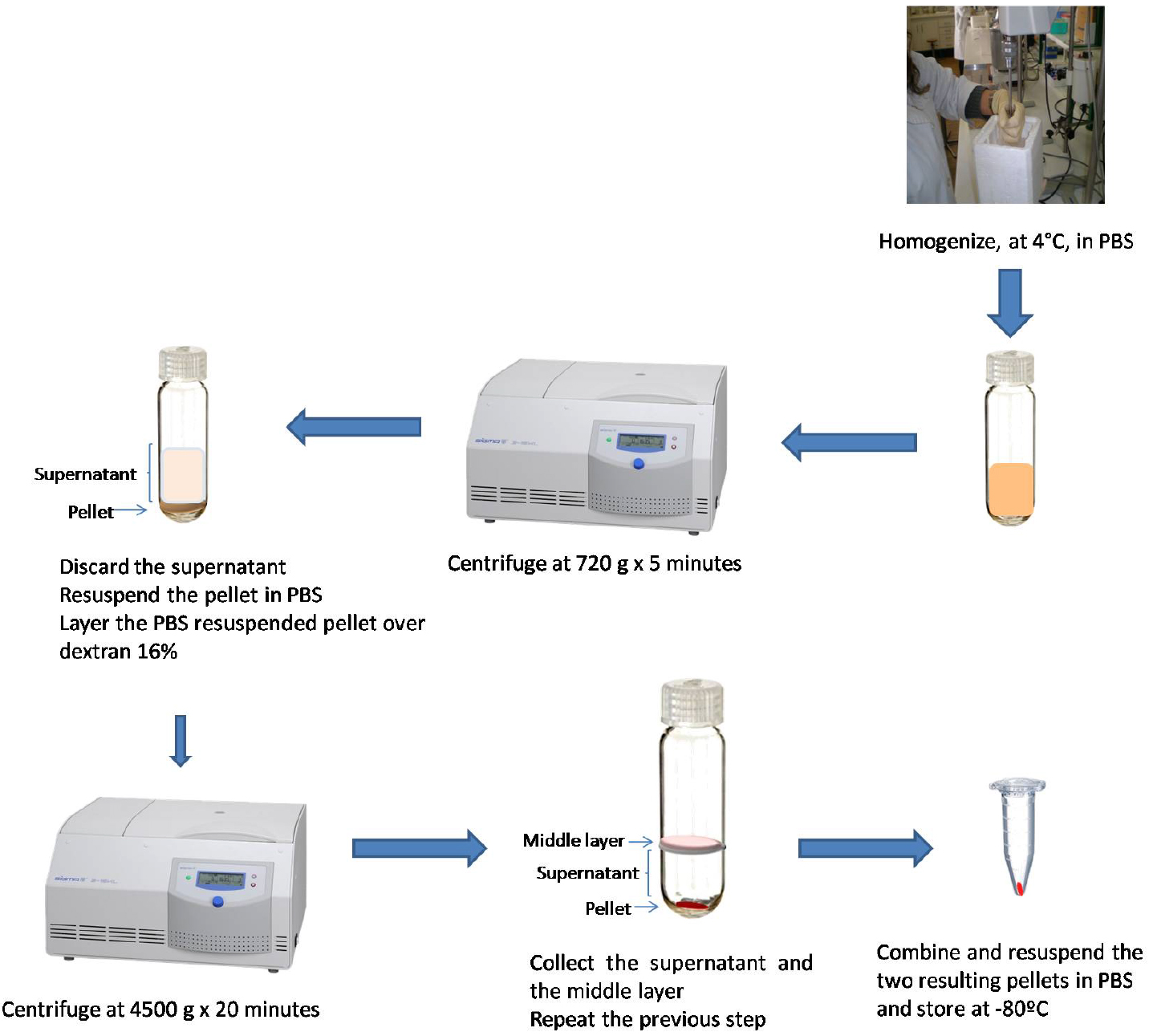
Figure 3. Schematic illustration of mice brain vessels isolation - Centrifuge at 720 x g for 5 min, at 4 °C (Figure 3).
- Discard the supernatant by decantation (Figure 3).
- Resuspend the pellet in 10 ml PBS using a soft hair brush, 3 mm dia (Figure 3).
- Repeat the steps 8-10 two more times.
- Layer the PBS resuspended pellet over 20 ml 16% dextran (Video 2; see Recipes).
Note: This step must be gently done to avoid mixing the two layers (Figure 3).Video 2. Brain vessels isolation - Centrifuge at 4,500 x g for 20 min, at 4 °C (Figure 2).
- Collect the supernatant and the middle layer, and keep the pellet on ice (Figure 3).
- Repeat step 14.
- Discard the supernatant and resuspend the two resulting pellets in 100 μl PBS.
Note: Resuspend the first pellet in 100 μl PBS and use the suspension to resuspend the second pellet. The vessels can be visualized at optic microscope (Figure 4) using the DiffQuick staining as described by Mota and Ramalho-Santos (2006). - Store the vessels at -80 °C until use (used until 2 years upon isolation; Figure 3).
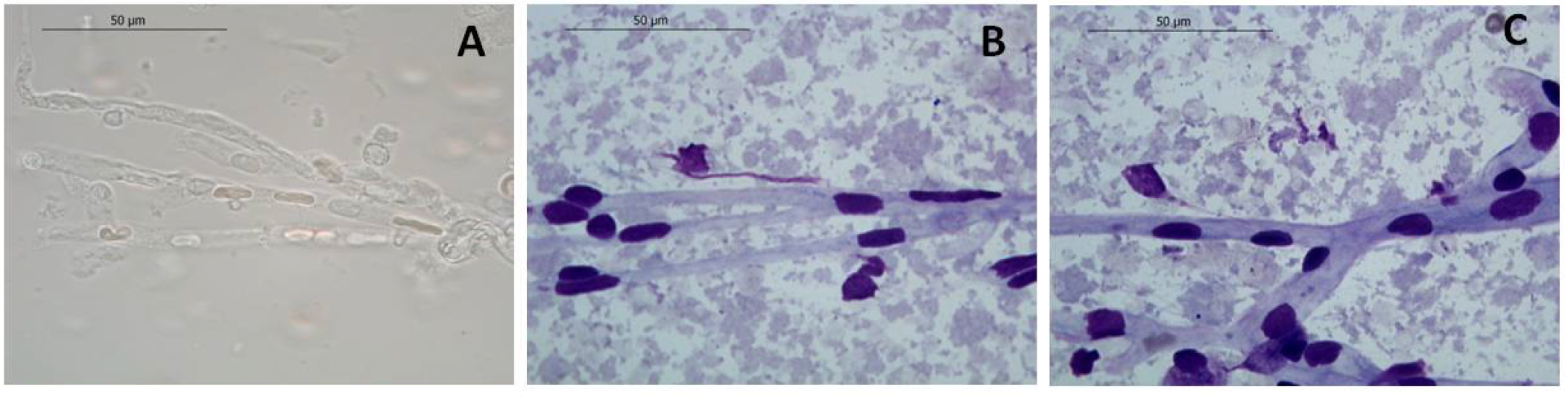
Figure 4. Representative images of isolated mice brain vessels after DiffQuik staining. Unstained (A) and stained (B, C) brain vessels observed under the optic microscope. 100x amplification; scale bars = 50 μM.
Notes
- In order to minimize the day-to-day variability that can bias the results, in studies using animal models of disease, researchers must always isolate vessels from control and diseased animal models.
- Minimum number of animals required for the isolation of brain vessels: 1 rat brain or 3 mice brains. It is possible to use only 1 mice brain. However, the pellet size will be very small, which will difficult its visualization with the naked eye.
Recipes
- Phosphate buffer (0.01 M)
8.5 g/L NaCl
1.42 g/L Na2HPO4
pH = 7.4 - Dextran (16%)
160 g/L Dextran from Leuconostoc mesenteroides
Notes:
- Distilled water type 1 must be used in the preparation of the solutions.
- For short periods of time solutions must be conserved at 4 °C (e.g., one week); for longer periods solutions must be conserved at -20 °C (except Dextran solution).
- Dextran solution must not be frozen; it is stable for one week at 4 °C.
Acknowledgments
The authors’ work is supported by ‘FEDER funds through the Operational Programme Competitiveness Factors–COMPETE 2020 and national funds by FCT–Foundation for Science and Technology under the strategic project with COMPETE-attributed reference: POCI-01-0145-FEDER-007440’. Cristina Carvalho has a Postdoc fellowship from FCT (SFRH/BPD/107741/2015). The authors of the manuscript have no conflicts of interest to declare. This protocol was adapted from a previous protocol described by McNeill et al. (1999). Reprinted from Journal of Alzheimer’s Disease, vol. 35, Carvalho, Cristina; Machado, Nuno; Mota, Paula; Correia, Sónia C.; Cardoso, Susana; Santos, Renato X.; Santos, Maria S.; Oliveira, Catarina R.; Moreira, Paula I., Type 2 Diabetic and Alzheimer’s Disease Mice Present Similar Behavioral, Cognitive, and Vascular Anomalies, pp. 623-635, Copyright (2013), with permission from IOS Press.
References
- Bennett, D. A., Wilson, R. S., Arvanitakis, Z., Boyle, P. A., de Toledo-Morrell, L. and Schneider, J. A. (2013). Selected findings from the Religious Orders Study and Rush Memory and Aging Project. JAlzheimers Dis 33 Suppl 1: S397-403.
- Carvalho, C., Machado, N., Mota, P. C., Correia, S. C., Cardoso, S., Santos, R. X., Santos, M. S., Oliveira, C. R. and Moreira, P. I. (2013). Type 2 diabetic and Alzheimer's disease mice present similar behavioral, cognitive, and vascular anomalies. JAlzheimers Dis 35(3): 623-635.
- Carvalho, C., Santos, M. S., Baldeiras, I., Oliveira, C. R., Seica, R. and Moreira, P. I. (2010). Chronic hypoxia potentiates age-related oxidative imbalance in brain vessels and synaptosomes. Curr Neurovasc Res 7(4): 288-300.
- Chui, H. C., Zarow, C., Mack, W. J., Ellis, W. G., Zheng, L., Jagust, W. J., Mungas, D., Reed, B. R., Kramer, J. H., Decarli, C. C., Weiner, M. W. and Vinters, H. V. (2006). Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol 60(6): 677-687.
- DeCarli, C. (2013). Clinically asymptomatic vascular brain injury: a potent cause of cognitive impairment among older individuals. JAlzheimers Dis 33 Suppl 1: S417-426.
- Gorelick, P. B., Scuteri, A., Black, S. E., Decarli, C., Greenberg, S. M., Iadecola, C., Launer, L. J., Laurent, S., Lopez, O. L., Nyenhuis, D., Petersen, R. C., Schneider, J. A., Tzourio, C., Arnett, D. K., Bennett, D. A., Chui, H. C., Higashida, R. T., Lindquist, R., Nilsson, P. M., Roman, G. C., Sellke, F. W., Seshadri, S., American Heart Association Stroke Council, C. o. E., Prevention, C. o. C. N. C. o. C. R., Intervention, Council on Cardiovascular, S. and Anesthesia (2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42(9): 2672-2713.
- Librizzi, L., de Cutis, M., Janigro, D., Runtz, L., de Bock, F., Barbier, E. L. and Marchi, N. (2017). Cerebrovascular heterogeneity and neuronal excitability. Neurosci Lett.
- McNeill, A. M., Kim, N., Duckles, S. P., Krause, D. N. and Kontos, H. A. (1999). Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke 30(10): 2186-2190.
- Mota, P. C. and Ramalho-Santos, J. (2006). Comparison between different markers for sperm quality in the cat: Diff-Quik as a simple optical technique to assess changes in the DNA of feline epididymal sperm. Theriogenology 65(7): 1360-1375.
- Plácido, A. I., Pereira, C. M., Correira, S. C., Carvalho, C., Oliveira, C. R. and Moreira, P. I. (2017). Phosphatase 2A inhibition affects endoplasmic reticulum and mitochondria homeostasis via cytoskeletal alterations in brain endothelial cells. Mol Neurobiol 54(1): 154-168.
- Saraiva, C., Praca, C., Ferreira, R., Santos, T., Ferreira, L. and Bernardino, L. (2016). Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 235: 34-47.
- Schneider, J. A., Arvanitakis, Z., Bang, W. and Bennett, D. A. (2007). Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69(24): 2197-2204.
- Toledo, J. B., Arnold, S. E., Raible, K., Brettschneider, J., Xie, S. X., Grossman, M., Monsell, S. E., Kukull, W. A. and Trojanowski, J. Q. (2013). Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136(Pt 9): 2697-2706.
- Wharton, S. B., Brayne, C., Savva, G. M., Matthews, F. E., Forster, G., Simpson, J., Lace, G., Ince, P. G., Medical Research Council Cognitive, F. and Aging, S. (2011). Epidemiological neuropathology: the MRC Cognitive Function and Aging Study experience. JAlzheimers Dis 25(2): 359-372.
- Yarchoan, M., Xie, S. X., Kling, M. A., Toledo, J. B., Wolk, D. A., Lee, E. B., Van Deerlin, V., Lee, V. M., Trojanowski, J. Q. and Arnold, S. E. (2012). Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 135(Pt 12): 3749-3756.
- Yates, P. A., Desmond, P. M., Phal, P. M., Steward, C., Szoeke, C., Salvado, O., Ellis, K. A., Martins, R. N., Masters, C. L., Ames, D., Villemagne, V. L., Rowe, C. C. and Group, A. R. (2014). Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82(14): 1266-1273.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Carvalho, C. I. and I. Moreira, P. (2017). Isolation of Rodent Brain Vessels. Bio-protocol 7(17): e2535. DOI: 10.21769/BioProtoc.2535.
Category
Neuroscience > Nervous system disorders > Blood brain barrier
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










