- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Lung Section Staining and Microscopy
Published: Vol 7, Iss 10, May 20, 2017 DOI: 10.21769/BioProtoc.2286 Views: 27402
Reviewed by: Ivan ZanoniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2900 Views

Development of a Novel Automated Workflow in Fiji ImageJ for Batch Analysis of Confocal Imaging Data to Quantify Protein Colocalization Using Manders Coefficient
Vikram Aditya [...] Wei Yue
Apr 5, 2025 2856 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 483 Views
Abstract
Our protocol describes immunofluorescent staining, hematoxylin and eosin staining and Masson’s trichrome staining on lung sections.
Keywords: Immunofluorescent stainingBackground
The primary function of the lungs is gas exchange. The lungs are composed of various specialized cells and tissues, including the bronchi, the bronchioles and the pulmonary alveoli to facilitate gas exchange. To study lung development or lung diseases in mouse models, the lungs can be removed from mice and either frozen and embedded in optimal cutting temperature (OCT) compound or chemically preserved and embedded in paraffin. To preserve lung tissue architecture, we filled the lung with 1 ml of OCT compound for preparing frozen sections, or 10% buffered formalin for paraffin sections (Zhou et al., 2016). Lung sections are then sliced from frozen or paraffin-embedded lungs and mounted onto slides for preparation of staining. We used frozen tissue sections for antibody-based immunofluorescent staining and used paraffin-embedded sections for hematoxylin and eosin (H&E) staining or Masson’s trichrome staining. Frozen sections are quicker to prepare for immunofluorescent staining and most antibodies work well on frozen sections. Paraffin sections can also be used for immunofluorescent staining, but they require deparaffinization, rehydration and antigen retrieval. Some antibodies do not work well on paraffin sections even after antigen retrieval. However, paraffin samples can be stored at room temperate for very long periods and can be easily cut into very thin sections. Paraffin sections preserve better tissue morphology than frozen sections, so they are better for H&E or trichrome staining.
Immunofluorescent staining is a type of immunohistochemistry that makes use of fluorophores to visualize the location of the antibodies that specifically bind to their target proteins. H&E staining is the most widely used stain in histology and medical diagnosis. This staining method involves application of hemalum and eosin Y that color cell nuclei blue and cytoplasm pink to red. Masson’s trichrome staining is a three-color staining that is used for detecting collagen fibers in tissues. The staining produces blue collagen, dark brown to black cell nuclei and red background.
Materials and Reagents
- Fisherbrand Superfrost Plus microscope slides (Fisher Scientific, catalog number: 12-550-15 )
- Disposable mold
- Coverslip slides
- C57BL/6 mice
- Tissue-Tek OCT compound (SAKURA FINETEK, catalog number: 4583 )
- Formaldehyde, 37-40% ACS (Newcomer Supply, catalog number: 1089 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- Phosphate buffered saline (PBS, pH 7.4) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- Serum from secondary antibody’s host
- Primary antibodies: Rabbit anti α-smooth muscle actin (α-SMA) antibody (Abcam, catalog number: ab5694 )
- Secondary reagents: Texas Red conjugated goat anti-rabbit IgG antibody (Abcam, catalog number: ab6719 )
- Prolong® Gold anti-fade reagent (Cell Signaling Technology, catalog number: 9071 ), or with DAPI (Cell Signaling Technology, catalog number: 8961 )
- Paraffin
- Ethyl alcohol (Newcomer Supply, catalog number: 10841 )
- Xylene (Newcomer Supply, catalog number: 1446 )
- S-mounting medium (Newcomer Supply, catalog number: 6750 )
- Bouin Fluid (Newcomer Supply, catalog number: 1020 )
- Biebrich scarlet-acid fuchsin solution (Sigma-Aldrich, catalog number: HT151 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3912 )
- Fetal bovine serum (FBS) (GE Healthcare, HycloneTM, catalog number: SH30396.03 )
- Eosin Y (Sigma-Aldrich, catalog number: E4009 )
- Acetic acid, glacial (Sigma-Aldrich, catalog number: A6283 )
- Aluminum potassium sulfate (Sigma-Aldrich, catalog number: 237086 )
- Hematoxylin (Sigma-Aldrich, catalog number: H3136 )
- Sodium iodate (Sigma-Aldrich, catalog number: S4007 )
- Citric acid (Sigma-Aldrich, catalog number: 251275 )
- Ferric chloride (Sigma-Aldrich, catalog number: 157740 )
- Hydrochloric acid (Sigma-Aldrich, catalog number: 320331 )
- Phosphomolybdic acid solution (Sigma-Aldrich, catalog number: HT153 )
- Phosphotungstic acid solution (Sigma-Aldrich, catalog number: HT152 )
- Aniline blue (Sigma-Aldrich, catalog number: 415049 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: S8282 )
- Blocking buffer (see Recipes)
- Eosin Y solution (see Recipes)
- Eosin Y working solution (0.25%) (see Recipes)
- Mayer’s hematoxylin solution (see Recipes)
- Weigert’s iron hematoxylin solution (see Recipes)
- Phosphomolybdic-phosphotungstic acid solution (see Recipes)
- Aniline blue solution (see Recipes)
Equipment
- Cryostat microtome
- -70 °C freezer
- Fluorescence microscope or a confocal microscope
- Staining jar and slide rack, for example EasyDip Slide Staining System (Newcomer Supply, catalog number: 5300KIT )
- Optical microscope
Procedure
- Immunofluorescent staining of lung frozen sections
Frozen lung tissues are prepared by inflating the lungs with optimum cutting temperature (OCT) compound through the trachea, tying off the trachea to maintain the fluid in the lung, and freezing the tissue in a disposable mold containing OCT. Frozen sections shall be cut at 5-10 µm in a cryostat microtome, mounted on Superfrost Plus microscope slides and stored in a -70 °C freezer. For detailed protocol of preparing frozen lung tissues and sections, please refer to Ling et al., 2009.- Upon removal of the frozen sections from the freezer, immediately add 50 µl of 4% formaldehyde in PBS to cover the sections.
- Fix the tissues for 15 min at room temperature.
- Cover lung sections with PBS for 5 min, then drip off PBS. Repeat 3 times.
- Permeabilize cells by covering the sections with 0.1% Triton X-100 in PBS for 10 min (if staining for proteins that are intracellular).
- Rinse in PBS 3 times, 5 min each.
- Incubate in blocking buffer of 5% serum from secondary antibody’s host in PBS pH 7.5 for 30 min to block unspecific binding of the antibodies. Alternative blocking buffers are 1% BSA or 1% FBS in PBS.
- While blocking, prepare primary antibody at appropriate dilution in blocking buffer. For example, use 1:200 dilution for rabbit anti α-smooth muscle actin (α-SMA) antibody (Abcam).
- Incubate lung sections with diluted primary antibodies for 60 min at room temperature (or overnight at 4 °C).
- Rinse slides 3 times in PBS, 15 min each.
- Incubate specimen in fluorochrome-conjugated secondary antibodies diluted in blocking buffer for 60 min at room temperature. For example, use 1:1,000 dilution for Texas Red conjugated goat anti-rabbit IgG antibody (Abcam). From this step forward, specimen should be protected from light.
- Rinse slides three times in PBS, 15 min each.
- Coverslip slides with Prolong® Gold anti-fade reagent with or without DAPI (Figure 1). DAPI (4’,6-diamidino-2-phenylindole) is a blue-fluorescent DNA stain that serves as an ideal nuclear counterstain for fixed cells. No additional step is required for DAPI staining.
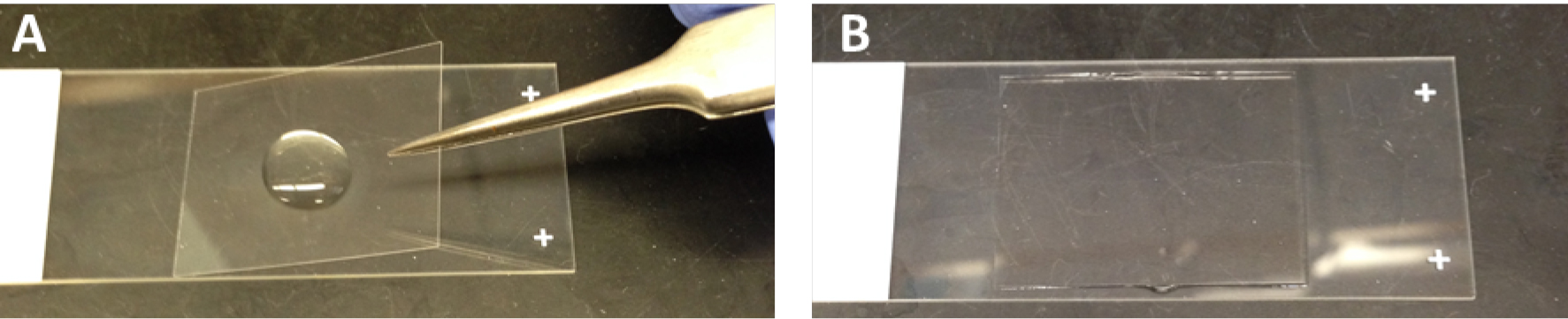
Figure 1. Mount a coverslip on glass slides with Prolong® Gold anti-fade reagent. A. Before mounting a coverslip; B. After mounting a coverslip. - Allow mounting media to cure overnight at room temperature.
- Observe (and photograph if necessary) an immunofluorescent stained slide by mounting it on the stage of a fluorescence microscope or a confocal microscope.
- Upon removal of the frozen sections from the freezer, immediately add 50 µl of 4% formaldehyde in PBS to cover the sections.
- H&E staining of lung sections
Lungs are fixed and embedded in paraffin as described in Baligar et al. (2016). Lung sections shall be cut at 4-5 µm from paraffin embedded lung tissues, and mounted on glass slides.- Place slides in glass staining racks. The solutions fill in square glass staining jars. For EasyDip Slide Staining System, each jar holds 80 ml reagent and 12 slides in a staining rack.
- Deparaffinize the sections by two successive xylene baths, 10 min each.
- Hydrate the lung sections by passing through decreasing concentration of alcohol baths: 2 changes of absolute alcohol, 5 min each, 95% alcohol for 2 min and 70% alcohol for 2 min.
- Wash briefly in distilled water.
- Stain in Mayer’s hematoxylin solution for 8 min.
- Wash in warm running tap water for 10 min.
- Rinse in distilled water.
- Counterstain in 1% eosin Y solution for 30 sec to 1 min.
- Dehydrate in 95% alcohol and absolute alcohol, 2 changes of 2 min each or until excess eosin is removed. Check under microscope.
- Clear in xylene, 2 changes of 5 min each.
- Coverslip slides with resinous mounting medium.
- Observe (and photograph if necessary) an H&E stained slide by mounting it on the stage of an optical microscope.
- Place slides in glass staining racks. The solutions fill in square glass staining jars. For EasyDip Slide Staining System, each jar holds 80 ml reagent and 12 slides in a staining rack.
- Masson’s trichrome staining of lung sections
- Deparaffinize and rehydrate lung sections as above.
- Wash the lung sections in distilled water.
- Mordant in preheated Bouin Fluid for one hour at 56-60 °C or overnight at room temperature.
- Stain in Weigert’s iron hematoxylin solution for 10 min.
- Wash in running warm tap water for 10 min.
- Rinse in distilled water.
- Stain in Biebrich scarlet-acid fuchsin solution for 10-15 min.
- Rinse in distilled water.
- Differentiate in phosphomolybdic-phosphotungstic acid solution for 10 to 15 min. Observe the slides frequently with naked eyes. Once collagen-rich areas lose red color and turn to clear proceed to the next step.
- Transfer slides directly to aniline blue solution and stain for 5 to10 min. Rinse in distilled water and differentiate in 1% acetic acid solution for 2 to 5 min.
- Wash in distilled water.
- Dehydrate very quickly through 95% alcohol and then absolute alcohol to wipe off Biebrich scarlet-acid fuchsin staining. Clear in xylene.
- Mount with resinous mounting medium.
- Observe (and photograph if necessary) a trichrome-stained slide by mounting it on the stage of an optical microscope.
- Deparaffinize and rehydrate lung sections as above.
Data analysis
Lung sectioning and staining are essential methods for studying lung development or lung pathology. Immunofluorescent staining visualizes the expression and localization of interested proteins. H&E staining is most widely used in histology studies and medical diagnosis. Masson’s trichrome staining detects collagen deposition in tissues. Figure 2 shows representative images of each staining on the lung sections from mice that had been undergone bone marrow transplantation and then infected with murine gamma herpesvirus 68 for three weeks.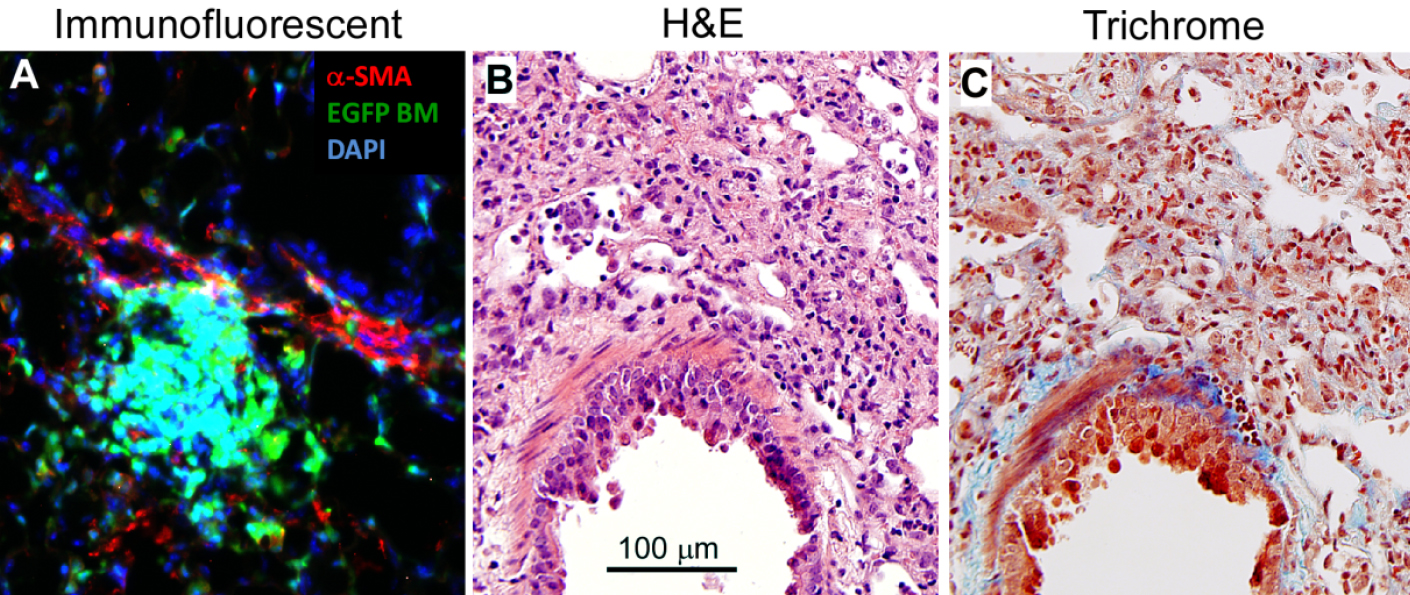
Figure 2. Lung section staining. The lungs were removed from C57BL/6 mice that had undergone transplantation of syngeneic EGFP-expressing bone marrow for 5 weeks, and followed by infection with murine gamma herpesvirus 68 (5 x 104 pfu/mouse) for 3 weeks. The lung had developed pneumonitis and pulmonary fibrosis as evidenced by infiltration of immune cells and deposition of collagen. A. Lung section is immunofluorescent stained with rabbit anti α-smooth muscle actin (α-SMA) antibody and α-SMA positive cells are visualized by Texas Red conjugated goat anti-rabbit IgG antibody in red. Donor bone marrow-derived cells express EGFP in green. DAPI counterstains all of the cell nuclei in blue. B. H&E staining colors cytoplasm pink and nuclei blue; C. Masson’s trichrome staining colors collagen blue, cytoplasm pink and nuclei dark brown to black.
Recipes
- Blocking buffer
5% serum from secondary antibody’s host, or 1% bovine serum albumin (BSA) or fetal bovine serum (FBS) in PBS pH 7.5 - Eosin Y solution
Eosin Y stock solution (1%):
10 g eosin Y
200 ml distilled water
800 ml of 95% ethanol
Mix to dissolve and store at room temperature - Eosin Y working solution (0.25%)
250 ml of eosin Y stock solution
750 ml of 80% ethanol
5 ml glacial acetic acid (concentrated)
Mix well and store at room temperature - Mayer’s hematoxylin solution
50 g aluminum potassium sulfate (alum)
1 g hematoxylin
0.2 g sodium iodate
1 g citric acid
1,000 ml distilled water
Stir to dissolve the chemicals in the order listed above - Weigert’s iron hematoxylin solution
Stock solution A:
1 g hematoxylin
100 ml of 95% alcohol
Stock solution B:
4 ml of 29% ferric chloride in distilled water
95 ml distilled water
1 ml hydrochloric acid, concentrated
Weigert’s iron hematoxylin working solution:
Mix equal parts of stock solution A and B. This working solution is stable for 3 months - Phosphomolybdic-phosphotungstic acid solution
25 ml of 5% phosphomolybdic acid
25 ml of 5% phosphotungstic acid - Aniline blue solution
2.5 g aniline blue
2 ml acetic acid, glacial
100 ml distilled water
Acknowledgments
This protocol was adapted from our publication (Zhou et al., 2016). This work was supported by NIH grants AI117229, HL115618, T32HL07749 and 2UL1TR000433.
References
- Baligar, P., Pokhrel, S. and Mukhopadhyay, A. (2016). Experimental liver fibrosis and intrasplenic transplantation of CD45+ bone marrow cells. Bio-protocol 6(20): e1972.
- Ling, L. H., Rogers, S. M., Tay, V., Limmon, G. V., Dahai, Z. and Rogers, K. (2009). Comparison of various tissue-preparation techniques for cryosectioning of frozen mouse tissues. J Histotechnol 32(4): 186-189.
- Zhou, X., Loomis-King, H., Gurczynski, S. J., Wilke, C. A., Konopka, K. E., Ptaschinski, C., Coomes, S. M., Iwakura, Y., van Dyk, L. F., Lukacs, N. W. and Moore, B. B. (2016). Bone marrow transplantation alters lung antigen-presenting cells to promote TH17 response and the development of pneumonitis and fibrosis following gammaherpesvirus infection. Mucosal Immunol 9(3): 610-620.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhou, X. and Moore, B. B. (2017). Lung Section Staining and Microscopy. Bio-protocol 7(10): e2286. DOI: 10.21769/BioProtoc.2286.
Category
Immunology > Immune cell staining > Immunodetection
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









