- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Differentiation of THP1 Cells into Macrophages for Transwell Co-culture Assay with Melanoma Cells
Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1638 Views: 50392
Reviewed by: HongLok LungNicoletta CordaniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vitro Regulatory T cells Differentiation From Naïve T Cells
Tomás Dalotto-Moreno [...] Marian Salatino
Mar 20, 2014 24292 Views

3D Co-culture System of Tumor-associated Macrophages and Ovarian Cancer Cells
Lingli Long [...] Wang Min
Apr 20, 2018 12161 Views

Peptide Loading on MHC Class I Molecules of Tumor Cells
Loredana Cifaldi [...] Doriana Fruci
Sep 20, 2016 11610 Views
Abstract
Understanding how immune cells such as macrophages interact with cancer cells is of increasing interest, as cancer treatments move towards combing both targeted- and immuno-therapies in new treatment regimes. This protocol is using THP-1 cells, a human leukemia monocytic cell line that can be differentiated into macrophages. This allows studying the effects of the macrophage secretome on cancer cells (on e.g., growth, drug response or gene expression) in co-cultures without direct cell contact interactions. This is an important aspect as it removes the presence of any phagocytic aspect to changes in the cancer cell number and behaviour. The in vitro THP-1 monocyte differentiation into polarized macrophages was used to study the effects of both M1 and M2 type populations of macrophages on melanoma cells (Smith et al., 2014; Tsuchiya et al., 1980). M1 type macrophages are classically thought to be tumour suppressing as opposed to M2 type macrophages, which are thought to possess tissue repairing and tumour growth promoting activities.
Keywords: Co-cultureMaterials and Reagents
- Transwell inserts 0.4 μM (BD Biosciences, catalog number: 353493 )
Note: Currently, it is “Corning, Falcon®, catalog number: 353493 ”. - Sterile 15 ml centrifuge tubes
- 20 µl and 1 ml tips
- Corning® 6 well cell culture plates (Sigma-Aldrich, catalog number: CLS3516-50EA )
- THP-1 cells (ATCC, catalog number: ATCC® TIB-202TM )
- Foetal bovine serum (500 ml) (heat inactivated by manufacturer) (Thermo Fisher Scientific, GibcoTM, catalog number: 10500064 )
- Penicillin/Streptomycin soln (100x) stabilised (100 ml) (Sigma-Aldrich, catalog number: P4333 )
- Dulbeccos PBS (1x) w/o Ca2+ or Mg2+ (500 ml) (Sigma-Aldrich, catalog number: PBS2541 )
- Trypsin-EDTA (1x) (100 ml) (Sigma-Aldrich, catalog number: T3924-100 ml )
- EDTA disodium salt AR E (500 gm) (Thermo Fisher Scientific, Fisher Scientific, catalog number: FKCF/0700/53 )
- DMEM w/L-Glutamine, Pyruvate & sodium bicarbonate (500 ml) (Sigma-Aldrich, catalog number: D6429-24x )
- RPMI 1640 w/L-Glutamine-Bicarbonate (500 ml) (Sigma-Aldrich, catalog number: R8758 )
- 12-O-tetradecanoylphorbol-l3-acetate (PMA) (Sigma-Aldrich, catalog number: P1585 )
- Recombinant human interferon type gamma (IFN-γ) (Peprotech, catalog number: 300-02 )
- Recombinant human interleukin 4 (IL-4) (Peprotech, catalog number: 200-04 )
- Recombinant human interleukin 13 (IL-13) (Peprotech, catalog number: 200-13 )
- Lipopolysaccharides from Escherichia coli (Sigma-Aldrich, catalog number: L2630 )
- DMEM/FCS media (see Recipes)
- RPMI/FCS media (see Recipes)
- IL4 stock solution (see Recipes)
- IL13 stock solution (see Recipes)
- IFN-γ stock solution (see Recipes)
- PMA (see Recipes)
- LPS (see Recipes)
Equipment
- Benchtop Centrifuge
- Aspirator
- Haemocytometer
- Tissue culture hood
- CO2 incubator
Procedure
Schematic of the protocol
Below is a diagram showing a brief outline of the steps of the protocol and a timeline indicating the order of events all carried out under sterile conditions.
Figure 1. A schematic diagram of the procedure. THP-1 cells (yellow) are placed into the insert, where they are activated by PMA; addition of LPS induces M1 macrophage (M1 MΦ), IL4/IL13 induces M2 MΦ differentiation (green cells).
Day 1
THP-1 cells are grown in suspension in RPMI/FCS media, and activation is performed once plated into transwell inserts. Inserts are handled at the sterile edges of the inserts using gloves.
- THP-1 cells are counted using a haemocytometer a cell suspension with a concentration of 1 x 105 cells/ml is made up.
- One 6 well-plate is then prepared by adding 2 ml of RPMI/FCS media per 6 well. The transwell inserts are then placed into the 6 well containing the 2 ml medium.
- 2 ml of the THP-1 cell suspension is added into the inserts (2 x 105 cells/insert).
- Once settled THP-1 cells are treated immediately with 10 ng/ml of 12-O-tetradecanoylphorbol-l3-acetate (PMA) for 24 h (1 µl of stock solution into 4 ml total volume) as described in Figure 1 Day 1.
Day 2
Activated THP-1 cells are differentiated in transwell inserts after 24 h in PMA.
- A new 6 well plate is prepared by adding 2 ml of fresh RPMI/FCS media.
- The PMA containing media is then aspirated gently from the inserts containing the activated THP-1 cells and the inserts are transferred to the new 6 well plate as described in Figure 1 Day 2.
- 2 ml of RPMI/FCS media is then added into the inserts and left for 1 h before treatment.
- The inserts are then treated with the desired mix of cytokines:
- M1 differentiation can be achieved either by treating with 15 ng/ml of lipopolysaccharide (LPS) (1.5 µl of stock solution into 4ml) or 50 ng/ml IFN-γ (2 µl of stock solution into 4 ml).
- M2 differentiation can be achieved by the combined treatment with 25 ng/ml IL4 and 25 ng/ml IL13 (1 µl of stock solution into 4 ml).
- Undifferentiated but activated THP-1 cells are left untreated in the insert to be used as a reference well for activated but non-differentiated monocytes.
- Correct differentiation into M1 and M2 type macrophages can be assessed at this stage by immunofluorescence, ELISA or gene expression analysis for specific markers (such as IL1, IL10, ARGINASE) as described in Smith et al. (2014).
- M1 differentiation can be achieved either by treating with 15 ng/ml of lipopolysaccharide (LPS) (1.5 µl of stock solution into 4ml) or 50 ng/ml IFN-γ (2 µl of stock solution into 4 ml).
Day 3
The human melanoma cell line to be investigated is pre-plated into 6 well plates. Melanoma cells are adherent and are usually grown in DMEM/FCS media. For example WM266-4 cells are plated at 2 x 105/insert for a 72 h co-culture experiment.
- Melanoma cells are washed once in 5 ml of PBS/EDTA.
- After PBS/EDTA aspiration 1 ml of trypsin/EDTA is added to the flask and then incubated in a 37 °C incubator for 3-5 min.
- Cells are then re-suspended in 10 ml of DMEM or RPMI (depending on cell line) media and then counted.
- Depending on the doubling rate of cell line used and the length of the experiment being performed the number of cells plated will vary but usually lie within the region of 0.5-2 x 105 cells per 6 well (2 ml per well).
Day 4
Setting up the co-culture by combining the differentiated macrophages and melanoma cells in the same plate as described in Figure 1 Day 4.
- 48 h after the addition of LPS or IL4/IL23, the differentiated THP-1cells are washed 3 times in fresh RPMI/FCS media. One wash consists of careful aspiration of the media and then adding 4 ml of fresh RPMI/FCS media.
- The inserts are then picked up using gloves and transferred onto the top of the melanoma cell culture with the addition of 2 ml of DMEM or RPMI (depending on the melanoma cell line) media into the inserts.
Note: Once transferred, the transwell assay is then ready to be used in several in vitro assays such as drug treatment survival assays or gene expression analysis after defined times of co-culture (Smith et al., 2014). Cells can be kept in this co-culture situation for a further 96 h if desired. This co-culture technique allows for the isolation and study of both populations of cells after the experiment. RNA or protein lysates can be obtained from the adherent melanoma cells at the bottom of the wells, in addition the macrophages can be lysed separately by isolating the insert.
Representative data
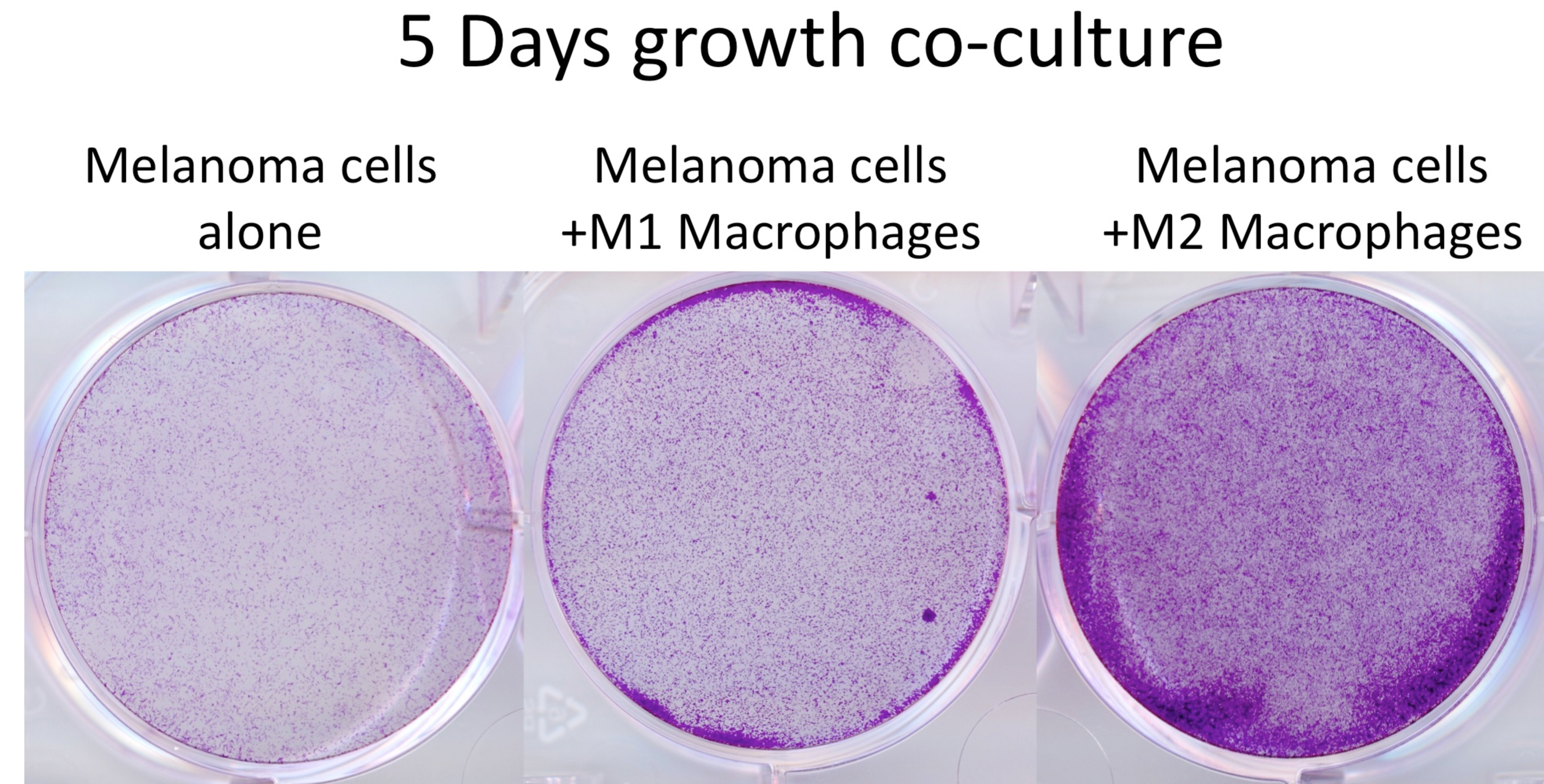
Figure 2. Cell number assessment of melanoma cells co-cultured with differentiated macrophages. Crystal violet staining of WM266-4 melanoma cells, which had been co-cultured with M1 and M2 macrophages for 5 days. After the removal of the insert, cells were fixed with 4% formaldehyde/PBS (15 min), stained with 0.5% crystal violet/PBS (1 h) then washed with water until wash-water was clear. Crystal violet very efficiently stains cells and the cell/colony number can be assessed by counting or optical density (OD540) measurement after solubilisation of the dye. This step does not require sterile conditions.
Notes
The most crucial factor in producing reliable and consistent results using this transwell technique is choosing the correct number of the respective melanoma cell line for plating so that they do not over-grow by the end-point of the experiment. There is also an increased risk of contamination due to the handling of the inserts above the wells, which can be reduced by working as swift and clean as possible.
Recipes
- DMEM/FCS media
Dulbecco's modified eagle medium supplemented with 1% of Penicillin-streptomycin solution and 10% FCS - RPMI/FCS media
RPMI supplemented with 1% of Penicillin-streptomycin solution and 10% FCS - IL4 stock solution
Made up to 100 µg/µl in sterile PBS 0.05% BSA and aliquoted and stored at -80 °C - IL13 stock solution
Made up to 100 µg/µl in sterile PBS 0.05% BSA and aliquoted and stored at -80 °C - IFN-γ stock solution
Made up to 100 µg/µl in sterile PBS 0.05%BSA and aliquoted and stored at -80 °C - PMA
Diluted in sterile PBS to concentrations of 40 µg/µl and then aliquoted and stored at -20 °C - LPS
Diluted in sterile PBS to concentrations of 40 µg/µl and then aliquoted and stored at -20 °C
Acknowledgments
The THP-1 differentiation protocol and development of this system was done with the guidance of Nadia Lunheshi and Richard Sainson at Medimmune Ltd, UK. Funding for this research was provided by CRUK C11591/A16416 awarded to C. Wellbrock. This protocol was developed and used in Smith et al. (2014).
References
- Smith, M. P., Sanchez-Laorden, B., O'Brien, K., Brunton, H., Ferguson, J., Young, H., Dhomen, N., Flaherty, K. T., Frederick, D. T., Cooper, Z. A., Wargo, J. A., Marais, R. and Wellbrock, C. (2014). The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov 4(10): 1214-1229.
- Tsuchiya, S., Yamabe, M., Yamaguchi, Y., Kobayashi, Y., Konno, T. and Tada, K. (1980). Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26(2): 171-176.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Smith, M. P., Young, H., Hurlstone, A. and Wellbrock, C. (2015). Differentiation of THP1 Cells into Macrophages for Transwell Co-culture Assay with Melanoma Cells. Bio-protocol 5(21): e1638. DOI: 10.21769/BioProtoc.1638.
Category
Cancer Biology > Tumor immunology > Cell biology assays > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









