- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessment of Brown Adipocyte Thermogenic Function by High-throughput Respirometry
Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1641 Views: 14688
Reviewed by: Hong-guang XiaValentine V TrotterAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Intestinal Co-culture System to Study TGR5 Agonism and Gut Restriction
Snehal N. Chaudhari and A. Sloan Devlin
Mar 20, 2021 6646 Views

OrganoPlate Micro-fluidic Microvessel Culture and Analysis
Abidemi Junaid and Thomas Hankemeier
Jul 5, 2021 4537 Views

Mass Spectrometry-based Lipidomics, Lipid Bioenergetics, and Web Tool for Lipid Profiling and Quantification in Human Cells
Liang Cui [...] Kuan Rong Chan
Aug 20, 2023 3005 Views
Abstract
Brown adipose tissue (BAT) has the unique ability to dramatically increase mitochondrial uncoupled fuel oxidation for thermogenesis in response to adrenergic stimulation. A key parameter in assessing brown adipocyte thermogenic capacity is mitochondrial uncoupling as determined by respiration. Measuring mitochondrial oxygen consumption rate (OCR) therefore provides valuable information to study the regulation and dysregulation of fuel metabolism and energy expenditure. Adding measurements of mitochondrial membrane potential allows for more in-depth interpretation of the respirometry data. Here we provide protocols for measuring respiration in adherent intact and plasma membrane permeabilized brown adipocytes using the Seahorse XF Analyzer. In the protocol Part I, a combination of norepinephrine and free fatty acids are used to induce uncoupled respiration. The ATP Synthase inhibitor oligomycin, the chemical uncoupler FCCP, and the complex III inhibitor Antimycin A are then used to measure coupled, maximal, and non-mitochondrial oxygen consumption, respectively. In the protocol Part II, the plasma membrane is permeabilized with recombinant perfringolysin O, a cholesterol-dependent cytolysin that oligomerizes into pores exclusively in the plasma membrane. This permits experimental control of metabolite availability without separating mitochondria from the native cell environment.
Part I. Intact brown adipocyte respiration
Materials and Reagents
Note: Rotenone, antimycin, oligomycin, and FCCP are toxic and light sensitive. Wear personal protective equipment when handling and store stocks in the dark.
- 3-4 weeks-old C57BL6/J mice
Note: Isolate primary pre adipocytes from brown adipose tissue, then differentiate them in an XF-24 well cell culture microplate for 7 days. - XF assay 24‐well cartridge and cell culture microplate (Seahorse Bioscience, catalog numbers: 100850-001 and 100777-004 )
- XF assay 24-well cartridge and utility plate
- DMEM (Thermo Fisher Scientific, GibcoTM, catalog number: 12100-046 )
- ewborn calf serum (Sigma-Aldrich, catalog number: N4887 )
- HEPES (Corning, catalog number: 25-060-Cl )
- L-Glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-164 )
- Penicillin-Streptomycin Solution (100x) (Corning, catalog number: 30-002-Cl )
- Sodium L-ascorbate (Sigma-Aldrich, catalog number: A4034 )
- Rosiglitazone (used as differentiation agent) (Toronto Research Chemicals, catalog number: R693500 )
- Insulin from Porcine Pancreas (Sigma-Aldrich, catalog number: I5523 )
- XF-assay/base medium (Seahorse Bioscience, Catalog number: 102365-100 )
- D-(+)-Glucose (Sigma-Aldrich, catalog numbers: G8270 )
- XF Calibrant solution (Seahorse Bioscience, Catalog number: 100840‐000 )
- L-(-)Norepinephrine(+)-bitartrate salt monohydrate (Sigma-Aldrich, Catalog number: N5785 )
- Free fatty acids (Palmitic acid or Oleate) (Sigma-Aldrich, catalog number: P0500 )
- DMSO (Sigma-Aldrich, catalog number: D2650 )
- RPMI medium (no glucose) (Thermo Fisher Scientific, GibcoTM, catalog number: 11879-020 )
- Fatty‐acid‐free BSA (CalBiochem, catalog number: 126575 )
- BAT differentiation media (see Recipes)
- Seahorse assay media (SH assay media) (see Recipes)
- Free fatty acid conjugated to the BSA (see Recipes)
- Norepinephrine (see Recipes)
- Oligomycin (Sigma-Aldrich, catalog number: 75371 ) (see Recipes)
- Antimycin A from Streptomyces sp. (Sigma-Aldrich, catalog number: A8674 ) (see Recipes)
- Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (Sigma-Aldrich, catalog number: C2920 ) (see Recipes)
- Sodium pyruvate (Sigma-Aldrich, catalog number: P5280 ) (see Recipes)
Equipment
- CO2-free incubator
- 8% CO2 incubator
- XF24 extracellular flux analyzer (Seahorse Bioscience)
- Cell culture hood
- pH meter
- 37 °C Water bath
Procedure
- Isolate preadipocytes from 3-4 weeks-old C57BL6/J mice according to Cannon and Nedergaard (2001). Resuspend cell pellet in 4 ml of BAT differentiation medium after BAT collagenase digestion and washing steps.
- Seed 70 μl of resuspended pellet into each well of a V7 XF 24-well cell culture plate, allow cells to attach for 1 h in cell culture hood, and then add 180 μl BAT differentiation medium and put in 8% CO2 incubator (getting an exact cell count is difficult at this stage because of the debris and red blood cells left over from isolation procedure). The next day, gently wash the cells twice with 150 μl medium to make sure that the red blood cells are washed out and add 250 μl of fresh medium. Replace the medium every other day for the next 7 days or until the preadipocytes differentiate. Differentiation can be visualized by lipid droplet formation in adipocytes or assessed by Western blot analysis of UCP1 expression (see Representative data Figures 1 and 2).
- Before beginning the respiratory assay, soak the cartridge with 1 ml of XF Calibrant per well for at least 3 h in CO2-free incubator.
- Wash cells once with SH assay media and then add 450 μl of SH assay medium to each of the 24 wells in the plate.
- Incubate plate for 30 min at 37 °C in CO2-free incubator before loading into the XF 24 extracellular analyzer.
- During these 30 min, load the ports of the cartridge containing the oxygen probes with the compounds to be injected during the assay (50 μl/portA, 55 μl/portB, 60 μl/portC, 65 μl/portD) and calibrate the cartridge.
- Run seahorse assay with 2 min mix and 4 min measurement (no wait). 6 measurements under basal, 5 measurements after Oligomycin and FCCP injection and more than 5 measurements after Antimycin A injection are recommended.
Representative data
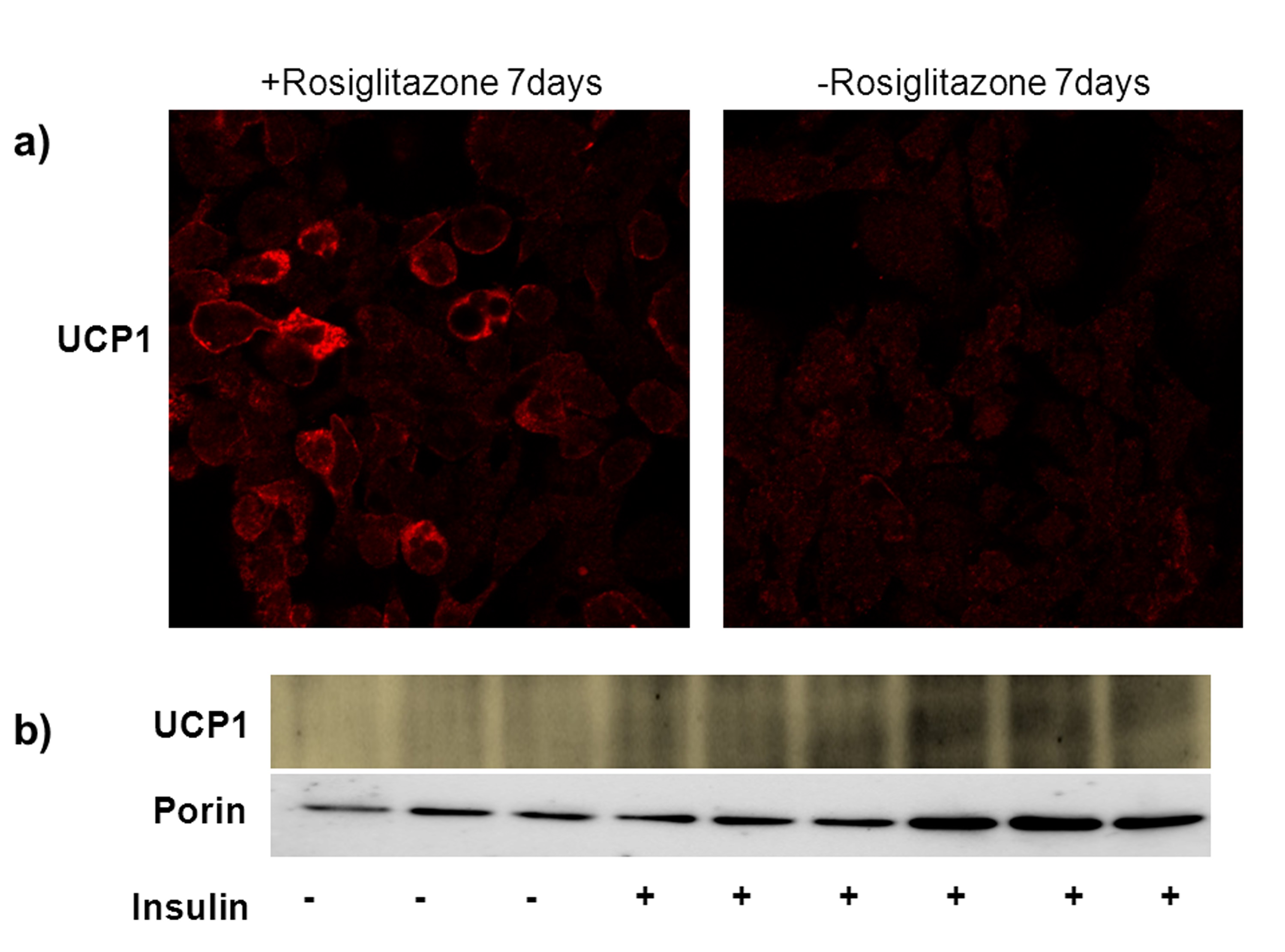
Figure 1. Brown adipocyte differentiation with insulin and rosiglitazone. a) UCP1 immunostaining of differentiated (with rosiglitazone) vs. undifferentiated cells (no rosiglitazone). Note the difference in fluorescence intensity. b) Western blot for UCP1and porin in differentiated brown adipocytes in the presence and absence of insulin and rosiglitazone. (Wikstrom et al., 2014)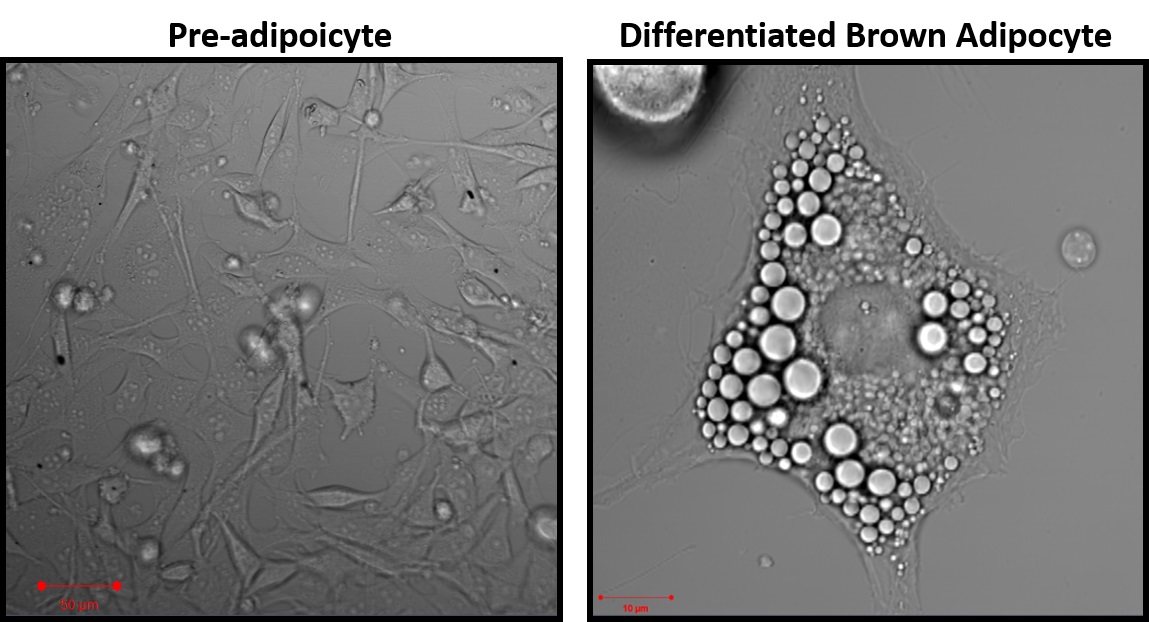
Figure 2. Representative images of pre-adipocytes 24 h after isolation and mature brown-adipocytes after 7 days of differentiation 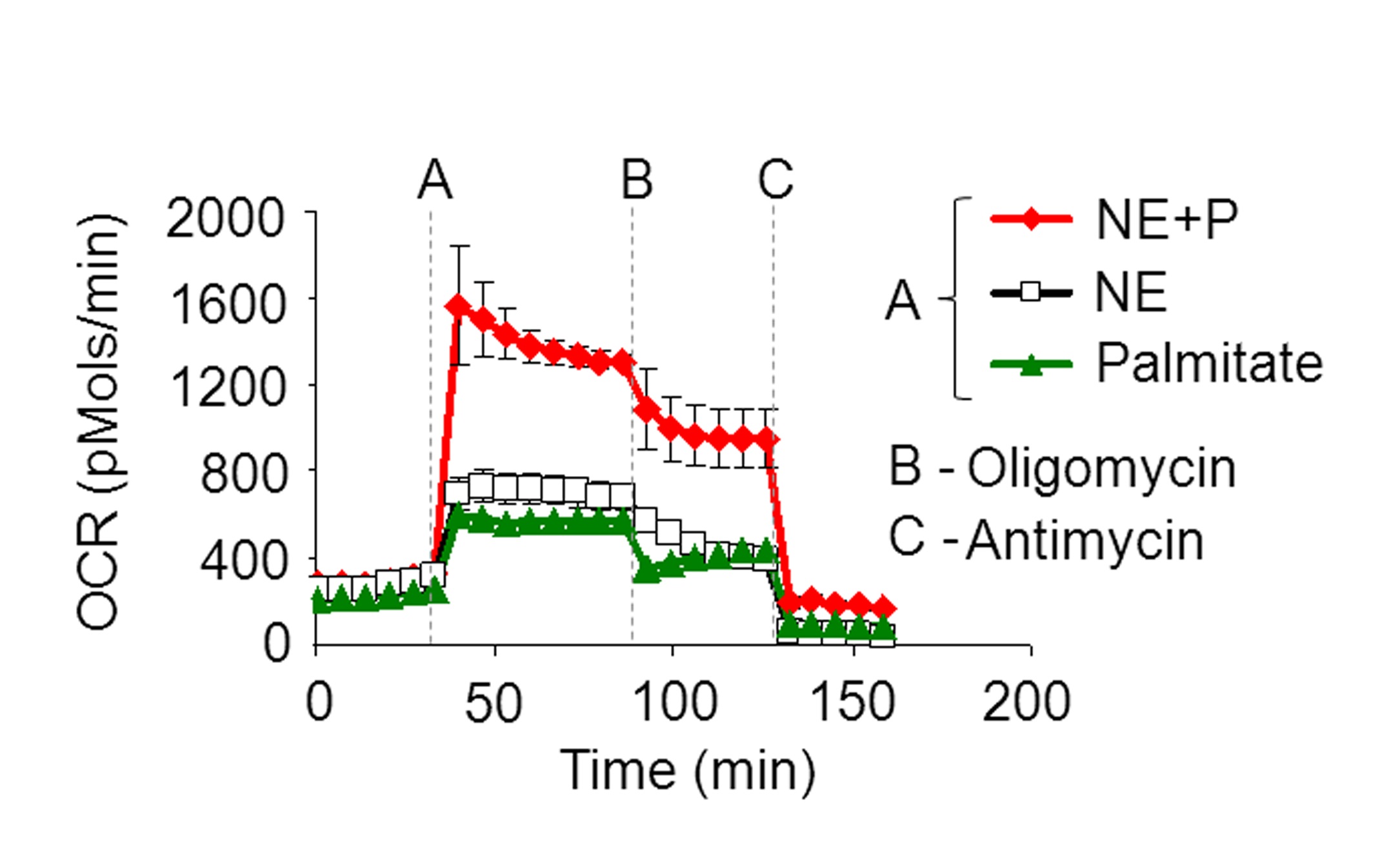
Figure 3. Representative OCR plot with injection of A) NE+P, NE, or palmitate alone, B) Oligomycin, and C) Antimycin A
Notes
- Successful NE activation of brown adipocytes expressing UCP1 results in a rapid increase in oxygen consumption rate. If OCR increase is <3-fold this suggests the brown adipocytes have not been sufficiently differentiated.
Recipes
- BAT differentiation media
Prepare stock (good for 6 weeks at 4 °C) of DMEM supplemented with:
10% newborn calf serum
10 mM HEPES
4 mM glutamine
50 IU of penicillin
50 μg streptomycin
100 μg/ml sodium L-ascorbate
Prepare 50 ml of BAT differentiation media (good for 1 week at 4 °C):
50 ml of Stock
60 nM insulin
1 μM rosiglitazone - Seahorse assay media (SH assay media) (PH=7.4 and filtered)
XF-assay medium
Stock contains:
0.8 mM Mg2+
1.8 mM Ca2+
143 mM NaCl
5.4 mM KCl
0.91 mM NaH2PO4
2 mM L-Ala-Gln (Glutamax)
Phenol red 3 mg/ml
On day of experiment: Add 3 mM glucose freshly to XF-assay medium, pH to 7.4 at 37 °C and sterile filter - Free fatty acid conjugated to the BSA (0.4 mM; FFA: BSA, 4:1)
Dissolve free fatty acids (Palmitate or Oleate) in DMSO to a final concentration of 0.4 M and then dissolve this solution at 43 °C in RPMI medium (No Glucose) containing 6.7% fatty‐acid‐free BSA to make a 10x stock (4 mM)
Aliquot and store in -80 °C - Norepinephrine
Freshly prepare L-(-)Norepinephrine(+)-bitartrate salt monohydrate in SH assay media at stock concentrations of 10 mM and use at final concentrations of 1 μM - Oligomycin
Prepare stock of 20 mM in DMSO and store in -20 °C
On day of experiment, dilute in SH assay media to prepare 50 μM (10x) solution to be loaded into cartridge ports
Final concentration will be 5 μM - Antimycin A
Prepare stock of 40 mM in DMSO and store in -20 °C
On day of experiment, dilute in SH assay media to prepare 80 μM (10x) solution to be loaded into cartridge ports
Final concentration will be 8 μM - Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP)
Prepare stock of 20 mM in DMSO and store in -20 °C
On day of experiment, dilute in SH assay media containing 100 mM Na pyruvate to prepare 50 μM (10x) solution to be loaded into cartridge ports
Final concentration will be 5 μM with 10mM sodium pyruvate - Sodium pyruvate
Dissolve in SH assay media at the concentration of 100 mM (pH=7.4 and filtered)
Final concentration is 10 mMSubstrate Solvent Concentration (10x)/Media Final concentration Comment Norepinephrine - 10 μM/SH assay media 1 μM FFA: BSA RPMI no glucose 4 mM/RPMI no glucose 0.4 mM; 4:1 Oligomycin DMSO 50 μM/SH assay media 5 μM Antimycin A DMSO 80 μM/SH assay media 8 μM FCCP DMSO 50 μM/SH+ Na Pyruvate 5 μM Na pyruvate - 100 mM/SH assay media 10 mM pH=7.4 and filter
Part II. Permeabilized brown adipocyte respiration
Materials and Reagents
- D-Mannitol (Sigma-Aldrich, catalog number: M9647 )
- Sucrose (Thermo Fisher Scientific, catalog number: S5 )
- Potassium Phosphate Monobasic (KH2PO4) (Thermo Fisher Scientific, catalog number: P285 )
- Magnesium Chloride Hexahydrate (MgCl2) (Thermo Fisher Scientific, catalog number: M33 )
- HEPES (Sigma-Aldrich, catalog number: H4034 )
- Ethylene glycol-bis(β-aminoethyl ether)-N,N,N,N'-tetraacetic acid tetrasodium salt (EGTA) (Sigma-Aldrich, catalog number: E8145 )
- Fatty-Acid Free BSA (EMD Millipore Corporation, CalBiochem, catalog number: 126575 )
- L-(−)-Malic acid (Sigma-Aldrich, catalog number: 02288 )
- Succinate (Sigma-Aldrich, catalog number: S3674 )
- Oligomycin A (Sigma-Aldrich, catalog number: 75371 )
- Antimycin A from Streptomyces sp. (Sigma-Aldrich, catalog number: A8674 )
- Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) (Sigma-Aldrich, catalog number: C2920 )
- Rotenone (Sigma-Aldrich, catalog number: R8875 )
- (+)-Etomoxir sodium salt hydrate (Sigma-Aldrich, catalog number: E1905 )
- Adenosine 5’-diphosphate monopotassium salt dihydrate (Sigma-Aldrich, catalog number: A5285 )
- L-(-)-Carnitine (Thermo Fisher Scientific, Acros Organics, catalog number: 241040100 )
- Succinate (Sigma-Aldrich, catalog number: S3674 )
- L-(−)-Malic acid (Sigma-Aldrich, catalog number: 02288 )
- Sodium pyruvate (Sigma-Aldrich, catalog number: P5280 )
- Palmitoyl-L-carnitine chloride (Sigma-Aldrich, catalog number: P1645 )
- Palmitoyl coenzyme A lithium salt (Sigma-Aldrich, catalog number: P9716 )
- 3x MAS buffer (see Recipes)
- XF PMP reagent (Seahorse Bioscience, catalog number: 102504-100) (see Recipes)
Equipment
- Same equipment as intact brown adipocyte respiration protocol
Procedure
- Prepare cells as described in steps 1-2 of intact brown adipocyte respiration protocol.
- On the day of experiment, thaw 1x MAS and pre-made substrate solutions described in Recipes section 1.
- Prepare 10x solutions of inhibitors in 1x MAS. If using fatty acid substrates, prepare them freshly as described in Recipes section 2b.
- Soak the cartridge for at least 3 h in CO2-free incubator before loading.
- Load ports: 50 μl in Port A, 55 μl in Port B, 60 μl in Port C, 65 μl in Port D and calibrate cartridge.
- Prepare 7.5 nM PMP in 1x MAS in a volume sufficient for 450 μl per well.
- Gently wash cells with 1x MAS buffer one time.
- Completely evacuate wash and add 450 μl 1x MAS with 7.5 nM PMP. Incubation in CO2-free incubator is not necessary.
- Immediately load the plate and run seahorse assay with 3 min mix and 2 min measurement (no wait). Make 2-3 measurements per condition. Do not exceed 1 h of assay time preceding Antimycin A injection as mitochondrial integrity will be compromised.
- Soak the cartridge for at least 3 h in CO2-free incubator before loading.
Representative data
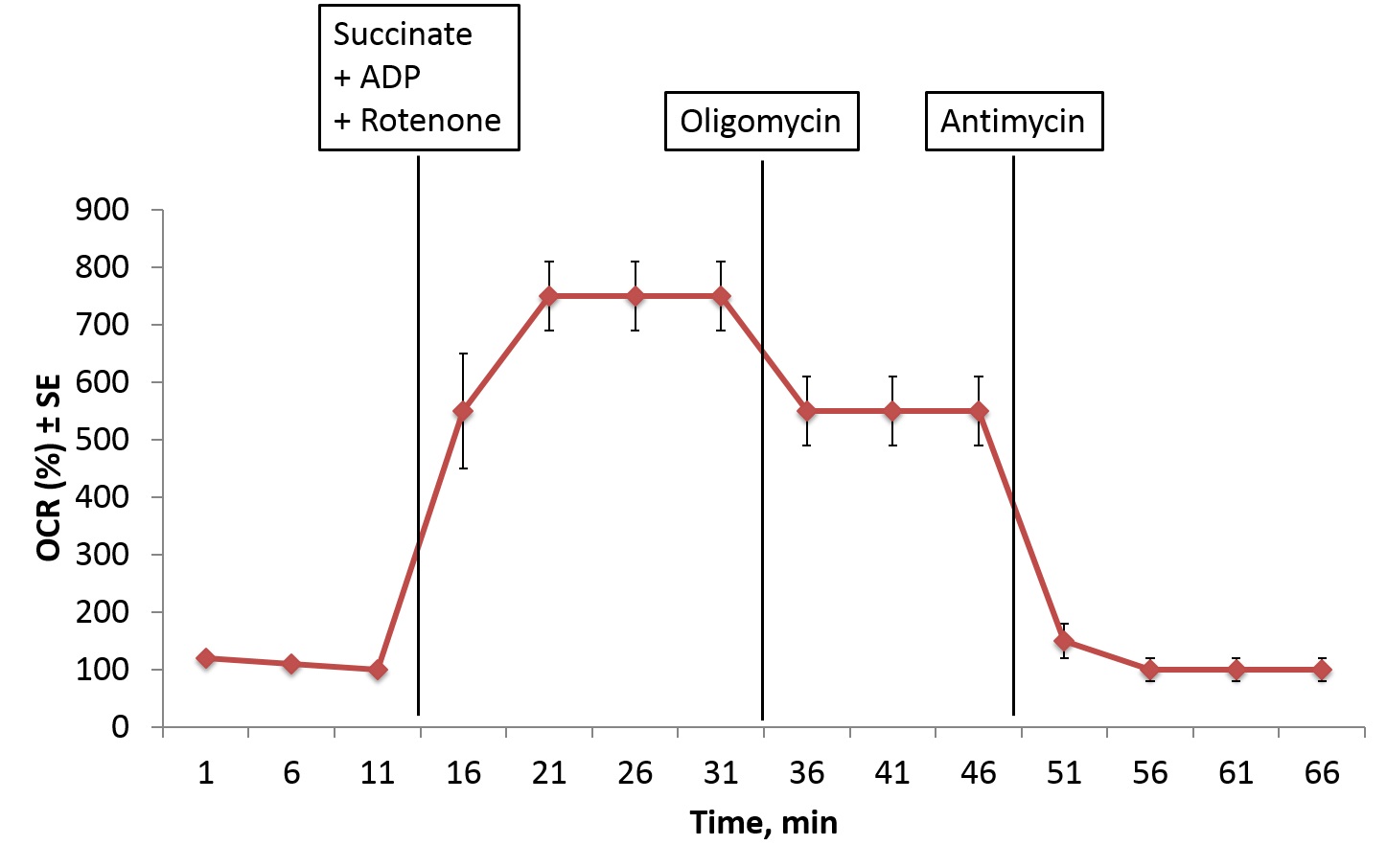
Figure 4. Representative OCR plot of permeabilized BAT injected with A) Succinate+ADP+Rotenone, B) Oligomycin, and C) Antimycin
Notes
- The simplest way to ensure permeabilization is to detect respiration elicited by the membrane-impermeable substrate succinate. Succinate may also be used to titrate the optimal XF PMP reagent concentration
- Always load all four injections ports. If one port is not used, load with 1x MAS buffer.
- Use at least three technical replicates per condition.
- To ensure the quality of the data, make sure that pH is stable throughout the run and that oxygen tension returns to base level after every measurement.
- If OCR exceeds 2,000 pmol/min, go to ‘Special Operations’ tab in the seahorse excel data viewer, click on ‘Change O2 calc method’ and select the ‘Gain (Fixed)’ algorithm as the AKOS algorithm will not process high OCR data correctly. Always express Gain (Fixed) data as percent of baseline measurement.
- For more information on permeabilization see Divakaruni et al. (2014) and Salabei et al. (2014).
Recipes
- 3x MAS buffer
Dissolve reagents in double-distilled water, pH to 7.2 with KOH, sterile filter, and store in -20 °CReagent Concentration Amount 1 L MAS Mannitol 660 mM 120.23 g Sucrose 210 mM 71.88 g KH2PO4 30 mM 4.08 g MgCl2 15 mM 15 ml of 1.0 M solution HEPES 6 mM 6 ml of 1.0 M solution EGTA 3 mM 12 ml of 0.25 M solution Fatty-Acid Free BSA 0.6% (w/v) 6.0 g
Optional: Dilute to 1X in double-distilled water, pH to 7.2 with KOH, sterile filter, and store in -20 °C - XF PMP reagent
Stock solution: 10 μM
Storage: -20 °C
Stock solutionsReagent MW Concentration Solvent Comments Malate 134.09 1.0 M ddH2O pH to 7.2 with KOH. Solution is strongly acidic, calculate molarity according to final volume. Succinate 270.1 1.0 M ddH2O pH to 7.2 with KOH Oligomycin 791.06 20 mM DMSO Complex V inhibitor Antimycin A 548.63 40 mM DMSO Complex III inhibitor FCCP 254.17 40 mM DMSO Chemical uncoupler Rotenone 394.4 40 mM DMSO Complex I inhibitor Etomoxir 338.76 40 mM DMSO CPT-1 inhibitor, blocks conversion of palmitoyl-coA to palmitoyl-carnitine ADP, K+ salt 501.32 Use as powder L-(-)-Carnitine 161.2 Use as powder
SubstratesSubstrate MW 10x Conc. for port injection Additives (10x conc. for port injection) Succinate 270.1 100 mM 40 mM ADP, 50 μM Rotenone Malate 134.09 30 mM 40 mM ADP Pyruvate 110.94 100 mM 40 mM ADP, 30 mM malate Palmitoyl-carnitine 436.07 1 mM 40 mM ADP, 30 mM malate, 5 mM carnitine Palmitoyl-CoA 1,004.94 1 mM 40 mM ADP, 30 mM malate, 5 mM carnitine - Prepare succinate, malate, and pyruvate in 1x MAS buffer and pH with KOH to 7.2 prior to day of experiment. Sterile filter, aliquot, and store in -20 °C.
- For example, for a 5 ml solution of succinate, add 0.5 ml 1.0 M succinate, 100.2 mg ADP, 1.67 ml 3x MAS, and 2.83 ml ddH2O and adjust pH to 7.2 with KOH. (Add rotenone on day of experiment)
- For every 100 μl of additional volume as a result of pH titration, add 50 μl 3x MAS to maintain osmotic strength.
- For example, if final volume of the 5 ml succinate solution is 5.1 ml, add 50 μl 3x MAS. This will result in a slight dilution of reagents that should not significantly affect the assay. However, given this variability, we advise preparing a large batch of solution and use the same stocks for all experiments within a given project.
- For example, for a 5 ml solution of succinate, add 0.5 ml 1.0 M succinate, 100.2 mg ADP, 1.67 ml 3x MAS, and 2.83 ml ddH2O and adjust pH to 7.2 with KOH. (Add rotenone on day of experiment)
- For fatty acids, prepare solutions of additives (except carnitine) in 1x MAS buffer and pH with KOH to 7.2 prior to day of the experiment. Sterile filter, aliquot, and store in -20 °C. Add carnitine and fatty acid to solutions on the day of the experiment.
- Prepare succinate, malate, and pyruvate in 1x MAS buffer and pH with KOH to 7.2 prior to day of experiment. Sterile filter, aliquot, and store in -20 °C.
Acknowledgments
Permeabilized brown adipocyte respiration protocol adapted from Divakaruni et al. (2014). This work was supported by National Institutes of Health grants R 01 DK074778, R01 DK56690-11 and by the Swedish Research Council as well as DIABAT FP7, collaborative EU Grant HEALTH-F2-2011-278373 and the Wallenberg Foundation.
References
- Cannon, B. and Nedergaard, J. (2001). Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol Biol 155: 213-224.
- Divakaruni, A. S., Rogers, G. W. and Murphy, A. N. (2014). Measuring mitochondrial function in permeabilized cells using the seahorse XF analyzer or a clark-type oxygen electrode. Curr Protoc Toxicol 60: 25 22 21-25 22 16.
- Salabei, J. K., Gibb, A. A. and Hill, B. G. (2014). Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc 9(2): 421-438.
- Wikstrom, J. D., Mahdaviani, K., Liesa, M., Sereda, S. B., Si, Y., Las, G., Twig, G., Petrovic, N., Zingaretti, C. and Graham, A. (2014). Hormone‐induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33(5): 418-436.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Mahdaviani, K., Benador, I. and Shirihai, O. (2015). Assessment of Brown Adipocyte Thermogenic Function by High-throughput Respirometry. Bio-protocol 5(21): e1641. DOI: 10.21769/BioProtoc.1641.
- Wikstrom, J. D., Mahdaviani, K., Liesa, M., Sereda, S. B., Si, Y., Las, G., Twig, G., Petrovic, N., Zingaretti, C. and Graham, A. (2014). Hormone‐induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33(5): 418-436.
Category
Cell Biology > Cell metabolism > Carbohydrate
Cell Biology > Cell metabolism > Lipid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link