- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
EAE Induction by Passive Transfer of MOG-specific CD4+ T Cells
(*contributed equally to this work) Published: Vol 7, Iss 13, Jul 5, 2017 DOI: 10.21769/BioProtoc.2370 Views: 13490
Reviewed by: Andrés AlloattiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1367 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1536 Views

Non-Enzymatic Isolation of Cancer-Associated Fibroblasts From Human Prostate Tumor Explants
Giulia Gangarossa [...] Paola Chiarugi
Mar 5, 2026 74 Views
Abstract
Experimental autoimmune encephalomyelitis (EAE) is an animal model of multiple sclerosis (MS), which is a chronic inflammatory disease of the central nervous system (CNS). It is characterized by focal demyelination and inflammatory responses mediated by myelin-specific autoreactive CD4+ T cells. Using a passive transfer model of EAE in mice, we have demonstrated that regional specific neural signals by sensory-sympathetic communications create gateways for immune cells at specific blood vessels of the CNS, a phenomenon known as the gateway reflex (Arima et al., 2012; Tracey, 2012; Arima et al., 2013; Sabharwal et al., 2014; Arima et al., 2015b). Here we describe protocols for passive transfer model of EAE using freshly isolated (MOG)-specific CD4+ T cells or periodically restimulated MOG-specific CD4+ T cell lines, which are suitable for tracking pathogenic CD4+ T cells in vivo, particularly in the CNS (Ogura et al., 2008; Arima et al., 2012 and 2015b).
Keywords: Experimental autoimmune encephalomyelitisBackground
It is widely accepted that autoreactive CD4+ T cells play a significant role in the pathogenesis of MS and EAE (Reboldi, 2009; International Multiple Sclerosis Genetics et al., 2011; Steinman, 2014), which are chronic inflammatory diseases of the CNS. The CNS is protected by the blood-brain barrier (BBB), which limits immune cell infiltration from the periphery (Liu et al., 2012). Until recently, where and how CD4+ T cells enter the CNS from the peripheral blood was unclear. Although EAE can be induced by immunization of animals with CNS-autoantigens emulsified in complete Freund’s adjuvant (CFA) and pertussis toxin (PTx) (Andreasen et al., 2009; Lu et al., 2016), unwanted systemic inflammation occurs by injection of CFA and PTx, which potentially affects the integrity of the BBB (Schellenberg et al., 2012; Marbourg et al., 2017). Alternatively, we recommend a passive transfer EAE model, in which activated CD4+ T cells specific for MOG are injected into naïve mice without treatments with CFA or PTx. Using this passive transfer EAE model, we have identified the dorsal vessels of the fifth lumbar (L5) cord as an initial gateway for autoreactive CD4+ T cells to reach the CNS (Arima et al., 2012). Mechanistically, gravity-mediated constant activation of sensory neurons in the soleus muscles induces sympathetic nerve activation that connects to the L5 dorsal vessels. The resulting noradrenaline secretion at the vessels enhances NF-κB activity, leading to the production of chemokines that recruit the CNS autoreactive CD4+ T cells (Arima et al., 2012). This sensory-sympathetic communication driven by anti-gravity responses through the soleus muscles is called ‘gravity-gateway reflex’ (Arima et al., 2012; Tracey, 2012; Sabharwal et al., 2014). In addition, this passive transfer EAE model enabled us to discover that other neural activators such as weak electric stimulation or pain sensation create unique gateways for immune cells at different sites (Arima et al., 2015a and 2015b). Here, we describe detailed protocols for the passive transfer EAE model using MOG-specific CD4+ T cells, which are suitable for tracking autoreactive CD4+ T cells in vivo. Although protocols for EAE have been reported (Racke, 2001), we particularly focus on the passive transfer EAE models and describe the methods in detail. The protocols here induce a transient EAE, in which after adoptive transfer, paralyzed tail (score 1) is expected to appear around 7 days, the clinical signs peak around 10-14 days with score 2 (uneven gait) to 2.5 (one paralyzed rear leg), and then the clinical symptoms will disappear around 20-25 days (Arima et al., 2015b). In this remission phase, the mice look healthy. However, activated monocytes remain in the spinal cords, and paralysis returns upon specific neural activation including pain sensation (Arima et al., 2015b).
Materials and Reagents
- Three-way connector (TERUMO Medical, catalog number: TS-TR1K )
- 1 ml syringe (TERUMO Medical, catalog number: SS-01T )
- Needle (25 G x 1) (TERUMO Medical, catalog number: NN-2525R )
- Needle (27 G x ¾) (TERUMO Medical, catalog number: NN-2719S )
- Cell strainer (100 μm) (Corning, Falcon®, catalog number: 352360 )
- 50 ml polypropylene conical tube (Corning, Falcon®, catalog number: 352070 )
- 2.5 ml syringe (TERUMO Medical, catalog number: SS-02SZ )
- 10 cm dish (Corning, catalog number: 430167 )
- Needle (18 G x 1 ½) (TERUMO Medical, catalog number: NN-1838R )
- 96-well U-bottom plate (Corning, catalog number: 3799 )
- Nylon wool
- 20 ml syringe
- MACS LS columns (Miltenyi Biotec, catalog number: 130-042-401 )
- C57BL/6 mouse (Japan SLC)
- M. Tuberculosis H37 RA (BD, catalog number: 231141 )
- MOG peptide 35-55 (MEVGWYRSPFSRVVHLYRNGK) (Sigma-Aldrich), stock solution = 4 mg/ml
- Incomplete Freund’s adjuvant (IFA) (Sigma-Aldrich, catalog number: F5506 )
- Isoflurane (Pfizer)
- Pertussis toxin from Bordetella pertussis (PTx) (Sigma-Aldrich, catalog number: P7208-50UG )
- CD4 (L3T4) Microbeads, mouse (Miltenyi Biotec, catalog number: 130-049-201 )
- Saline (Otsuka Pharmaceutical Factory, catalog number: 0815 )
- Mouse IL-1β (BioLegend, catalog number: 575102 ), stock solution = 10 μg/ml
- Mouse IL-23 (BioLegend, catalog number: 589002 ), stock solution = 10 μg/ml
- Human IL-6 (Toray, order-made) stock solution = 100 μg/ml (Commercially available mouse IL-6 will work)
- CellBanker (Takara Bio, Clontech, catalog number: CB021 )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: A4514 )
- DDW
- EDTA-2Na
- Fetal bovine serum (FBS) (GE Healthcare, HyCloneTM, catalog number: SH30910.03 )
- RPMI medium 1640 basic (1x) (Thermo Fisher Scientific, GibcoTM, catalog number: C11875500BT )
- Penicillin/streptomycin (Sigma-Aldrich, catalog number: P4333-100ML )
- 2-mercaptoethanol (NACALAI TESQUE, catalog number: 21417 )
- Iscove’s modified Dulbecco’s medium (Sigma-Aldrich, catalog number: I3390-500ML )
- GlutaMAX-1 (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9625-5KG )
- Potassium chloride (KCl) (Wako Pure Chemical Industries, catalog number: 163-03545 )
- Sodium hydrogen phosphate (Na2HPO4) (Wako Pure Chemical Industries, catalog number: 197-02865 )
- Potassium dihydrogen phosphate (KH2PO4) (Wako Pure Chemical Industries, catalog number: 169-04245 )
- Red blood cell (RBC) lysis buffer (see Recipes)
- Phosphate buffered saline (PBS) (see Recipes)
- MACS buffer (see Recipes)
- RP10 medium (see Recipes)
- Nylon wool column (see Recipes)
- IM20 medium (see Recipes)
Equipment
- X-ray irradiator (Hitachi, model: MBR-1520R ) or equivalent
- Scissors (BONIMED, catalog number: 669-060-72 )
- Micro-dissecting scissors (Karl Hammacher, catalog number: HSB 014-11 )
- Angled serrated tip forceps (Karl Hammacher, catalog number: HSC 187-11 )
- Pipet-aid (Corning, Falcon®, catalog number: 357471 )
- Glass syringe (Tsubasa Industry, 5 ml, lock type), autoclaved
- Centrifuge (Hitachi, model: CF7D2 )
- Cell culture incubator, 37 °C, 5% CO2 (Panasonic Healthcare, model: MCO-175 )
- Multichannel pipette
- Water bath
- Autoclave
Part I. Passive transfer method with MOG-specific CD4+ T cells isolated from MOG immunized mice
Procedure
- MOG immunization in mice (Figure 1)
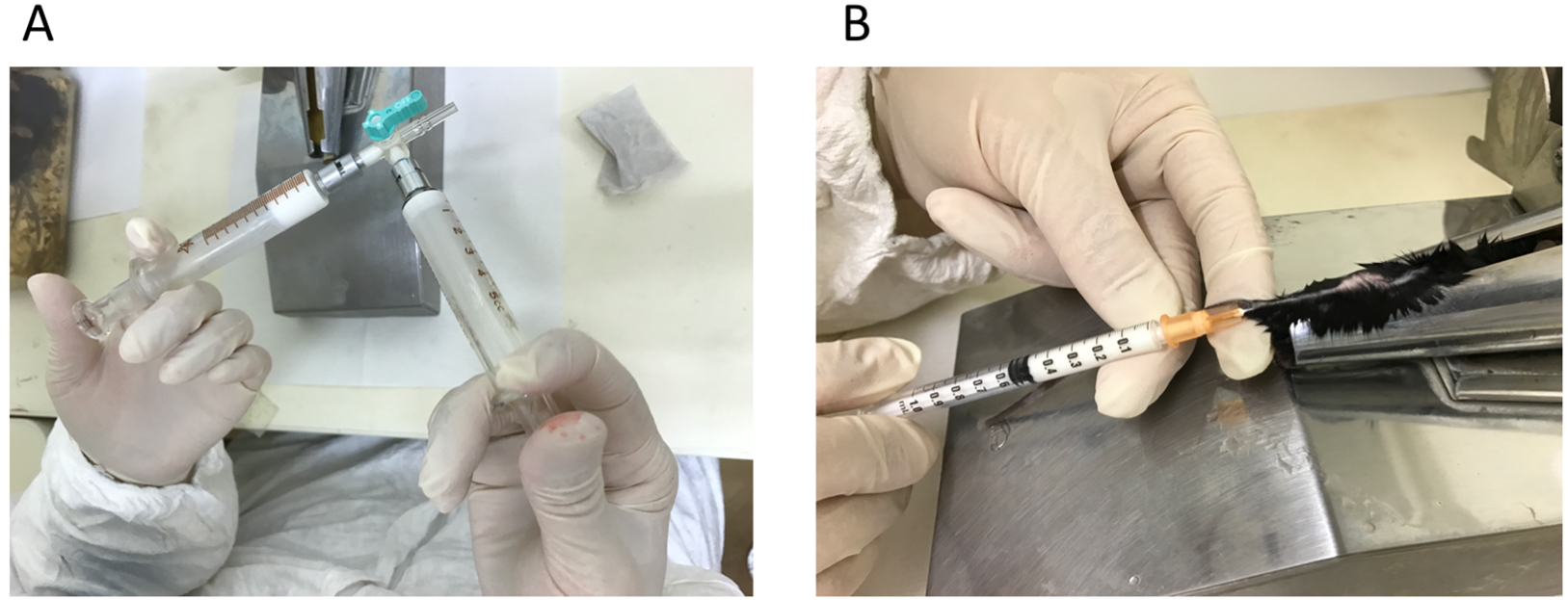
Figure 1. Making emulsion and immunization in C57BL/6 mice. A. MOG peptide (35-55) and CFA mixed at 1:1 and emulsified; B. Tail base immunization with 200 μg/100 μl emulsion.- Take sufficient volume (2 ml for 30 mice) of 4 mg/ml MOG peptide (35-55) in one glass syringe, take the same volume of CFA in the other glass syringe, and connect two syringes with a two- or three-way connector. Then, emulsify the two solutions by pushing plungers of the two syringes until the MOG/CFA solution becomes white, uniform emulsion (about 50 strokes).
Note: CFA is prepared by mixing 10 ml IFA and 2 vials of 10 mg M. Tuberculosis H37 RA. - Move all the emulsion to one-side of the glass syringe, unlock the other empty glass syringe, and attach a new disposable 1-ml syringe to the open side of the connector.
- Load the emulsion to the 1-ml syringe, and put a 25 G x 1 needle for immunization.
- Anesthetize a mouse with isoflurane. It is easier for injections if the body of the mouse is fixed in a restrainer (optional). Intravenously administer (i.v.) 200 ng/200 μl PTx to the tail vein of C57BL/6 mice (6-8 weeks old) with 27 G x ¾ needle, followed by immunization by subcutaneous administration (s.c.) of 200 μg/100 μl MOG peptide emulsified in CFA at tail base on day 0. Perform the PTx injection and MOG immunization on the same day.
- Inject i.v. 200 ng/200 μl PTx on days 2 and 7.
- Take sufficient volume (2 ml for 30 mice) of 4 mg/ml MOG peptide (35-55) in one glass syringe, take the same volume of CFA in the other glass syringe, and connect two syringes with a two- or three-way connector. Then, emulsify the two solutions by pushing plungers of the two syringes until the MOG/CFA solution becomes white, uniform emulsion (about 50 strokes).
- Separation of CD4+ T cells from MOG immunized mice by using CD4 Microbeads.
- Collect spleens from MOG immunized mice (30 mice) on day 9 or 10.
- Homogenize the spleens on a cell strainer attached to a 50-ml tube using a plunger from a 2.5 ml syringe (7-8 spleens/tube, total four strainers and four 50-ml tubes are used for 30 mice). Add plain RPMI medium up to 50 ml during homogenization.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the cell pellet in 10 ml/tube (4 tubes if 30 mice are used) of RBC lysis buffer (see Recipe 1) and incubate on ice for about 1 min.
- Add plain RPMI medium up to 50 ml/tube.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the pellet in 1 ml/tube of MACS buffer (see Recipe 2).
- Add 100 μl/tube of CD4 MACS beads and incubate on ice for 30 min.
- Add plain RPMI medium up to 50 ml/tube.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the pellet in 5 ml/tube MACS buffer (4 tubes if 30 mice are used). Use the same number of MACS LS columns as that of 50-ml tubes used. Apply the cell suspension to MACS LS columns (5 ml/column). Refer to the manufacturer guide for the use of MACS LS columns.
- Wash the column with 3 ml/column of MACS buffer twice.
Note: Do not discard the flow-through cells, which are used as antigen-presenting cells. - Elute the CD4 positive selected cells with 5 ml/column MACS buffer and pool the eluted fractions to a 50 ml tube.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the CD4+ T cells in 20 ml RP10 medium (see Recipe 3) and adjust to 8 x 106 cells/ml.
- Collect spleens from MOG immunized mice (30 mice) on day 9 or 10.
- Irradiation of splenocytes
- Collect the flow-through cells obtained in the STEP 12 of the previous section.
- Centrifuge the flow-through cells depleted of CD4+ T cells (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the cells in 10 ml of RP10 medium.
- Irradiate the cells at 35 Gy, centrifuge (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the cells in 20 ml of RP10 medium and adjust to 2 x 107 cells/ml.
- Collect the flow-through cells obtained in the STEP 12 of the previous section.
- In vitro restimulation and adoptive transfer
- Co-culture 4 x 107 cells CD4+ T cells and 1 x 108 irradiated splenocytes in 10 ml RP10 medium in a 10-cm dish containing 2 ng/ml IL-23 and 25 μg/ml MOG peptide for 2 days.
- Add 10 ml RP10 medium in each dish on day 1.
- Collect the cells by pipetting up and down on day 2, centrifuge (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the cells in 10 ml RP10 medium pre-warmed at 37 °C and add the suspension to a nylon wool column (see Recipe 4) (Video 1).
Note: Put an 18 G x 1 ½ inches needle to a nylon wool column, and stand the column in a 50 ml tube without a cap. To equilibrate the column, add 20 ml RP10 medium pre-warmed at 37 °C until the medium spreads uniformly in nylon wool. Incubate the nylon wool column with the co-cultured cells in a CO2 incubator (37 °C, 20 min). After 20 min, elute the cells with 30 ml RP10 medium.Video 1. Making a nylon wool column. 20 ml of RP10 medium are added to nylon wool and stirred until the medium uniformly spreads in the wool. - Centrifuge the eluted cells (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the pellets in 1 ml MACS buffer.
- Add 100 μl/tube of CD4 MACS beads and incubate on ice for 30 min.
- Add plain RPMI medium up to 50 ml/tube.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the pellet in 10 ml/tube MACS buffer and apply the suspensions to two MACS columns (5 ml/column).
- Wash with 3 ml/column MACS buffer twice.
- Elute the CD4 positive selected cells with 5 ml/column MACS buffer and pool the eluted fractions to a 50 ml tube.
- Centrifuge the tube (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the eluted CD4+ T cells in sterile saline.
Note: Resuspended volume should not exceed the maximal volume of i.v. injection allowed in the ethics guideline of your institute. - Inject 1.5 x 107 cells/mouse, i.v.
Note: Usually, 6-8 mice can be injected using 30 spleens. - Measure clinical scores as described previously (Ogura et al., 2008; Huseby et al., 2001; Arima et al., 2012 and 2015b).
- Co-culture 4 x 107 cells CD4+ T cells and 1 x 108 irradiated splenocytes in 10 ml RP10 medium in a 10-cm dish containing 2 ng/ml IL-23 and 25 μg/ml MOG peptide for 2 days.
Part II. Passive transfer model with MOG-specific T cells lines
Passive transfer EAE can also be induced using a MOG-specific T cell line generated by periodical antigen stimulations in vitro. Once the T cell line is established, this method is useful to reduce the number of mice used to induce the transfer EAE.
Procedure
- MOG immunization
- Perform MOG immunization in the same way as Part I. A. MOG immunization in mice, except for the number of mice to be immunized. Usually, 3-4 mice are sufficient to obtain MOG-specific T cells lines.
- Perform MOG immunization in the same way as Part I. A. MOG immunization in mice, except for the number of mice to be immunized. Usually, 3-4 mice are sufficient to obtain MOG-specific T cells lines.
- Culture and passage of T cell lines
- Collect inguinal lymph nodes (iLNs) from MOG immunized mice (3-4 mice) on day 9 or 10 (Figure 2).
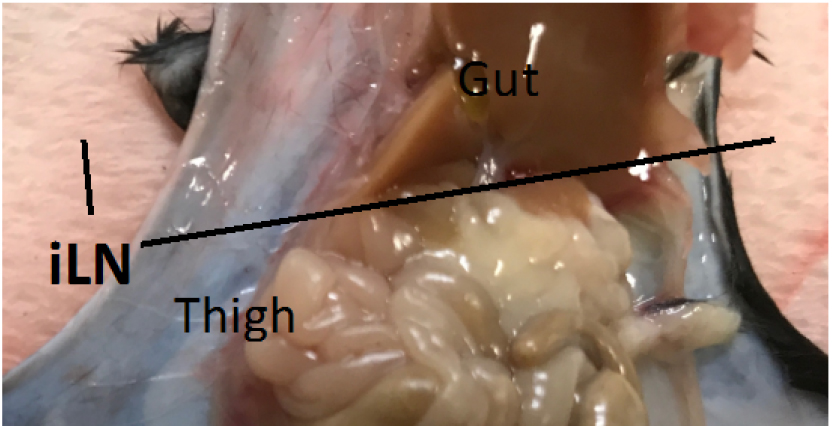
Figure 2. The location of the inguinal lymph nodes (iLN). iLN of the immunized C57BL/6 mouse. - Homogenize the iLNs on a cell strainer in a 50 ml tube using the plunger from a 2.5 ml syringe.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the cell pellet in 10 ml/tube RBC lysis buffer (see Recipe 1) and incubate on ice for 1 min.
- Add plain RPMI medium up to 50 ml/tube.
- Centrifuge the tubes (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Resuspend the cells in 2 ml IM20 medium (see Recipe 5).
- Filter the cells using a cell strainer.
- Count the cells.
- Seed 2.5 x 105 cells/well/200 μl iLN cells in the presence of 4 μg/ml MOG peptide, 0.5 ng/ml IL-1β, 5 ng/ml IL-6 and 0.5 ng/ml IL-23 in 96-well U-bottom plates for 10 to 14 days.
- Proceed to step C1.
- Collect inguinal lymph nodes (iLNs) from MOG immunized mice (3-4 mice) on day 9 or 10 (Figure 2).
- T cell preparation
- Collect CD4+ T-cell-rich iLN cells from 96U plates in a 10-cm dish by gently pipetting 2 to 3 times using a multichannel pipette.
- Collect the cells into 50 ml tubes and centrifuge (600 x g, 5 min, 4 °C).
- Aspirate the supernatant and resuspend the T cells in 5 ml IM20 medium.
- Count the cells and adjust the concentration to 2.5 x 105 cells/ml.
- Collect CD4+ T-cell-rich iLN cells from 96U plates in a 10-cm dish by gently pipetting 2 to 3 times using a multichannel pipette.
- Irradiation of splenocytes
- Collect the spleens of naive C57BL/6 mice and homogenize using the plunger from a 2.5 ml syringe.
- Add plain RPMI medium up to 50 ml/tube and centrifuge (600 x g, 5 min, 4 °C).
- Aspirate the supernatant and add 1 ml/spleen RBC lysis buffer.
- Add plain RPMI medium up to 50 ml and centrifuge (600 x g, 5 min, 4 °C).
- Irradiate the cells at 35 Gy, centrifuge (600 x g, 5 min, 4 °C) and aspirate the supernatant.
- Suspend the irradiated splenocytes in 10 ml IM20 medium, count and adjust the concentration to 2.5 x 106 cells/ml.
- Collect the spleens of naive C57BL/6 mice and homogenize using the plunger from a 2.5 ml syringe.
- In vitro stimulation
- Mix 2.5 x 105 cells/ml CD4+ T-cell-rich iLN cells and 2.5 x 106 cells/ml irradiated splenocytes at 1:1.
- Seed the cells at 200 μl/well containing 4 μg/ml MOG peptide, 0.5 ng/ml IL-1β, 5 ng/ml IL-6 and 0.5 ng/ml IL-23 in 96-well U-bottom plates.
- Incubate the co-cultured cells at 37 °C, 5% CO2 for 10-14 days.
- Repeat steps C1 to E3 to enrich the percentage of MOG-specific CD4+ T cells from total iLN cells (Figure 3). Use fresh irradiated splenocytes in each repeating cycle.
Notes:- T cells can be frozen after step E3 in CellBanker or equivalent solution at -80 °C. When thawing, use a 37 °C water bath, and wash the cells in plain RPMI medium. Then, the T cell line can be used for culture (start from step E1).
- Typically, more than 95% of live cells will be CD4+ T cells after four rounds of in vitro stimulation (Figure 3).
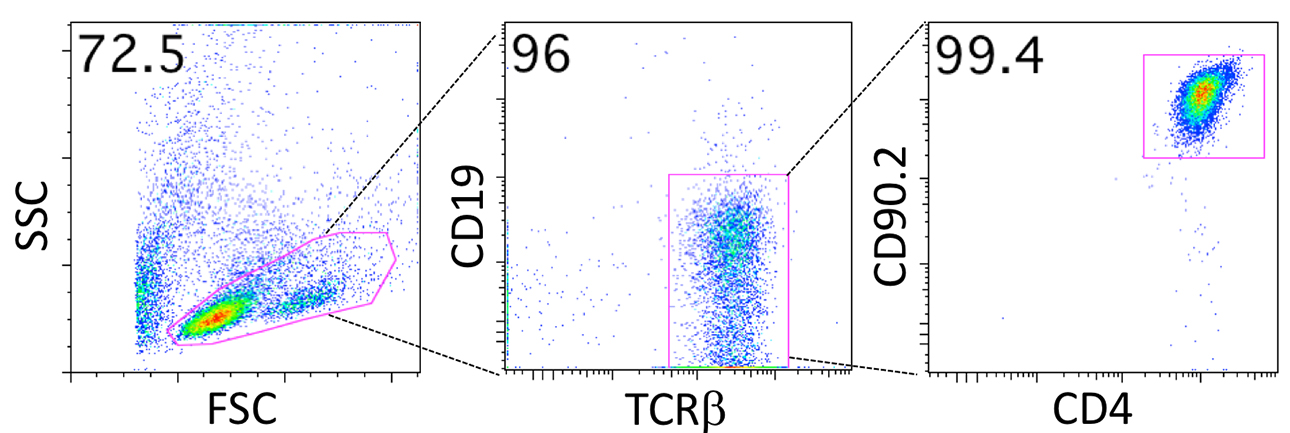
Figure 3. Representative FACS plots of a T cell line. Most living cells (> 95%) are CD4+ T cells (CD19- T cell receptor (TCR)β+CD90.2+CD4+ T cells) after 5 days of the fourth round of in vitro stimulation (Procedure E). Irradiated splenocytes can be seen as forward scatter (FSC)low, side scatter (SSC)low dead cells.
- T cells can be frozen after step E3 in CellBanker or equivalent solution at -80 °C. When thawing, use a 37 °C water bath, and wash the cells in plain RPMI medium. Then, the T cell line can be used for culture (start from step E1).
- Mix 2.5 x 105 cells/ml CD4+ T-cell-rich iLN cells and 2.5 x 106 cells/ml irradiated splenocytes at 1:1.
- Adoptive transfer of T cell lines
- Set up culture the same way as ‘E. In vitro stimulation’.
Note: Do not forget to add 4 μg/ml MOG peptide, 0.5 ng/ml IL-1β, 5 ng/ml IL-6 and 0.5 ng/ml IL-23. - Seed these cells at 200 μl/well in 96-well U-bottom plates (about 5 plates for 1 mouse).
- Incubate the co-cultured cells in 37 °C, 5% CO2 for 2 days.
- Collect the cells from the 96-well U-bottom plates in a 10-cm dish by gently pipetting 2 to 3 times with a multichannel pipette.
- Collect the cells in 50 ml tubes and centrifuge (600 x g, 5 min, 4 °C).
- Aspirate the medium and resuspend the cells in 1 ml plain RPMI medium.
- Count the cells and adjust the concentration to 2.5-3.75 x 107/ml.
Note: Count only living cells. - Inject 1-1.5 x 107 cells i.v. into C57BL/6 mice.
- Measure clinical scores as described previously (Huseby et al., 2001; Ogura et al., 2008; Arima et al., 2012 and 2015b).
- Set up culture the same way as ‘E. In vitro stimulation’.
Data analysis
The clinical symptoms of EAE are evaluated as follows: grade 1, paralyzed tail; grade 2, uneven gait; grade 2.5, one paralyzed rear leg; grade 3, rear limb paralysis; grade 4, paralyzed front and rear legs; and grade 5, moribund (Huseby et al., 2001; Ogura et al., 2008; Arima et al., 2012 and 2015b).
Recipes
- Red blood cell (RBC) lysis buffer (500 ml)
4.41 g NH4Cl
500 ml DDW, then autoclave - Phosphate buffered saline (PBS)
1.46 g NaCl
1.86 g KCl
3.5 g Na2HPO4
3.4 g KH2PO4 - MACS buffer (1,000 ml)
950 ml PBS
1.86 g EDTA-2Na
Add 50 ml heat-inactivated FBS after autoclaving - RP10 medium (500 ml)
500 ml RPMI medium 1640 basic
50 ml heat-inactivated FBS
5 ml 100x penicillin/streptomycin
1.86 μl 2-mercaptoethanol - Nylon wool column
Nylon wool (1.2 g) is unraveled with a brush, put into a 20 ml syringe, and sterilized by autoclave - IM20 medium (600 ml)
500 ml Iscove’s modified Dulbecco’s medium
100 ml FBS
5 ml GlutaMAX-1 (100x)
1.8 μl 2-mercaptoethanol
5 ml 100x penicillin/streptomycin
Acknowledgments
We appreciate the excellent technical assistance provided by Ms. Ezawa, and Ms. Nakayama, and thank Ms. Fukumoto for her excellent secretarial assistance. We thank Dr. P. Karagiannis (CiRA, Kyoto University, Kyoto, Japan) for carefully reading the manuscript and important discussion. The protocol Part I was used in our previous reports (Arima, 2012 and 2015b; Mori, 2014). This work was supported by KAKENHI (D. K., Y. A., T. A., and M. M.), Takeda Science Foundation (M. M.), Institute for Fermentation Osaka (M. M.), Mitsubishi Foundation (M. M.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (D. K.), Suzuken Memorial Foundation (Y. A.), Japan Prize Foundation (Y. A.), Ono Medical Research Foundation (Y. A.), Kanzawa Medical Research Foundation (Y. A.), Kishimoto Foundation (Y. A.), Nagao Takeshi Research Foundation (Y. A.), Japan Multiple Sclerosis Society (Y. A.), Kanae Foundation (Y. A.), Tokyo Medical Research Foundation (M. M. and Y. A.), Uehara Memorial Foundation (Y. A.), Japan Brain Foundation (Y. A.), Kao Foundation(Y. A.), Nagao Memorial Fund(Y. T.), Suzuken Memorial Foundation (D. K.), Suhara Memorial Foundation (D. K.), Yasuda Memorial Foundation (D. K.), and Novartis Pharma Research Grants (D. K.).
References
- Andreasen, C., Powell, D. A. and Carbonetti, N. H. (2009). Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One 4(9): e7079.
- Arima, Y., Harada, M., Kamimura, D., Park, J. H., Kawano, F., Yull, F. E., Kawamoto, T., Iwakura, Y., Betz, U. A., Marquez, G., Blackwell, T. S., Ohira, Y., Hirano, T. and Murakami, M. (2012). Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell 148(3): 447-457.
- Arima, Y., Higuchi, K., Nishikawa, N., Stofkova, A., Ohki, T., Kamimura, D. and Murakami, M. (2015a). Pain is an inducer for relapse in multiple sclerosis models via a regional neural signal. Clin Exp Neuroimmunol 6: 343-344.
- Arima, Y., Kamimura, D., Atsumi, T., Harada, M., Kawamoto, T., Nishikawa, N., Stofkova, A., Ohki, T., Higuchi, K., Morimoto, Y., Wieghofer, P., Okada, Y., Mori, Y., Sakoda, S., Saika, S., Yoshioka, Y., Komuro, I., Yamashita, T., Hirano, T., Prinz, M. and Murakami, M. (2015b). A pain-mediated neural signal induces relapse in murine autoimmune encephalomyelitis, a multiple sclerosis model. Elife 4.
- Arima, Y., Kamimura, D., Sabharwal, L., Yamada, M., Bando, H., Ogura, H., Atsumi, T. and Murakami, M. (2013). Regulation of immune cell infiltration into the CNS by regional neural inputs explained by the gate theory. Mediators Inflamm 2013: 898165.
- Huseby E. S., Sather, B., Huseby, P. G. and Goverman, J. (2001). Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity 14: 471-481.
- International Multiple Sclerosis Genetics, C., Wellcome Trust Case Control, C., Sawcer, S., Hellenthal, G., Pirinen, M., Spencer, C. C., Patsopoulos, N. A., Moutsianas, L., Dilthey, A., Su, Z., Freeman, C., Hunt, S. E., Edkins, S., Gray, E., Booth, D. R., Potter, S. C., Goris, A., Band, G., Oturai, A. B., Strange, A., Saarela, J., Bellenguez, C., Fontaine, B., Gillman, M., Hemmer, B., Gwilliam, R., Zipp, F., Jayakumar, A., Martin, R., Leslie, S., Hawkins, S., Giannoulatou, E., D'Alfonso, S., Blackburn, H., Martinelli Boneschi, F., Liddle, J., Harbo, H. F., Perez, M. L., Spurkland, A., Waller, M. J., Mycko, M. P., Ricketts, M., Comabella, M., Hammond, N., Kockum, I., McCann, O. T., Ban, M., Whittaker, P., Kemppinen, A., Weston, P., Hawkins, C., Widaa, S., Zajicek, J., Dronov, S., Robertson, N., Bumpstead, S. J., Barcellos, L. F., Ravindrarajah, R., Abraham, R., Alfredsson, L., Ardlie, K., Aubin, C., Baker, A., Baker, K., Baranzini, S. E., Bergamaschi, L., Bergamaschi, R., Bernstein, A., Berthele, A., Boggild, M., Bradfield, J. P., Brassat, D., Broadley, S. A., Buck, D., Butzkueven, H., Capra, R., Carroll, W. M., Cavalla, P., Celius, E. G., Cepok, S., Chiavacci, R., Clerget-Darpoux, F., Clysters, K., Comi, G., Cossburn, M., Cournu-Rebeix, I., Cox, M. B., Cozen, W., Cree, B. A., Cross, A. H., Cusi, D., Daly, M. J., Davis, E., de Bakker, P. I., Debouverie, M., D'Hooghe M, B., Dixon, K., Dobosi, R., Dubois, B., Ellinghaus, D., Elovaara, I., Esposito, F., Fontenille, C., Foote, S., Franke, A., Galimberti, D., Ghezzi, A., Glessner, J., Gomez, R., Gout, O., Graham, C., Grant, S. F., Guerini, F. R., Hakonarson, H., Hall, P., Hamsten, A., Hartung, H. P., Heard, R. N., Heath, S., Hobart, J., Hoshi, M., Infante-Duarte, C., Ingram, G., Ingram, W., Islam, T., Jagodic, M., Kabesch, M., Kermode, A. G., Kilpatrick, T. J., Kim, C., Klopp, N., Koivisto, K., Larsson, M., Lathrop, M., Lechner-Scott, J. S., Leone, M. A., Leppa, V., Liljedahl, U., Bomfim, I. L., Lincoln, R. R., Link, J., Liu, J., Lorentzen, A. R., Lupoli, S., Macciardi, F., Mack, T., Marriott, M., Martinelli, V., Mason, D., McCauley, J. L., Mentch, F., Mero, I. L., Mihalova, T., Montalban, X., Mottershead, J., Myhr, K. M., Naldi, P., Ollier, W., Page, A., Palotie, A., Pelletier, J., Piccio, L., Pickersgill, T., Piehl, F., Pobywajlo, S., Quach, H. L., Ramsay, P. P., Reunanen, M., Reynolds, R., Rioux, J. D., Rodegher, M., Roesner, S., Rubio, J. P., Ruckert, I. M., Salvetti, M., Salvi, E., Santaniello, A., Schaefer, C. A., Schreiber, S., Schulze, C., Scott, R. J., Sellebjerg, F., Selmaj, K. W., Sexton, D., Shen, L., Simms-Acuna, B., Skidmore, S., Sleiman, P. M., Smestad, C., Sorensen, P. S., Sondergaard, H. B., Stankovich, J., Strange, R. C., Sulonen, A. M., Sundqvist, E., Syvanen, A. C., Taddeo, F., Taylor, B., Blackwell, J. M., Tienari, P., Bramon, E., Tourbah, A., Brown, M. A., Tronczynska, E., Casas, J. P., Tubridy, N., Corvin, A., Vickery, J., Jankowski, J., Villoslada, P., Markus, H. S., Wang, K., Mathew, C. G., Wason, J., Palmer, C. N., Wichmann, H. E., Plomin, R., Willoughby, E., Rautanen, A., Winkelmann, J., Wittig, M., Trembath, R. C., Yaouanq, J., Viswanathan, A. C., Zhang, H., Wood, N. W., Zuvich, R., Deloukas, P., Langford, C., Duncanson, A., Oksenberg, J. R., Pericak-Vance, M. A., Haines, J. L., Olsson, T., Hillert, J., Ivinson, A. J., De Jager, P. L., Peltonen, L., Stewart, G. J., Hafler, D. A., Hauser, S. L., McVean, G., Donnelly, P. and Compston, A. (2011). Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359): 214-219.
- Liu, W. Y., Wang, Z. B., Zhang, L. C., Wei, X. and Li, L. (2012). Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 18(8): 609-615.
- Lu, K. W., Hsu, C. K., Hsieh, C. L., Yang, J. and Lin, Y. W. (2016). Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci Rep 6: 22123.
- Marbourg, J. M., Bratasz, A., Mo, X. and Popovich, P. G. (2017). Spinal cord injury suppresses cutaneous inflammation: implications for peripheral wound healing. J Neurotrauma 34(6): 1149-1155.
- Mori, Y., Murakami, M., Arima, Y., Zhu, D., Terayama, Y., Komai, Y., Nakatsuji, Y., Kamimura, D. and Yoshioka, Y. (2014). Early pathological alterations of lower lumbar cords detected by ultrahigh-field MRI in a mouse multiple sclerosis model. Int Immunol 26(2): 93-101.
- Ogura, H., Murakami, M., Okuyama, Y., Tsuruoka, M., Kitabayashi, C., Kanamoto, M., Nishihara, M., Iwakura, Y. and Hirano, T. (2008). Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29(4): 628-636.
- Racke, M. K. (2001). UNIT 9.7 Experimental Autoimmune Encephalomyelitis (EAE). Curr Protoc in Neurosci.
- Reboldi, A., Coisne, C., Baumjohann, D., Benvenuto, F., Bottinelli, D., Lira, S., Uccelli, A., Lanzavecchia, A., Engelhardt, B. and Sallusto, F. (2009). C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10(5): 514-523.
- Sabharwal, L., Kamimura, D., Meng, J., Bando, H., Ogura, H., Nakayama, C., Jiang, J. J., Kumai, N., Suzuki, H., Atsumi, T., Arima, Y. and Murakami, M. (2014). The Gateway Reflex, which is mediated by the inflammation amplifier, directs pathogenic immune cells into the CNS. J Biochem 156(6): 299-304.
- Schellenberg, A. E., Buist, R., Del Bigio, M. R., Toft-Hansen, H., Khorooshi, R., Owens, T. and Peeling, J. (2012). Blood-brain barrier disruption in CCL2 transgenic mice during pertussis toxin-induced brain inflammation. Fluids Barriers CNS 9(1): 10.
- Steinman, L. (2014). Immunology of relapse and remission in multiple sclerosis. Annu Rev Immunol 32: 257-281.
- Tracey, K. J. (2012). Immune cells exploit a neural circuit to enter the CNS. Cell 148(3): 392-394.
Article Information
Copyright
Tanaka et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tanaka, Y., Arima, Y., Higuchi, K., Ohki, T., Elfeky, M., Ota, M., Kamimura, D. and Murakami, M. (2017). EAE Induction by Passive Transfer of MOG-specific CD4+ T Cells. Bio-protocol 7(13): e2370. DOI: 10.21769/BioProtoc.2370.
- Arima, Y., Kamimura, D., Atsumi, T., Harada, M., Kawamoto, T., Nishikawa, N., Stofkova, A., Ohki, T., Higuchi, K., Morimoto, Y., Wieghofer, P., Okada, Y., Mori, Y., Sakoda, S., Saika, S., Yoshioka, Y., Komuro, I., Yamashita, T., Hirano, T., Prinz, M. and Murakami, M. (2015b). A pain-mediated neural signal induces relapse in murine autoimmune encephalomyelitis, a multiple sclerosis model. Elife 4.
Category
Immunology > Animal model > Mouse
Immunology > Inflammatory disorder > Multiple sclerosis
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










