- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Olfactory Bulb (OB) Transplants
Published: Vol 6, Iss 17, Sep 5, 2016 DOI: 10.21769/BioProtoc.1921 Views: 8263
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1843 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3794 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2675 Views
Abstract
Transplantation in mouse brain slices is a powerful tool in order to study axon targeting and migrational events during development. Taking advantage of donors and recipients belonging to different genotypes, this technique allows researchers to assess the contribution of donor and/or recipient tissue by performing various combinations and to study cell-autonomous functions or effects that are influenced by the recipient’s environment (Bastakis, et al., 2015). Here we describe the transplantation procedure on sagittal brain slices containing olfactory bulb (OB). Specifically, we have transplanted the proximal-to-the-cortex part of dorsal OB to the same region on a recipient slice. Transplanted slices can be cultured for up to 3 days before their morphology is disfigured due to growth in 3D. Re-sectioning of these slices allows for a more detailed immunohistochemical analysis.
Materials and Reagents
- Millicell cell culture insert, 30 mm, hydrophilic PTFE, 0.4 µm (Merck Millipore, catalog number: PICM0RG50 )
- Disposable transfer pipettes (SARSTEDT, catalog number: 86.1172.001 )
- 5 ml stripette disposable serological pipette (Sigma-Aldrich, catalog number: CLS4051 )
- 50 ml Falcon tube
- Sterile 24-well plate
- Stainless surgical blade (Swann Morton, Lance, model: No. 24 )
- Vibratome blade (Campden Instruments, catalog number: 752-1-SS )
- 60 mm culture dishes
- Straight triangle insert pin (Fine Science Tools, catalog number: 26007-04 )
- Stainless steel minutien pins 0.10 mm (Fine Science Tools, catalog number: 26002-10 )
- Filters (0.2 μm, 0.45 μm)
- Mouse embryos at embryonic days E13.5-E15.5
- Leibovitz’s L-15 medium (Thermo Fisher Scientific, GibcoTM, catalog number: 11415-064 )
- SeaPlaqueTM low melting temperature agarose (Lonza, catalog number: 50101 )
- Sterile water
- 100 units/ml penicillin-streptomycin (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15070-063 )
- NaCl (Merck Millipore, catalog number: 106404 )
- KCl (Merck Millipore, catalog number: 104936 )
- Na2HPO4 (Merck Millipore, catalog number: 106586 )
- NaH2PO4 (Sigma-Aldrich, catalog number: S-0751 )
- NaOH (Merck Millipore, catalog number: 106498 )
- Glucose (Sigma-Aldrich, catalog number: G8270 )
- DMEM/F12 medium (Thermo Fisher Scientific, GibcoTM, catalog number: 21041-025 )
- Fetal bovine serum (FBS) (Merck Millipore, Biochrom, catalog number: S0115 )
- 1x glutamax (Thermo Fisher Scientific, GibcoTM, catalog number: 35050-038 )
- N-2 supplement (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17502-048 )
- Neurobasal medium (Thermo Fisher Scientific, GibcoTM, catalog number: 12348-017 )
- B-27 supplement (50x) (Thermo Fisher Scientific, GibcoTM, catalog number: 17504-044 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: P6148 )
- Leibovitz’s L-15/penicillin-streptomycin medium (see Recipes)
- 3% SeaPlaque agarose in Leibovitz’s L-15 (see Recipes)
- 10x phosphate buffer solution (PBS) (see Recipes)
- 10 M NaOH (see Recipes)
- Sterile 1x PBS (0.1 M PBS) with 100 units/ml penicillin-streptomycin (see Recipes)
- 25% glucose in 1x PBS (see Recipes)
- DMEM/F12 plus medium (see Recipes)
- Neurobasal/ B-27 plus medium (See Recipes)
- 4% paraformaldehyde (PFA) (see Recipes)
Equipment
- 37 °C, 5% CO2 forced-air incubator (Thermo Fisher Scientific, model: 3110 )
- Dissecting microscope (Leica Microsystems, model: MZ125 )
- 2 Dumont #5 forceps (Fine Science Tools, catalog number: 11251-10 )
- Moria perforated spoon (Fine Science Tools, catalog number: 10370-17 )
- 250 ml glass beaker
- Two-in-One micro spatula (Fine Science Tools, catalog number: 10091-12 )
- Leica vibratome (VT 1000S)
- Nickel plated pin holder (Fine Science Tools, catalog number: 26018-17 )
Note: All surgical tools should be UV-sterilized before use.
Procedure
Housing and all animal procedures used were according to the European Union policy (Directive 86/609/EEC) and institutionally approved protocols. E0.5: day of the vaginal plug.
- Pregnant mice of recipient and donor genotypes are sacrificed with cervical dislocation at the same day of gestation. Embryos at embryonic day 14.5 (E14.5) are easier to handle.
- Embryos are removed from their yolk sacs and their brain is dissected under a dissecting microscope in Leibovitz’s L-15/penicillin-streptomycin medium using Dumont #5 forceps (Video 1).
Video 1. Brain dissection from a mouse E14.5 embryo. The video illustrates step by step instructions for the dissection of the brain from a mouse embryo at the embryonic stage E14.5. - Cortices and OBs are separated from the midbrain (Video 1). An illustration of the cortices and OBs as well as the site of the transplant is shown in Figure 1. We add 2 ml of pre-warmed 3% SeaPlaque agarose in Leibovitz’s/L-15 (60 °C) per well of a 24-well plate placed on ice. Each sample is transferred with a perforated spoon as shown in Figure 2A.
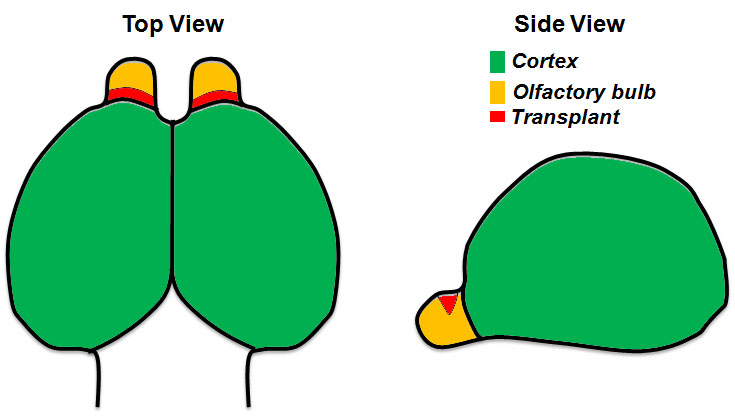
Figure 1. Illustration showing a top and side view of the forebrain from a mouse E14.5 embryo. The area of transplantation is indicated in red. - Swirl the liquid using a 200 μl pipette tip to prevent the sample from touching the bottom of the well (Figure 2B). It is preferable that you set the orientation of your sample in the agarose with the tip before it solidifies, after approximately 10-15 min. In our case the olfactory bulbs are facing up in a vertical position.
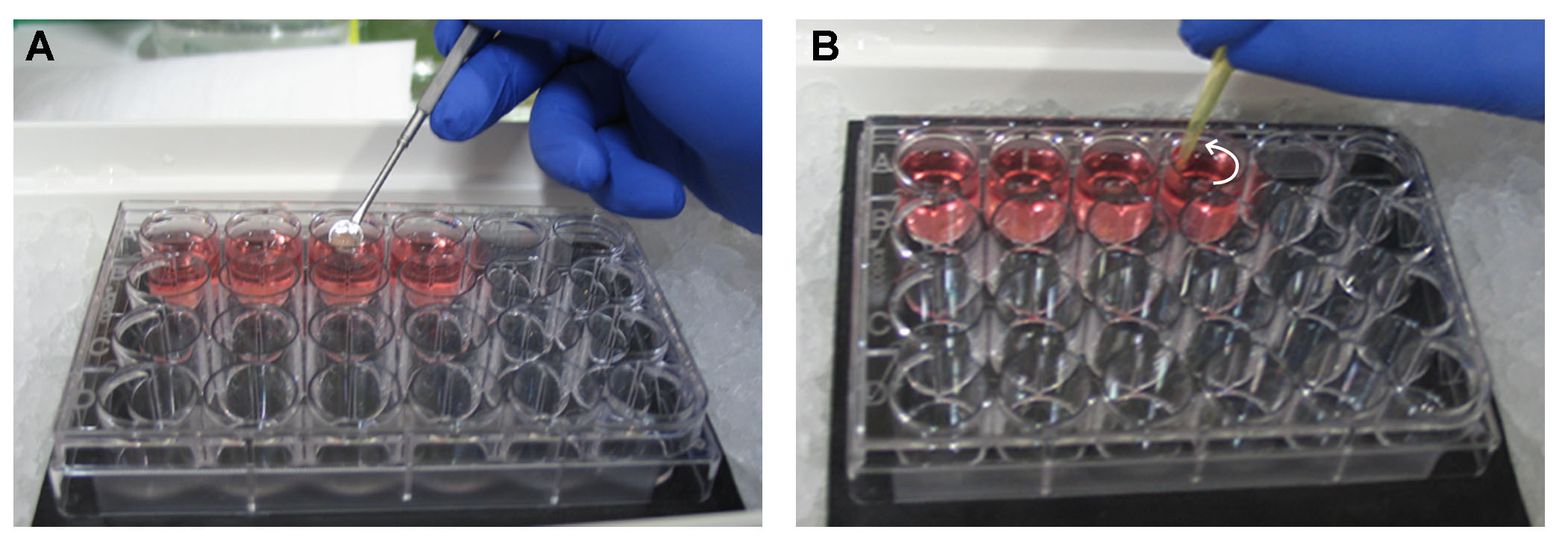
Figure 2. Embedding of a tissue in 3% SeaPlaque agarose in Leibovitz’s L-15 on ice. A. A dissected brain is transferred with a perforated spoon in a well with 3% SeaPlaque agarose in Leibovitz’s L-15. The 24-well plate with the agarose has previously been put on ice. B. Swirl the liquid using a 200 μl pipette tip. Without disrupting the tissue, orient it with the tip on a vertical position. Swirl for approximately 10 min until the agarose starts solidifying. - Fill the tank of the vibratome with ice-cold 1x PBS with penicillin-streptomycin.
- Use a new UV-sterilized vibratome blade.
- Remove the agarose block containing the tissue from the plastic well using a micro spatula.
- Cut the agarose block with a steel blade in order to create a trapezoid with the tissue placed horizontally in its center (Figure 3A).
- Use instant glue and fix the tissue block to the metallic specimen plate of the vibratome with the big base of the trapezoid facing down.
- Adapt the specimen plate in the vibratome and set the speed to 5, the frequency to 5 and the feed to 250 μm.
- Perform continuous cutting and transfer the sections that contain the olfactory bulb to a 60 mm culture dish containing 2 ml of Leibovitz’s L-15/penicillin-streptomycin medium, using a perforated spoon (Figure 3B).
- Put the cell culture insert with the correct orientation in a new 60 mm culture dish containing 2.2 ml of DMEM/F12 plus medium. On the top surface of the membrane add 100 μl of DMEM/F12 plus medium in spots as shown in Figure 3C.
- Remove the surrounding agarose from the tissue sections using the dissecting forceps and transfer them on top of the cell culture insert membrane with a sterile disposable transfer pipette (Figure 3D). Use separate membranes for the recipient and the donor sections.
- Incubate at 37 °C, 5% CO2 for 1 h.
- Take the dish out of the incubator and remove 2 ml of the medium from the plate, using a 5 ml sterile disposable pipette. Afterwards, add 2 ml of prewarmed (at 37 °C) Neurobasal/B27 plus medium to the plate. The sections on top of the membrane remain wet during the whole procedure, and their medium will be gradually replaced with the new one through the membrane pores that are in contact with it.
- Under the dissecting microscope cut a small triangular piece of the OB in the recipient section using the straight triangle insert pin (adapted to the Pin Holder) and remove it from the section with a 200 μl pipette.
- Cut a slightly bigger piece of the donor OB section and transfer it with a 200 μl pipette (transplant in medium) close to the recipient section (Figure 3E).
- Push the transplant (Red* in Figure 3E) towards the missing part of the recipient and adjust it using a minutien pin (adapted to the pin holder) (Figure 3F).
- Incubate the transplanted sections (Figure 3F) at 37 °C, 5% CO2 for 24, 48 or 72 h. Change medium every 24 h.
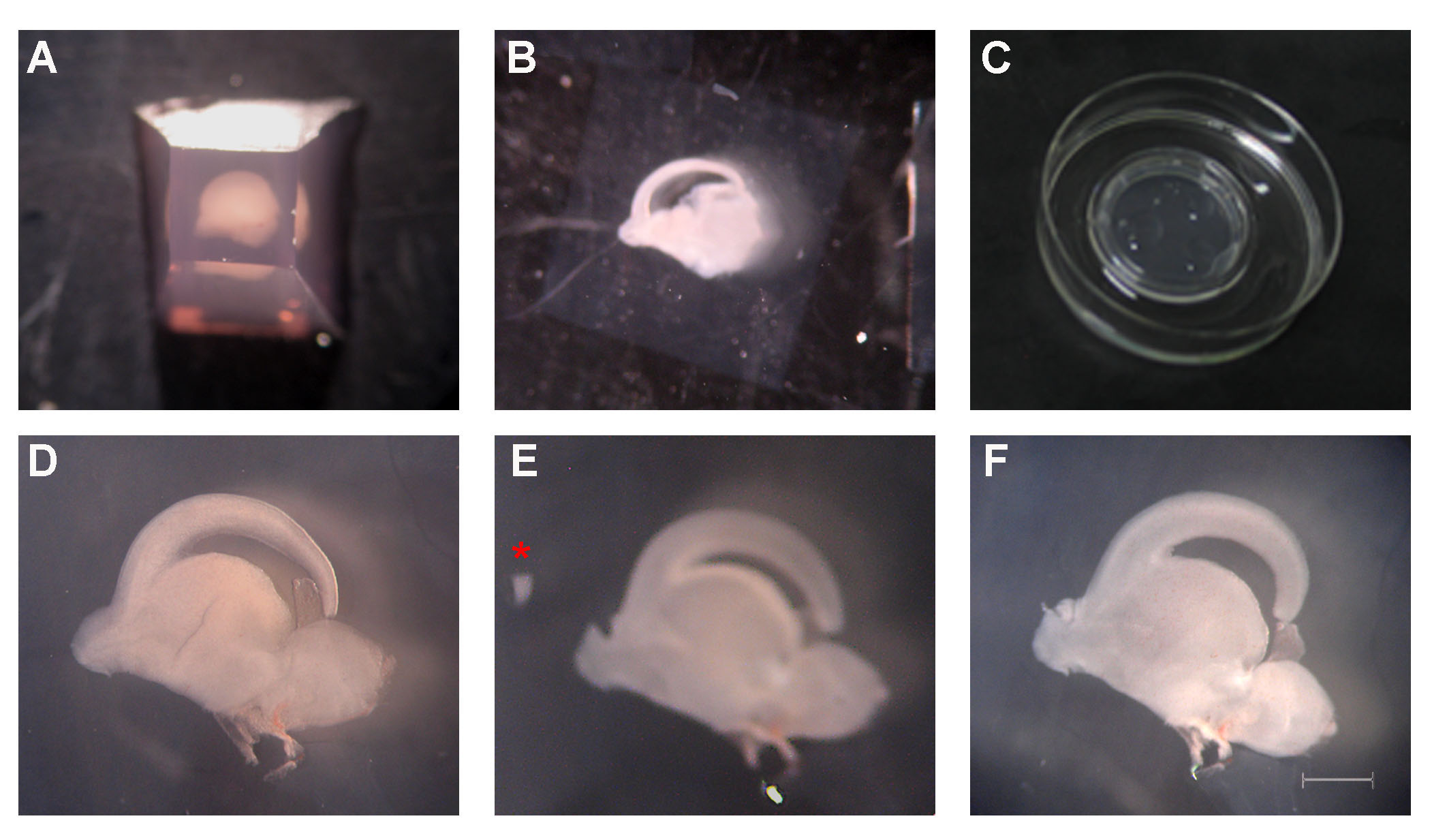
Figure 3. Transplantation procedure in steps. A. A trapezoid is prepared with the tissue placed horizontally in its center. B. Sagittal section containing the forebrain. C. A cell culture insert is applied in a 60 mm culture dish containing 2.2 ml of DMEM/F12 plus medium. Add in spots 100 μl of DMEM/F12 plus medium. D. A brain section, clean of agarose, on the top surface of the cell culture insert. E. The proximal-to-the-cortex part of dorsal OB has been removed from the recipient slice and a slightly larger piece from the same site of the donor is transferred close to the recipient (red asterisk). F. The transplant has been applied to the recipient slice with the correct orientation. Scale: 1 mm in D, E, F. - For further sectioning fix the tissue section with 4% PFA for 1 h at RT, wash in 1x PBS and transfer it to the bottom of a clean well in a 24-well plate.
- Dry the section and re-embed it in 3% of SeaPlaque agarose as described before (the section now should stay at the bottom of the well).
- Re-section the tissue in a similar way to the one described before in the vibratome at a 50 μm thickness.
- Continue with immunostaining as described in Bastakis et al. (2015).
Representative data
Representative images of the expected results regarding migration of projection neurons (Tbr1+) from the proximal-to-the-cortex part of dorsolateral OB derived from a GFP-labelled mouse (BrdU pulsed at E11.5) is shown below (Figure 4, adapted from Bastakis et al., 2015).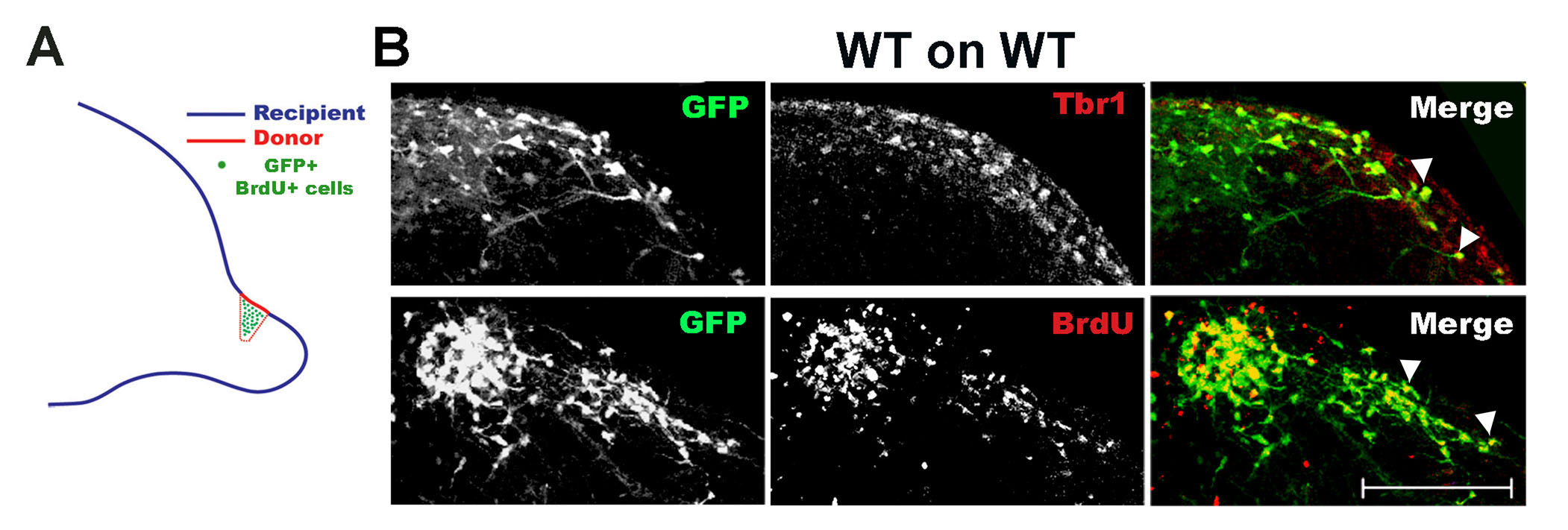
Figure 4. Transplantation of dorsal OB from mouse brain sections for the study of migrating projection neurons. A. Transplants were generated on sagittal E14.5 sections as shown in the scheme, with donor cells expressing GFP or being BrdU-pulsed at E11.5. B. WT GFP+, BrdU+ (BrdU injection at E11.5) cells were transplanted on WT recipients and immunostained for GFP and Tbr1 (marker of immature OB projection neurons) or GFP and BrdU. Migrating cells (GFP+) were projection neurons (co-localization with Tbr1, arrowheads, upper images) and were born at E11.5 (co-localization with BrdU, arrowheads, lower images). The dorsal part of the slice is shown in B. Scale: 150 μm.
Recipes
- Leibovitz’s L-15/penicillin-streptomycin (50 ml)
0.5 ml 100x penicillin-streptomycin
49.5 ml Leibovitz’s L-15
Filter sterilize (0.2 μm)
Store at 4 °C - 3% SeaPlaque agarose in Leibovitz’s L-15 (10 ml)
- Use the microwave at high power to warm up 10 ml of Leibovitz’s L-15 medium in a 50 ml Falcon tube until bubbles appear (just before boiling).
Note: The Falcon tube should be sunk in a 250 ml beaker containing 100 ml of water and the cup should be slightly loose. - Dissolve 0.3 g of SeaPlaque agarose in the pre-warmed medium by vigorous shaking. If there are still undissolved particles, boil for 1 more min as previously described.
- Cool the solution in a water bath to 60 °C prior to use.
- Use the microwave at high power to warm up 10 ml of Leibovitz’s L-15 medium in a 50 ml Falcon tube until bubbles appear (just before boiling).
- 10x PBS (1 liter)
Mix 70.1 g of NaCl with 2 g of KCl, 12.8 g of Na2HPO4, 4.4 g of NaH2PO4
If necessary, adjust pH to 7.4 with 10 M NaOH
Add dH2O to 1 liter
Filter sterilize (0.2 μm)
Store at RT - 10 M NaOH
Weight 40 g of NaOH in a beaker
Add dH2O to make solution up to 100 ml
Store at RT - 1x PBS with 100 units/ml penicillin-streptomycin
Add 100 ml of 10x PBS to 890 ml of sterile water and mix
Add 10 ml of 100x penicillin-streptomycin stock
Mix and filter sterilize (0.2 μm)
Store at 4 °C - 25% glucose in 1x PBS (50 ml)
Dissolve 12.5 g of glucose in 30 ml of 1x PBS
Add 1x PBS to 50 ml
Filter sterilize (0.2 μm)
Store at 4 °C - DMEM/F12 plus (25 ml)
0.6 ml 25% glucose
1.25 ml FBS
0.25 ml 100x penicillin-streptomycin
0.25 ml 100x glutamax
0.25 ml N2 supplement
22.4 ml DMEM/F12
Filter sterilize (0.2 μm)
Store at 4 °C - Neurobasal/B27 plus (50 ml)
1.2 ml 25% glucose
0.5 ml 100x penicillin-streptomycin
0.5 ml 100x glutamax
1 ml B27 supplement
47.75 ml neurobasal
Filter sterilize (0.2 μm)
Store at 4 °C - 4% paraformaldehyde (PFA) in 1x PBS
Dissolve 4 g PFA in a bottle with 100 ml of prewarmed 1x PBS (at 65 °C)
Add 20 μl of 10 M NaOH and mix well until the solution is clear
Filter with a 0.45 μm filter and store at 4 °C for 1 week or at -20 °C for several months
Acknowledgments
This protocol was adapted from the previously published study, de Diego et al. (2002) and it was performed by Bastakis et al. (2015). This work was supported by the European Commission FP7 programme “Translational Potential” [contract number 285948], InnovCrete [316223], ARISTEIA I [Project 593 MyelinTag] and by Institute of Molecular Biology and Biotechnology intramural grants.
References
- Bastakis, G. G., Savvaki, M., Stamatakis, A., Vidaki, M. and Karagogeos, D. (2015). Tag1 deficiency results in olfactory dysfunction through impaired migration of mitral cells. Development 142(24): 4318-4328.
- de Diego, I., Kyriakopoulou, K., Karagogeos, D. and Wassef, M. (2002). Multiple influences on the migration of precerebellar neurons in the caudal medulla. Development 129(2): 297-306.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Savvaki, M. and Karagogeos, D. (2016). Olfactory Bulb (OB) Transplants. Bio-protocol 6(17): e1921. DOI: 10.21769/BioProtoc.1921.
Category
Developmental Biology > Cell growth and fate > Neuron
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link