- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse CD8+ T Cell Migration in vitro and CXCR3 Internalization Assays
Published: Vol 7, Iss 6, Mar 20, 2017 DOI: 10.21769/BioProtoc.2185 Views: 17951
Reviewed by: Ivan ZanoniMeenal SinhaYang Fu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Functional Phenotyping of Lung Mouse CD4+ T Cells Using Multiparametric Flow Cytometry Analysis
Céline M. Maquet [...] Bénédicte D. Machiels
Sep 20, 2023 4200 Views

T-Cell-Based Platform for Functional Screening of T-Cell Receptors Identified in Single-Cell RNA Sequencing Data Sets of Tumor-Infiltrating T-Cells
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
Apr 20, 2024 6396 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3547 Views
Abstract
Chemokines are molecules that regulate the positioning of cells during homeostasis and inflammation. CXCL10 is an interferon-induced chemokine that attracts cells that express the chemokine receptor CXCR3 on their surface. CXCL10 expression is often induced upon inflammation and guides lymphocytes, such as T and NK cells, into the injured tissues. Notably, CXCL10 binding to CXCR3 induces receptor internalization and, therefore, low CXCR3 levels in cells positive for CXCR3 expression can be indicative of chemokine signaling.
Here, we describe an in vitro method to evaluate the ability of murine CD8+ T cells to migrate towards recombinant murine CXCL10; and a flow cytometry assay to measure CXCR3 expression levels at the surface of T cells, after exposure to different doses of chemokine.
Background
Chemokine-mediated T cell trafficking is an important process during homeostasis and inflammation. Activated CD8+ T cells express chemokine receptors, such as CXCR3, allowing them to migrate towards the chemokines CXCL9, 10 and 11, often upregulated at the injured tissue. The evaluation of molecular cues that modulate T cell migration is important to understand the biology behind their functions but the complex mechanisms operating in vivo are sometimes hard to deconvolve. Here, we provide detailed information on an in vitro method to evaluate chemokine functions on CD8+ T cells, focusing on CXCL10-mediated chemo-attraction and CXCR3 internalization. We use antigen-specific transgenic CD8+ T cells that can be easily expanded and activated in vitro, therefore providing enough number of phenotypically identical lymphocytes (e.g., high chemokine-receptor expression on their surface), required to perform an assay using enough replicates for biologically significant observations and statistical analysis. The cells used per assay originate from one single animal, therefore accounting for reduction of animal usage. This assay is combined with flow cytometry analysis, permitting simultaneous evaluation of 1) number of migrating CD8+ T cells; and 2) phenotypic characterization of chemokine receptor levels on their surface.
Materials and Reagents
- 6 well plate (Fisher Scientific, catalog number: 08-772-33 )
- Sterile pipettes
5 ml (Corning, catalog number: 4051 )
10 ml (Corning, catalog number: 4101 )
25 ml (Corning, catalog number: 4251 ) - 70 μm cell strainer (Corning, catalog number: 431751 )
- 5 ml syringes, without needle (BD, catalog number: 309646 )
- Sterile conical tubes
15 ml (Corning, catalog number: 352099 )
50 ml (Corning, catalog number: 352098 ) - 24 well plate (Corning, catalog number: 353226 )
- Corning® HTS Transwell® 96 well permeable supports, 5.0 μm pore size (Corning, catalog number: 3387 )
- 96 well plates, U bottom (Fisher Scientific, catalog number: 12-565-500 )
- 1.5 ml Eppendorf tubes (Fisher Scientific, catalog number: 05-408-129 )
- Flow cytometry tubes (Round-Bottom Polystyrene Tubes, Corning, catalog number: 352052 )
- CD8+ T cell TCR transgenic mouse, such as OT-1 (C57BL/6-Tg[TcraTcrb]1100Mjb/J, THE JACKSON LABORATORIES, catalog number: 003831 ) or Pmel-1 (B6.Cg-Thy1a/Cy Tg[TcraTcrb]8Rest/J, THE JACKSON LABORATORIES, catalog number: 005023 ) mouse (age 8-12 weeks)
- TCR-specific peptide
If using OT-1 cells – SIINFEKL (AnaSpect, catalog number: AS-60193-1 )
If using Pmel-1 cells – human gp100 (AnaSpec, catalog number: AS-62589 ) - 1x PBS (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- Recombinant human IL-2 (Miltenyi Biotec, catalog number: 130-097-742 )
- Recombinant murine CXCL10 (Peprotech, catalog number: 250-16 )
- AccuCheck Counting Beads reagent (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: PCB100 )
- RPMI 1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 11875093 )
- Fetal bovine serum (Seradigm, catalog number: 1500-100 )
- Non-essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number: 11140-050 )
- Sodium pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11360-070 )
- HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 15630-080 )
- Beta-mercaptoethanol (Thermo Fisher Scientific, GibcoTM, catalog number: 21985-023 )
- Gentamycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15710-064 )
- Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14025092 )
- Protease-free BSA (GE Healthcare, catalog number: SH30574.02 )
- Mouse CD16/CD32 Fc Blocking antibodies (BD, catalog number: 553142 )
- LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit, for 405 nm excitation (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: L34957 ). Reconstitute each vial with 50 μl of DMSO provided with the kit
- Pacific Blue-conjugated anti-mouse CD3 (clone 145-2C11) (BioLegend, catalog number: 100334 )
- APC-conjugated CD8 (clone 53-6.7) (BD, catalog number: 553035 )
- PE-conjugated CXCR3 (clone CXCR3-173) (BioLegend, catalog number: 126506 )
- R10 media (see Recipes)
- Migration media (see Recipes)
- Blocking buffer (see Recipes)
- Staining mix (see Recipes)
Equipment
- Sterile dissection tools such as small scissors and tweezers (tools can be purchased from Carolina or Sigma, for example)
- Bench-top refrigerated centrifuge (such as Thermo Fisher Scientific, Thermo ScientificTM, model: Thermo ScientificTM SorvallTM LegendTM X1 )
- Incubator (37 °C, 5% CO2, 95% humidity, such as Thermo Fisher Scientific, Thermo ScientificTM, model: Forma Steri-Cycle CO2 incubator )
- Tissue culture hood (Biosafety cabinet, such as The Baker Company, model: SterilGARD e3 )
- Bench-top orbital shaker (such as Thermo Fisher Scientific, model: Thermo Barnstead 4625 Titer Plate Shaker , catalog number: THERMO BARNSTEAD 4625 TITER PLATE SHAKER-1478)
- Optical microscope (such as Leica, model: DM IL LED )
- Neubauer cell counting chamber (such as InCyto, catalog number: DHC-N01 )
- Flow cytometer (such as the BD, model: LSRFortessa )
Software
- Flow cytometer software: DIVA
- Computer analysis software: FlowJo (Treestar)
Procedure
- Obtaining CXCR3-expressing CD8+ T cells
Note: This step of the protocol explains how to generate enough numbers of CD8+ T cells used in the subsequent steps.- Sacrifice one OT-1 or Pmel-1 transgenic mouse.
- Collect spleen in sterile conditions using sharp scissors and tweezers.
- Place spleen in a well of a sterile 6 well plate, containing 2 ml of sterile ice cold 1x PBS.
- Mash spleen using the back part of a 5 ml syringe plunger.
- Collect cells with a 5 ml pipette (pipette up and down at least 3 times in order to homogenize the cell suspension) and pass them through a 70 μm cell strainer, into a 50 ml sterile conical tube.
- Wash filter with 7 ml of ice cold sterile 1x PBS and collect. Wash it into the same conical tube.
- Centrifuge cells at 350 x g, 5 min, 4 °C.
- Discard supernatant and resuspend cells in 10 ml of R10.
- Add 1 nM of TCR-specific peptide (SIINFEKL or human gp100, respectively if you use an OT-1 transgenic or Pmel-1 transgenic mouse).
Note: The TCR-specific peptide will initiate T cell activation. - Incubate at room temperature, in a bench-top orbital shaker with agitation for 1 h.
Note: Set up shaker speed so the liquid inside the conical tube is moving – speed 3 or 4 in the orbital shaker indicated in the Equipment section. - Add 10 ml of fresh R10 (room temperature) and centrifuge cells at 350 x g, 5 min, 4 °C.
- Discard supernatant and resuspend cells in 50 ml of fresh R10 (room temperature), supplemented with 30 U/ml of recombinant human IL-2.
Note: IL-2 promotes T cell expansion and survival. - Place cells in a 24 well plate (2 ml per well) and place plate in an incubator at 37 °C, 5% CO2, 95% humidity.
Note: There is no need to isolate CD8+ T cells during this procedure. The percentage of CD8+ T cells found in a spleen of a TCR transgenic mouse is approximately 10-20% of total hematopoietic cells, and they will expand during the next steps. - Split cells every two to three days by collecting 500 μl of cell suspension from each well and placing them in a new 24 well plate, filled with 1.5 ml of fresh R10 (room temperature), supplemented with 30 U/ml of recombinant human IL-2 (dilution of cells is 1 to 4).
- After 6-7 days (about 2 cell splits), the cells collected should be over 90% CD8+ T cells and harbor high CXCR3 expression on their surface (please refer to the flow cytometry protocol below for evaluation of CXCR3 expression).
Note: By the end of the expansion, each plate should have at least 10 million CD8+ T cells.
- In vitro migration assay
Note: This step of the protocol explains how to evaluate the ability of CXCL10 to induce migration of CD8+ T cells generated in Procedure A.- Collect activated CD8+ T cells (around 45 ml total volume per plate) in a 50 ml conical tube and spin at 350 x g, 5 min, 4 °C. Cells are collected by pipetting up and down 3-5 times per well.
- Discard supernatant and resuspend cells in 5 ml of migration media.
- Count cells.
- Adjust volume so you have 5 x 105 cells/100 μl of migration media.
- Remove transwell insert from the Transwell 96 well plate, exposing the wells from the receiving plate.
- Prepare dilutions of chemokine, in migration media, and add them to the wells of the receiving plate. We advice to do triplicates for each condition.
Note: Final volume in the receiving plate is 200 μl. Please refer to the notes at the end of this step of the protocol for suggested dilutions of CXCL10. - Carefully place the transwell onto the receiving plate. The liquid from each well will be in contact with the transwell.
- Carefully add 100 μl of CD8+ T cell suspension (5 x 105 cells) to each transwell. Close the plate and place in the incubator at 37 °C, 5% CO2, 95% humidity for 1 h and 30 min.
- After the incubation time, carefully remove the transwell plate away from the receiving plate.
- Collect the cells from the wells of the receiving plate (by pipetting up and down 3-5 times) onto a new 96-well U-bottom plate. We advise that this step is performed with a multichannel pipet.
- Spin the 96 U bottom plate at 350 x g, 2 min and discard supernatant.
- Resuspend cells with blocking buffer (50 μl per well). Incubate for 20 min, 4 °C in the dark.
- Add 150 μl of 1x PBS, spin the plate at 350 x g, 2 min and discard supernatant.
- Resuspend cells with staining mix (50 μl per well). Incubate for 30 min, 4 °C in the dark.
- Add 150 μl of 1x PBS, spin the plate at 350 x g, 2 min and discard supernatant.
- Resuspend cells in 200 μl of 1x PBS.
- Transfer cells to flow cytometry tubes.
- Add 10 μl of counting beads to each tube and run samples in the flow cytometer. Acquire at least 2,000 events on the fluorescent beads gate and 2,000 events on the CD3+CD8+ T cell gate, as 1,000 events is the minimum number of events required for accurate cell counting using the AccuCheck Counting Beads Kit.
Note: In cases where T cell migration is residual (e.g., conditions without chemoattractant) it might be difficult to reach the required number of events on the T cell gate, but make sure you get the maximum events possible, by running the entire sample. - Analysis of flow cytometry data is done using the Flow Jo software.
- Collect activated CD8+ T cells (around 45 ml total volume per plate) in a 50 ml conical tube and spin at 350 x g, 5 min, 4 °C. Cells are collected by pipetting up and down 3-5 times per well.
- CXCR3 internalization assay
Note: This step of the protocol explains how to evaluate the ability of CXCL10 to induce internalization of the chemokine receptor CXCR3, expressed at the surface of CD8+ T cells generated in the Procedure A.- Collect activated CD8+ T cells in a 50 ml conical tube and spin at 350 x g, 5 min, 4 °C.
- Discard supernatant and resuspend cells in 5 ml of migration media.
- Count cells.
- Adjust volume so you have 5 x 105 cells/100 μl of migration media.
- Plate cells in a 96-well U-bottom well plate (100 μl per well).
- Add 100 μl of migration media supplemented with different concentrations of murine CXCL10 (200 μl total volume per well). Use the same concentration range as in Procedure B. Alternatively, you can do 10 fold dilutions, including 1,000; 100; 10; and 1 ng/ml.
- Place plate in the incubator at 37 °C 5% CO2 95% humidity for 1 h and 30 min.
Note: The time point for this assay (1 h and 30 min) was chosen to match with the time point used in migration assay, described in Procedure B of this protocol. For new chemotatic stimuli, we recommend a time course study to be done (e.g., evaluation of chemokine-receptor internalization after different time points of incubation with chemokines). - After the incubation time, spin the plate at 350 x g, 2 min and discard supernatant.
- Resuspend cells with blocking buffer (50 μl per well). Incubate for 20 min, 4 °C in the dark.
- Add 150 μl of cold 1x PBS, spin the plate at 350 x g, 2 min and discard supernatant.
- Resuspend cells with staining mix (50 μl per well). Incubate for 30 min, 4 °C in the dark.
- Add 150 μl of cold 1x PBS and resuspend them in 200 μl of 1x PBS.
- Transfer cells to flow cytometry tubes and acquire at least 10,000 events per tube.
- Analysis of flow cytometry data is done using the Flow Jo software.
- Collect activated CD8+ T cells in a 50 ml conical tube and spin at 350 x g, 5 min, 4 °C.
Data analysis
After exclusion of debris and dead cells (Aqua+ cells) the flow cytometry plots are visualized as represented in Figure 1. For calculation of the number of T cells that migrated into the lower chamber, gate on CD8+ cells (red gate) and on fluorescent beads (blue gate) and follow the instruction for calculation of cell numbers by the provider of the beads. For evaluation of CXCR3 levels on the surface of T cells, plot gated CD8+ T cells as histograms (Figure 2). You can obtain the values of mean fluorescence intensity (MFI) on the FlowJo software and plot them as graphs as well. It is recommended to run at least 3 technical replicates, per experiment.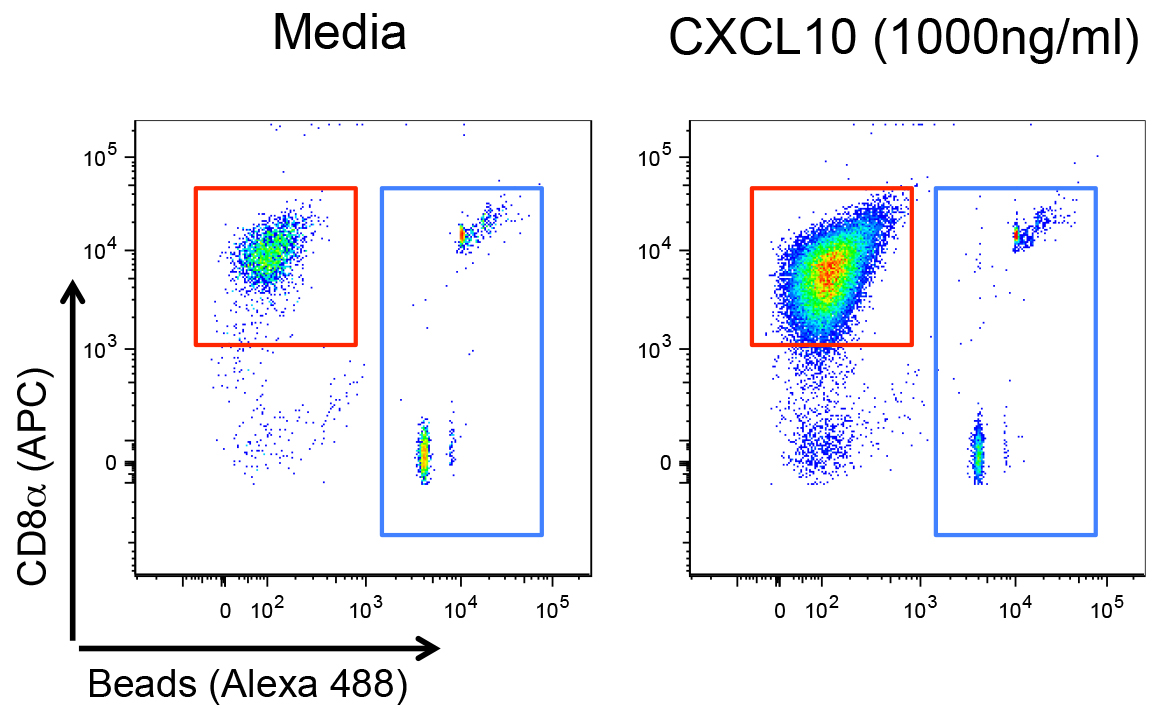
Figure 1. Example of flow cytometry plots corresponding to the cells collected from the receiving chambers of the transwell plate, after exposure to migration media (left plot) or migration media supplemented with 1,000 ng/ml of recombinant murine CXCL10 (right plot). Cells were stained and fluorescent counting beads (gated in blue) were added to the cells as described in the Procedure B section. Debris and Aqua+ (dead) cells were excluded before this representation. Live CD3+CD8+ T cells are gated in red. APC: Allophycocyanin.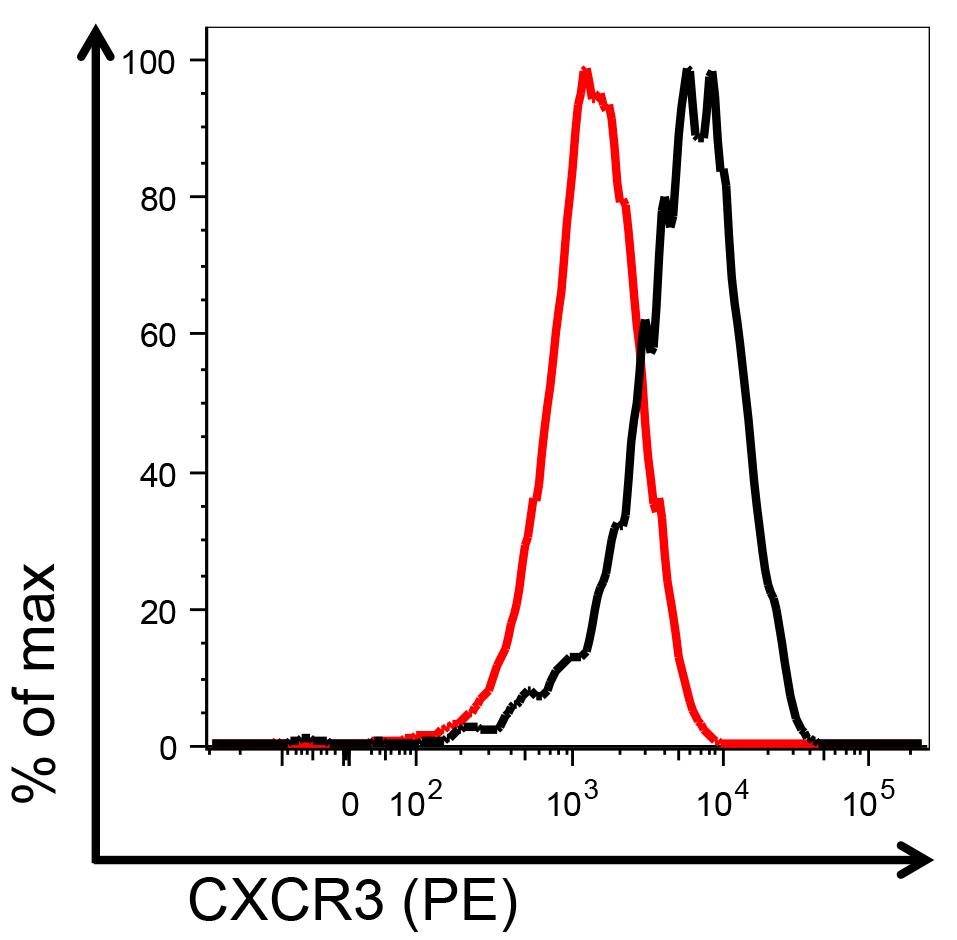
Figure 2. Example of flow cytometry histograms representing CXCR3 staining on CD3+CD8+ T cells collected from the receiving chambers of the transwell plate, after exposure to migration media (black) or migration media supplemented with 1,000 ng/ml of recombinant murine CXCL10 (red). % of max: percentage of maximum staining intensity; PE: R-Phycoerythrin.
Recipes
- R10 media – Store at 4 °C for 1 month
RPMI 1640
10% fetal bovine serum
0.1 mM non-essential amino acids
1 mM sodium pyruvate
10 mM HEPES
70 μM beta-mercaptoethanol
23 μg/ml gentamycin - Migration media – Prepare fresh for each experiment
1x HBSS
0.1% protease-free BSA - Blocking buffer – Use 50 μl per condition, prepare fresh for each experiment
1x PBS
Mouse CD16/CD32 Fc Blocking antibodies (used at 1 to 50 dilution)
Fixable Aqua Dead Cell Stain reagent (used at 1 to 500 dilution) - Staining mix – Use 50 μl per condition, prepare fresh for each experiment
1x PBS
Pacific Blue-conjugated anti-mouse CD3 (clone145-2C11, used at 1 to 100 dilution)
APC-conjugated CD8 (clone 53-6.7, used at 1 to 400 dilution)
PE-conjugated CXCR3 (clone CXCR3-173, used at 1 to 100 dilution)
Acknowledgments
When using this protocol, please refer to Barreira da Silva et al. (2015) Nat Immunol. Funding was provided by the Pasteur-Roux post-doctoral fellowship (RBdS), the Ligue Contre le Cancer and the Fondation ARC pour la recherche sur le cancer (MLA) and the French government’s Invest in the Future Program, managed by the Agence Nationale de la Recherche (LabEx Immuno-Onco [RBdS, MLA]). Animal experimental protocols were approved by the comité d’éthique pour l’expérimentation animale (The ethics committee for animal experimentation) Paris.
References
- Barreira da Silva, R., Laird, M. E., Yatim, N., Fiette, L., Ingersoll, M. A. and Albert, M. L. (2015). Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol 16(8): 850-858.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Barreira da Silva, R. and Albert, M. L. (2017). Mouse CD8+ T Cell Migration in vitro and CXCR3 Internalization Assays. Bio-protocol 7(6): e2185. DOI: 10.21769/BioProtoc.2185.
Category
Immunology > Immune cell function > Lymphocyte
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link