- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Antifungal and Zearalenone Inhibitory Activity of Ocimum sanctum L. Essential Oil on Fusarium graminearum Determined by UHPLC and RT-qPCR
Published: Vol 6, Iss 15, Aug 5, 2016 DOI: 10.21769/BioProtoc.1893 Views: 10639
Reviewed by: Arsalan DaudiArsheed Hussain SheikhPriyanka Das

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2635 Views

PhosphoLIMBO: An Easy and Efficient Protocol to Separate and Analyze Phospholipids by HPTLC From Plant Material
Louise Fougère [...] Yohann Boutté
Sep 5, 2025 1334 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1568 Views
Abstract
Fusarium graminearum has been given special attention in the context of agricultural commodities due to its ability to grow in diverse climatic conditions, and to produce different mycotoxins including zearalenone (ZEA) and type-B trichothecenes, which cause ill health effects on humans, animals and plants. The application of synthetic antifungal agents for the control of F. graminearum result in negative health impacts in livestock and humans and the upsurge of resistant organisms as well. Therefore, there is a need to propose proper food grain management practices, including the application of herbal antifungal and mycotoxin controlling agents, to reduce the growth of toxigenic F. graminearum as well as the production of ZEA in agricultural commodities. Ocimum sanctum also known as Holy Basil or Tulsi is widely used as a medicinal plant in Ayurveda. The current protocol demonstrates to quantify the antifungal activity of O. sanctum L. essential oil (OSEO) as reflected by the decreased F. graminearum growth and ZEA production. Antifungal activities of OSEO are carried out by micro well dilution method and further validated quantitatively by scanning electron microscopic methods. Effects of OSEO on ZEA production is analysed by Quantitative reverse transcription PCR (RT-qPCR) and Ultra high performance liquid chromatography (UHPLC) methods from a broth culture of F. graminearum. Anti-mycotoxic efficacy of OSEO is assessed directly on F. graminearum inoculated maize grains. The protocol efficiently assessed the activity of OSEO as an herbal antagonistic agent against fungal infestation and ZEA production by F. graminearum. The protocol can be used to test a wide variety of herbal compounds for antifungal activity against F. graminearum or with modifications on other mycotoxigenic fungi, an important intervention in food safety and processing industries where the fungal infestation is a major concern.
Keywords: ZearalenoneMaterials and Reagents
- 0.22 µm Millex-GP syringe filter unit (Sigma-Aldrich, catalog number: Z359904 )
- 96-well microtiter plates (Eppendorf, catalog number: 0030602200 )
- Column C18, 5 µm, 250 x 4.6 mm (Phenomenex, catalog number: 00G-4041-E0 )
- Carbon Conductive Tape (Ted Pella, Inc., catalog number: 16084-7 )
- Glass slides (HiMedia, catalog number: CG081 )
- Whatman No.1 paper (Sigma-Aldrich, catalog number: Z274852 )
- Zearalenone producing F. graminearum [The Microbial Type Culture Collection and Gene Bank, (MTCC), catalog number: 1893 ]
- Maize grains (Local agricultural market, Mysore, India)
- Zearalenone standard (Sigma-Aldrich, catalog number: Z2125 )
- Dimethyl sulfoxide (Merck Millipore, catalog number: 317275 )
- Distilled water
- Sodium chloride (NaCl) (Merck Millipore, catalog number: 1064040500 )
- Potassium chloride (KCl) (Merck Millipore, catalog number: 1049360250 )
- Sodium phosphate dibasic (Na2HPO4) (Merck Millipore, catalog number: 567550-1KG )
- Potassium phosphate monobasic (KH2PO4) (Merck Millipore, catalog number: 1048730250 )
- 0.1 M sodium cacodylate buffer, pH 6.5 (Sigma-Aldrich, catalog number: 70114 )
- 25% glutaraldehyde (Merck Millipore, catalog number: 354400 )
- Acetonitrile (Merck Millipore, catalog number: 100030 )
- Ethanol (Merck Millipore, catalog number: 100983 )
- Gold foil (Sigma-Aldrich, catalog number: 265829 )
- Immunoaffinity column of ZEA (Vicam, catalog number: G1026 )
- iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad Laboratories, catalog number: 1708892 )
- Liquid nitrogen (Local suppliers, Mysore, India)
- Nuclease-free water (Qiagen, catalog number: 129114 )
- Nystatin (Sigma-Aldrich, catalog number: N6261 )
- Ocimum sanctum L. essential oil (OSEO) (prepared as describe in Procedure step 2)
- Peptone (HiMedia, catalog number: RM001-500G )
- Porcelain mortar (Sigma-Aldrich, catalog number: Z529508 )
- RNA easy plant Mini kit (Qiagen, catalog number: 74903 )
- Sabouraud dextrose agar (HiMedia, catalog number: M063-500G )
- Sabouraud dextrose broth (HiMedia, catalog number: M033-500G )
- Synthesized primer sequences (Sigma-Aldrich, Bangalore, India)
- Tween 80 (Merck Millipore, catalog number: 822187 )
- Lactophenol-cotton blue (HiMedia, catalog number: S016-500ML )
- Phosphate-buffered saline (pH 7.4) (see Recipes)
- Lactophenol-cotton blue staining solution (see Recipes)
Equipment
- Milli-Q integral water purification system (Merck Millipore, catalog number: ZRXQ005WW )
- Aluminum stubs (Ted Pella, Inc., catalog number: 16111N )
- Autoclave (Medica Instrument Manufacturing Company, model: 7431PAD )
- Microcentrifuge (Sigma-Aldrich, Eppendorf, model: 5415 R )
- Centrifuge (Eppendorf, model: 5430 R )
- Hemocytometer (Sigma-Aldrich, catalog number: Z359629 )
- Hot-air oven (Memmert, model: UFP800DW )
- Incubator (Bio-age, model: BSR-R2 )
- Real-Time PCR system (Roche Diagnostics, Light cycler®, model: 480 )
- 0.5-10 µl micropipette (Eppendorf Research plus, catalog number: 3120000020 )
- 20-200 µl micropipette (Eppendorf Research plus, catalog number: 3120000054 )
- 100-1,000 µl micropipette (Eppendorf Research plus, catalog number: 3120000062 )
- Microscope (Leica Microsystems, model: Leica DM 1000 LED )
- 250 ml Erlenmeyer flasks (Duran Group, catalog number: 21 216 36 )
- 500 ml Erlenmeyer flasks (Duran Group, catalog number: 21 216 44 )
- NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, catalog number: ND-8000-GL )
- Nexera UHPLC system (Shimadzu Corporation, model: Nexera X2 )
- Scanning electron microscope (FEI, model: Quanta 200 )
Note: This product has been discontinued by the manufacturer [Replaceable items (FEI, model: Quanta 250/450/650 )]. - Shaker Incubator (Bio-age, model: BSR-R1 )
- Sputter coater (Quorum Technologies, model: SC7620 )
- Water bath (NUVE, model: NB 5 )
- Weighing balance (Denver instruments, model: TB-215D )
Software
- GeneRunner software version 5.0.47 Beta
Procedure
- Grow the zearalenone (ZEA) producing F. graminearum (MTCC, 1893) on Sabouraud dextrose agar (SDA) for 7 days at 28 °C and collect the spores in 10 ml of peptone water containing 0.001% Tween 80 with a soft scrape. Determine the number of spores using a hemocytometer and adjust the spore suspension to 1 x 106 per ml.
- Collect the Ocimum sanctum L. plant and identify its botanical nomenclature, and dry the plant at room temperature under dark condition. Extract the essential oil from dried aerial parts following the technique of European Pharmacopoeia (Council of Europe, 1997). Prepare a stock solution of 0.05% OSEO in DMSO.
Note: In the present study, Ocimum sanctum L. was collected from Mysore, Karnataka state, India and identification was done by Botanical Survey of India (Coimbatore, India). - Determine the minimum inhibitory (MIC) and minimum fungicidal concentrations (MFC) of OSEO on F. graminearum by micro-well dilution technique in 96-well microtiter plate implementing the methodology of Clinical and Laboratory Standards Institute (2008) and Vieira et al. (2014) with following minor modifications.
- Add 10 µl of spore suspension (1 x 106 spores per ml) to the different concentrations of OSEO and adjust the total volume to100 µl per well with Sabouraud dextrose broth (SDB).
- Consider the wells without OSEO as control and incubate the microplates for 3 days at 28 °C in the dark.
- Observe the minimum concentration of OSEO without detectable fungal growth and determine as minimum inhibitory concentration (MIC).
- Spread plate 10 µl from each well on SDA plates and incubate at 28 °C for 3 days.
- Identify the minimum concentration of OSEO with no detectable fungal growth and determine as the MFC, specifying 99.5% killing of the original inoculum in comparison to nystatin (positive control).
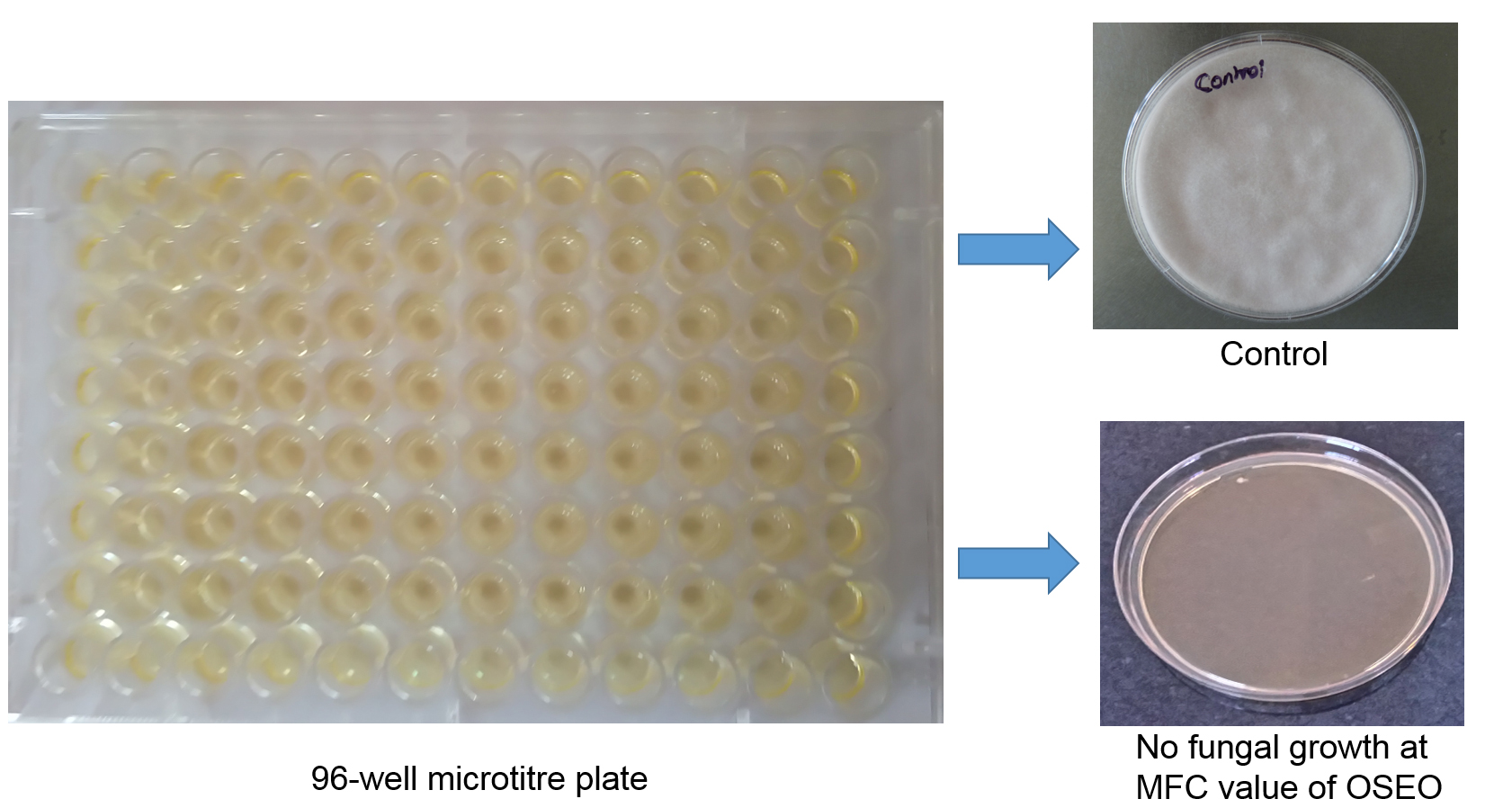
Figure 1. Determination of minimum inhibitory (MIC) and minimum fungicidal concentrations (MFC) of O. sanctum essential oil (OSEO) on F. graminearum by micro-well dilution method. Fungal growth is observed in control (OSEO untreated) and no detectable growth is observed at MFC value of OSEO. - Add 10 µl of spore suspension (1 x 106 spores per ml) to the different concentrations of OSEO and adjust the total volume to100 µl per well with Sabouraud dextrose broth (SDB).
- Analyse the effect of OSEO on spore germination of F. graminearum by the method of Rana et al. (1997) with minor modifications.
- Inoculate 10 µl of fungal spore suspension (1 x 106 spores per ml) on SDA slides containing different concentrations of OSEO (100-1,800 µg/ml) and incubate at 28 °C for 24 h.
- Consider SDA slide alone with fungal spores and without OSEO as control.
- Following the incubation period, stain each slide with lactophenol-cotton blue (100 µl) by dropping method at 28 °C for 15 min and observe for germ tubes under microscope.
- Examine at least 200 spores from each slide and calculate the percentage of spore germination using the formula,
% Spore germination = ST/SC x 100
Where, SC is number of spores germinated in control and ST is number of spores germinated in test.
- Inoculate 10 µl of fungal spore suspension (1 x 106 spores per ml) on SDA slides containing different concentrations of OSEO (100-1,800 µg/ml) and incubate at 28 °C for 24 h.
- Determine the effect of OSEO on mycelial and spore structure of F. graminearum by scanning electron microscope (SEM) observation according to the method of Yamamoto-Ribeiro et al. (2013) with minor modifications.
- Collect the mycelia disk of 1 cm2 from a seven-day culture of F. graminearum and inoculate aseptically at the middle of SDA dishes that contained different concentrations of OSEO and incubate at 28 °C for 7 days in the dark.
- Consider the SDA medium without OSEO as control.
- After the incubation period, collect mycelial disk of 1 cm2 and rinse in phosphate-buffered saline (pH 7.4) and fix with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 6.5 and dehydrate with gradient ethanol (20, 40, 70, 90 and 100%, keeping the mycelia for a longer duration in 100%).
- Paste the sample on dual side glue carbon conductive tape and fix to the surface of aluminum stubs.
- Further, expose the stubs towards critical-point dry out in CO2 and sputter-coat with gold to increase its conductivity.
- Observe the morphological quality of mycelia under scanning electron microscope at 20.0 KV in environmental mode.

Figure 2. Determination of the antifungal activity of O. sanctum L. essential oil (OSEO) on F. graminearum by scanning electron microscopic observation (Kalagatur et al., 2015). The control (OSEO untreated) hyphae (a) is smooth, turgid and homogenous. Whereas hyphae treated with MIC (b) and MFC (c) values of OSEO exhibited craters, protuberances and collapsed. The control (OSEO untreated) spores (d) are round and smooth, and on other hand spores treated with MIC (e) and MFC (f) values of OSEO are disrupted and wrinkled. - Collect the mycelia disk of 1 cm2 from a seven-day culture of F. graminearum and inoculate aseptically at the middle of SDA dishes that contained different concentrations of OSEO and incubate at 28 °C for 7 days in the dark.
- Determine the anti-mycotoxic activity of OSEO in liquid cultures. Add different concentrations of OSEO including, 250, 500, 1,000, 1,500 and 2,000 μg/ml to each 250 ml Erlenmeyer flask that contain 100 ml of SDB. Inoculate 10 μl of fungal suspension (1 x 106 spores/ml) of 7-day-old culture into these flasks under aseptic conditions. Consider the flask without OSEO as control and incubate the flasks under shaking condition (140-160 rpm) at 28 °C for 14 days in the dark.
- Following the incubation period, separate the culture media from fungal biomass by filtering through Whatman No.1 paper and use the broth for determination of ZEA. Wash the fungal mycelia twice with sterile distilled water and use 10 mg of mycelia for RNA extraction, and pack the leftover mycelia in pre-weighed Whatman no.1 filter paper and dry out at 60 °C for 24 h and determine the mycelial biomass by weighing.
- Detection and quantification of ZEA by UHPLC by the method of Ibáñez-Vea et al. (2011) with slight modifications.
- Blend the culture broth with an equivalent quantity of acetonitrile at 140 rpm under shaker incubator for 30 min.
- Subsequently, collect the supernatant of the sample by centrifugation at 4226.04 x g for 12 min and transfer 15 ml of the supernatant through an immunoaffinity column of ZEA, which is pre-conditioned under 10 ml of phosphate-buffered saline (pH 7.4).
- Next, Wash the column with 5 ml of PBS and 10 ml of distilled water.
- Finally, air-dry the column and elute the ZEA with 5 ml of acetonitrile. Maintain contact between acetonitrile and column antibodies at least for 5 min.
- Dry out the eluate completely over a water bath at 60 °C and redissolved the final residue in 1 ml of acetonitrile and filter through 0.22 µm of syringe filter.
- Use the filtrate for UHPLC determination and quantification of ZEA.
- Employ the Nexera UHPLC system attached with the column C18, 5 µm, 250 x 4.6 mm for detection and quantification of ZEA in reverse-phase with a fluorescence detector, and set excitation and emission wavelength at 334 and 450 nm, respectively.
- The mobile phase is acetonitrile-water (50:50, v/v) with a flow rate of 1 ml/min.
- Construct a five-point calibration curve for standard ZEA (100 ng-500 µg/ml) with peak area versus concentration of ZEA.
- The injection volume is 25 µl for both the standard solution and test sample.
- The sensing limitation of the technique is 100 ng/ml.
- Blend the culture broth with an equivalent quantity of acetonitrile at 140 rpm under shaker incubator for 30 min.
- Determination of the effect of OSEO on gene expression of PKS4 and PKS13, which are involved in ZEA biosynthesis of F. graminearum (Gaffoor and Trail, 2006; Kim et al., 2005) by RT-qPCR evaluation using GAPDH as an endogenous reference gene.
- Design the primers for target genes using the GeneRunner software version 5.0.47 Beta (Table 1).
- Briefly, flash-frozen the mycelia in liquid nitrogen and ground into a fine powder with a porcelain mortar. Extract the total RNA using RNEASY PLANT MINI KIT following manufacturer’s guidelines (Qiagen, Hilden, Germany).
- Quantify the total RNA by NanoDrop 8000 spectrophotometer.
- Carry out the RT-qPCR analysis of PKS4 and PKS13 in the Light Cycler 480 using iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad Laboratories, USA).
- Briefly, make 50 µl volume of reaction mixture consisting 25 µl of 2x SYBR Green RT-PCR reaction mix, 1 µl of iScript reverse transcriptase for one-step RT-PCR, 1 µl of primer (450 nM), 1 µl of template RNA (100 ng) and 22 µl of nuclease-free water (PCR grade).
- The thermal conditions for the reaction include 10 min of cDNA synthesis at 50 °C for 1 cycle, 5 min of polymerase activation at 95 °C and following by 35 cycles of PCR at 95 °C for 10 sec, 60 °C for 30 sec for combined annealing and extension.
- Attain individual narrow peak through melting curve analysis at distinct temperatures for each and every PCR product.
- Quantify the relative quantification levels of gene expression making use of second derivative maximum analysis with the determination of the crossing points for every single transcript.
- Normalize the crossing point values for each gene to the particular crossing point values with the reference gene GAPDH.
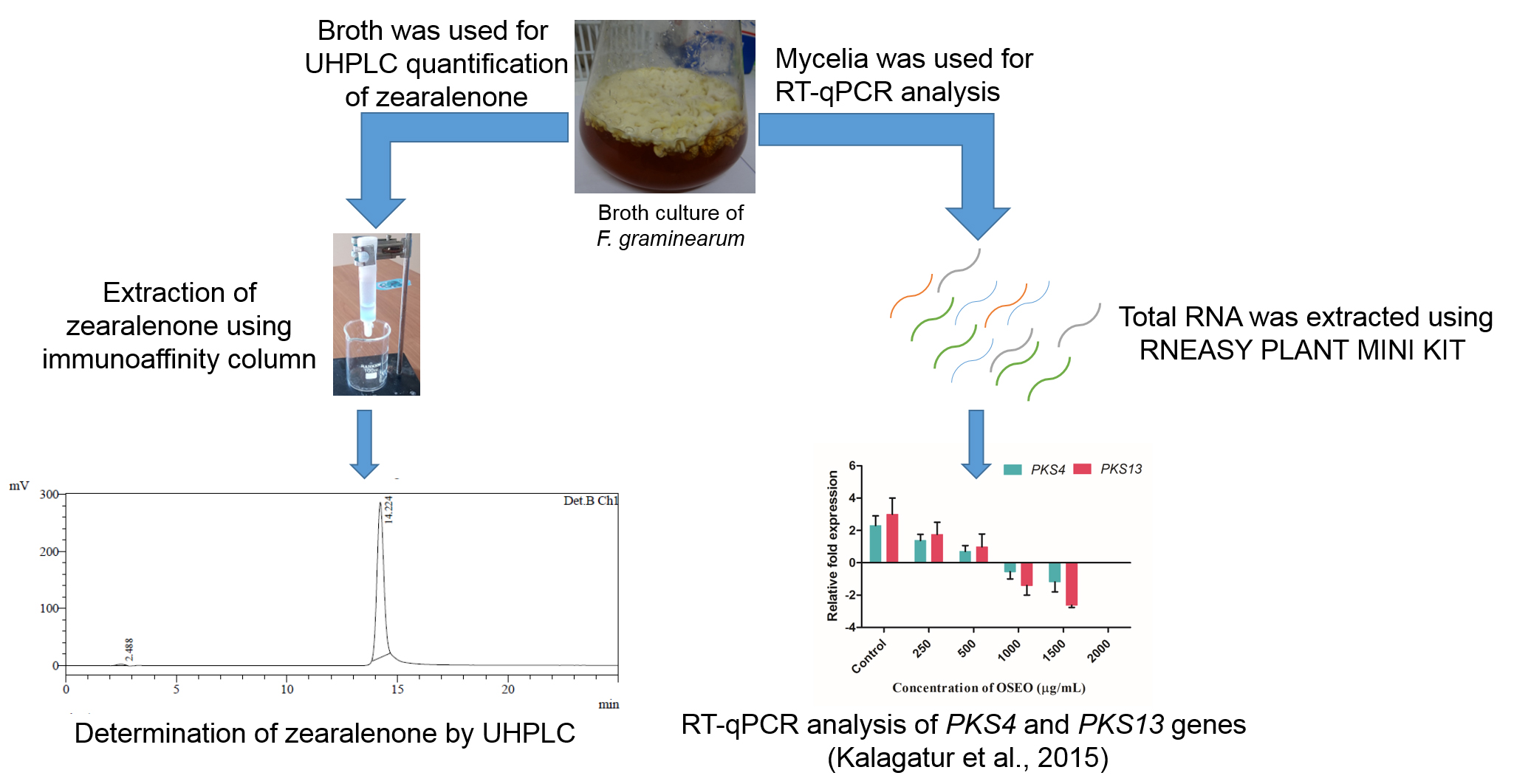
Figure 3. Determination of the anti-mycotoxic activity of O. sanctum L. essential oil (OSEO) on F. graminearum by UHPLC and RT-qPCR analysis. The quantification of ZEA present in broth culture is determined by UHPLC and concentration of ZEA is declined with increasing the concentration of OSEO. The relative fold expression of PKS4 and PKS13 are determined by RT-qPCR analysis and it is down-regulated with increasing the concentration of OSEO. - Design the primers for target genes using the GeneRunner software version 5.0.47 Beta (Table 1).
- Assessment of anti-mycotoxic efficacy of OSEO on F. graminearum in maize grains.
- Sterilize the seeds by autoclave and dry in a hot-air oven at 60 °C for 2 h.
- Treat 100 g of sterilized maize grains with various concentrations (250, 500, 1,000, 1,500 and 2,000 μg/g) of OSEO in 500 ml conical flask and inoculate 10 μl of fungal spore suspension (1 x 106 spores/ml) of 7-day-old culture into each conical flask and incubate for 14 days at 28 °C in the dark.
- Consider the grains not treated with OSEO as control and incubated for a period of 14 days at 28 °C in the dark condition.
- Following the incubation period, extract total RNA from fungal mycelia and carry out RT-qPCR evaluation for PKS4 and PKS13 genes as mentioned earlier.
- Further, ground the maize grains into a fine powder and dissolve in 500 ml of acetonitrile and centrifuge at 4226.04 x g for 30 min.
- Transfer 15 ml of supernatant through ZEA specific immunoaffinity column and quantify the ZEA by UHPLC as mentioned earlier.
Table 1. Primers used for RT-qPCR analysis of zearalenone production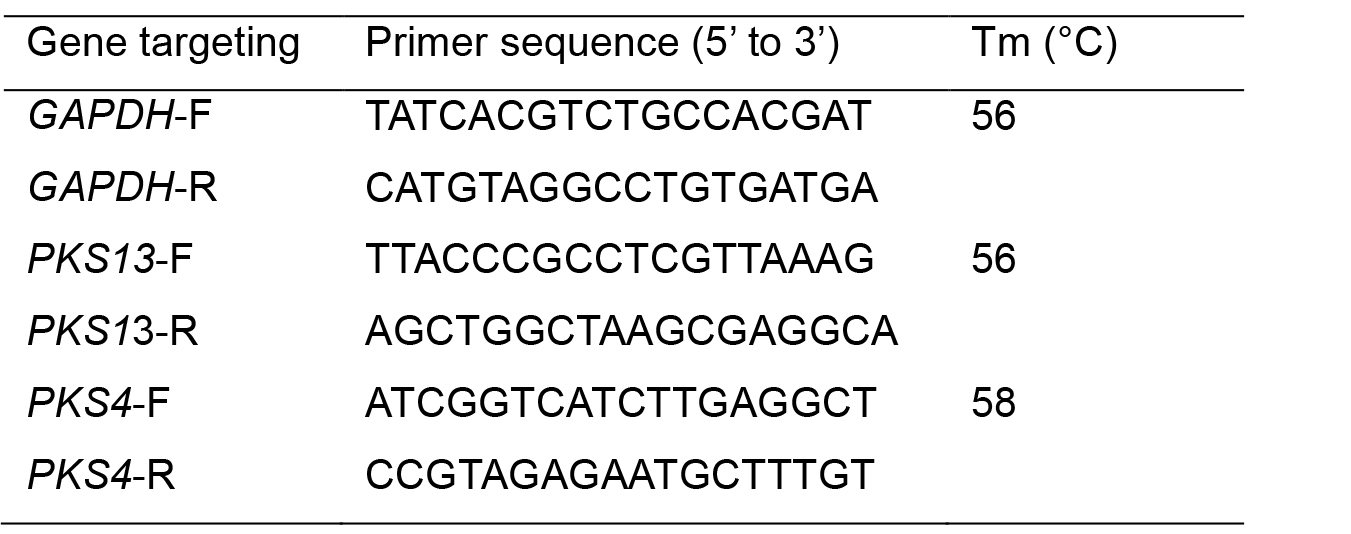
- Sterilize the seeds by autoclave and dry in a hot-air oven at 60 °C for 2 h.
Recipes
- Phosphate-buffered saline (pH 7.4)
8.0 g NaCl
0.2 g KCl
1.44 g Na2HPO4
0.24 g KH2PO4
Dissolve all these chemicals in 800 ml of distilled water and adjust pH to 7.4 with 1 N HCl, and make up the volume to 1,000 ml with distilled water and sterilize by autoclaving. - Lactophenol-cotton blue staining solution
20 ml lactic acid
20 g phenol crystals
0.05 g cotton blue
20 ml glycerol
20 ml distilled water
Notes
Zearalenone is toxic and classified as group 3 carcinogen by International Agency for Research on Cancer (IARC, 1999) and care should be taken.
Acknowledgments
Authors are thankful to the Director, DFRL and DRDO-BU-CLS for their support to carry out the study, and also acknowledge Rana et al. (1997), Clinical and Laboratory Standards Institute, (2008), Ibáñez-Vea et al. (2011), Yamamoto-Ribeiro et al. (2013) and Vieira et al. (2014). This protocol is invited from the report of Kalagatur et al. (2015) and Kumar et al. (2016).
References
- Council of Europe. (1997). “Methods of pharmacognosy,” in European Pharmacopoeia, 3rd Ed. European Department for the Quality of Medicines: 121-122.
- Gaffoor, I. and Trail, F. (2006). Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl Environ Microbiol 72(3): 1793-1799.
- Ibáñez-Vea, M., Corcuera, L. A., Remiro, R., Murillo-Arbizu, M. T., González-Peñas, E., and Lizarraga, E. (2011). Validation of a UHPLC-FLD method for the simultaneous quantification of aflatoxins, ochratoxin A and zearalenone in barley. Food Chemistry, 127(1), 351-358.
- John, H. and Mahmoud, A. (eds) (2008). Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard-Third Edition, M27-A3. CLSI.
- Kalagatur, N. K., Mudili, V., Siddaiah, C., Gupta, V. K., Natarajan, G., Sreepathi, M. H., Vardhan, B. H. and Putcha, V. L. (2015). Antagonistic activity of Ocimum sanctum L. essential oil on growth and zearalenone production by Fusarium graminearum in maize grains. Front Microbiol 6: 892.
- Kim, Y. T., Lee, Y. R., Jin, J., Han, K. H., Kim, H., Kim, J. C., Lee, T., Yun, S. H. and Lee, Y. W. (2005). Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol Microbiol 58(4): 1102-1113.
- Kumar, K. N., Venkataramana, M., Allen, J. A., Chandranayaka, S., Murali, H. S. and Batra, H. V. (2016). Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT-Food Science and Technology 69, 522-528.
- Rana, B. K., Singh, U. P. and Taneja, V. (1997). Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. J Ethnopharmacol 57(1): 29-34.
- Vieira, P. R. N., de Morais, S. M., Bezerra, F. H. Q., Ferreira, P. A. T., Oliveira, I. R. and Silva, M. G. V. (2014). Chemical composition and antifungal activity of essential oils from Ocimum species. Industrial Crops and Products 55: 267-271.
- Yamamoto-Ribeiro, M. M., Grespan, R., Kohiyama, C. Y., Ferreira, F. D., Mossini, S. A., Silva, E. L., Filho, B. A., Mikcha, J. M. and Machinski, M., Jr. (2013). Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem 141(3): 3147-3152.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kalagatur, N. K., Dhamodaran, N., Siddaiah, C., Mudili, V. and Sreepathi, M. H. (2016). Antifungal and Zearalenone Inhibitory Activity of Ocimum sanctum L. Essential Oil on Fusarium graminearum Determined by UHPLC and RT-qPCR. Bio-protocol 6(15): e1893. DOI: 10.21769/BioProtoc.1893.
Category
Microbiology > Antimicrobial assay > Antifungal assay
Microbiology > Microbe-host interactions > Fungus
Plant Science > Plant biochemistry > Lipid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












