- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Particles of Recombinant ASC and NLRP3
Published: Vol 5, Iss 10, May 20, 2015 DOI: 10.21769/BioProtoc.1480 Views: 12590
Reviewed by: Jia LiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for the Preparation of a Recombinant Treacle Fragment for Liquid–Liquid Phase Separation (LLPS) Assays
Nadezhda V. Petrova [...] Artem K. Velichko
Sep 20, 2025 1814 Views

Cluster FLISA—A Method to Compare Protein Expression Efficiency Between Cell Lines and Subunit Clustering of Proteins
Sabrina Brockmöller and Lara Maria Molitor
Nov 5, 2025 1207 Views

Optimized Secretome Sample Preparation From High Volume Cell Culture Media for LC–MS/MS Proteomic Analysis
Basil Baby Mattamana [...] Peter Allen Faull
Dec 20, 2025 1229 Views
Abstract
NLRP3 inflammasome is a multiprotein complex responsible for the activation of inflammatory caspase-1, resulting in processing and release of pro-inflammatory cytoquines IL-1β and IL-18 (Schroder and Tschopp, 2010). This inflammasome is composed of the sensor protein NLRP3 connected to caspase-1 through the adaptor protein ASC (apoptosis-associated speck-like protein with a caspase-recruitment domain) (Schroder and Tschopp, 2010). We and others have reported that upon inflammasome activation functional oligomeric inflammasome particles of NLRP3 and ASC were released from cells, acting as danger signals to amplify inflammation by promoting the activation of caspase-1 extracellularly (Baroja-Mazo et al., 2014; Franklin et al., 2014).
Studying the extracellular function of oligomeric ASC and NLRP3 inflammasome particles was possible by purification of recombinant particles of ASC or the constitutively activated NLRP3 mutant associated with cryopyrin-associated periodic syndromes (CAPS, mutation p.D303N), both tagged with the yellow fluorescent protein (YFP) and expressed in HEK293 cells. The purification process was facilitated by the fact that expression of recombinant ASC or mutant NLRP3 in HEK293 cells resulted in their spontaneous aggregation into specks (Baroja-Mazo et al., 2014) and the protocol was originally adapted from Fernandes-Alnemri and Alnemri (2008).
Materials and Reagents
- HEK293T cell line (ATCC® number: CRL-11268 )
- Dulbecco´s Modified Eagle´s medium F12 (DMEM-F12) (Biowest, catalog number: L0090 )
- 10% (v/v) Fetal Bovine Serum (Lonza, catalog number: DE14-801F )
- 200 mM L-Glutamine (Lonza, catalog number: 17-605E )
- Penicillin and streptomycin (Lonza, catalog number: 17-603E )
- Liquid nitrogen
- 1x DPBS (Life Technologies, Gibco®, catalog number: 14190-094 )
- 1x Percoll (see Recipes)
- Buffer A (see Recipes)
- 2x CHAPS (see Recipes)
- 1x CHAPS (see Recipes)
Equipment
- Tissue culture flasks with quick-release screw cap 75 ventilation (SARSTEDT AG, catalog number: 83.1813.002 )
- Tissue culture plate with lid, hydrophobic, sterile (SARSTEDT AG, catalog number: 83.1839 )
- 37 °C, 5% CO2 cell culture incubator
- Table top cooling centrifuge with rotor for 15 ml Falcon tubes (Sigma-Aldrich, catalog number: 3K30 )
- Table top cooling microcentrifuge with rotor for 2 ml tubes (HERMLE, catalog number: Z216MK )
- Water bath at 37 °C
- Syringe (1 ml) (Nipro Syringe, catalog number: SY31SCTU EC )
- 20 G and 25 G needles (Becton, Dickinson and Company, catalog number: 300600 )
- Tissue culture class II laminar flow hood (Telstar Bio II)
- Inverted microscope with epifluorescence (Nikon Eclipse Ti)
- Bürker counting chamber
- Ultrafree-CL low-binding Durapore PVDF membrane (5 µm) (EMD Millipore, catalog number: UFC40S25 )
Procedure
Perform all the steps on ice and all the centrifugations at 4 °C unless noted otherwise.
Start the purification from 107 HEK293 transiently expressing ASC-YFP or stably expressing NLRP3 (p.D303N)-YFP maintained in DMEM: F12 (1:1) supplemented with 10% FCS, 2 mM Glutamax and 1% penicillin-streptomycin.
- Wash the cells with warm PBS and detached them with 6 ml of cold PBS by pipetting up and down against the bottom of the flask. Transfer the cell suspension into a 15 ml tube (representative scheme of steps from 1 to 8 is shown in Figure 1).
- The cells were pelleted by centrifugation at 2,000 x g for 3 min. Discard supernatant and re-suspended the pellet in 600 µl of cold Buffer-A and transfer it into a 1.5 ml tube.
- Cells in buffer A were lysed on ice by passing through a 1 ml syringe with a 20 G needle (10 times) and then through a 25 G needle (20 times). After that, the lysate was freeze in liquid nitrogen (keep in liquid nitrogen around 30-40 sec or until the lysate is frozen) and subsequent thawed in a water bath at 37 °C (repeat this freeze/thaw cycle 3 times). The cell lysate was passed again through a 25 G needle (10 times).
- The lysates were then centrifuged at 400 x g for 8 min. The supernatant was recovered and the pellet discarded.
- Supernatant was transferred into a 15 ml tube, diluted with 2 volumes of CHAPS buffer 2x and then filtered using a 5 μm centrifugal filters at 2,000 x g during 10 min. The centrifugal filter was discarded and the filtrate kept.
- The filtrate was diluted with 1 volume of CHAPS buffer 2x and mixed by gentle pipetting up and down (this step is to lysate organelles such as mitochondria). Afterwards, the diluted lysate was centrifuged at 2,300 x g for 8 min and the pellet was recovered, discarding the supernatant.
- The pellet was re-suspended in 1 ml of CHAPS buffer 1x and centrifuged at 5,000 x g for 8 min, discarding the supernatants and keeping the pellet. Repeat this wash step twice.
- After the last centrifugation step, the pellet was re-suspended in 1 ml CHAPS buffer 1x and loaded carefully on the top of 200 µl of 40% Percoll (prepared previously in a 1.5 ml tube).
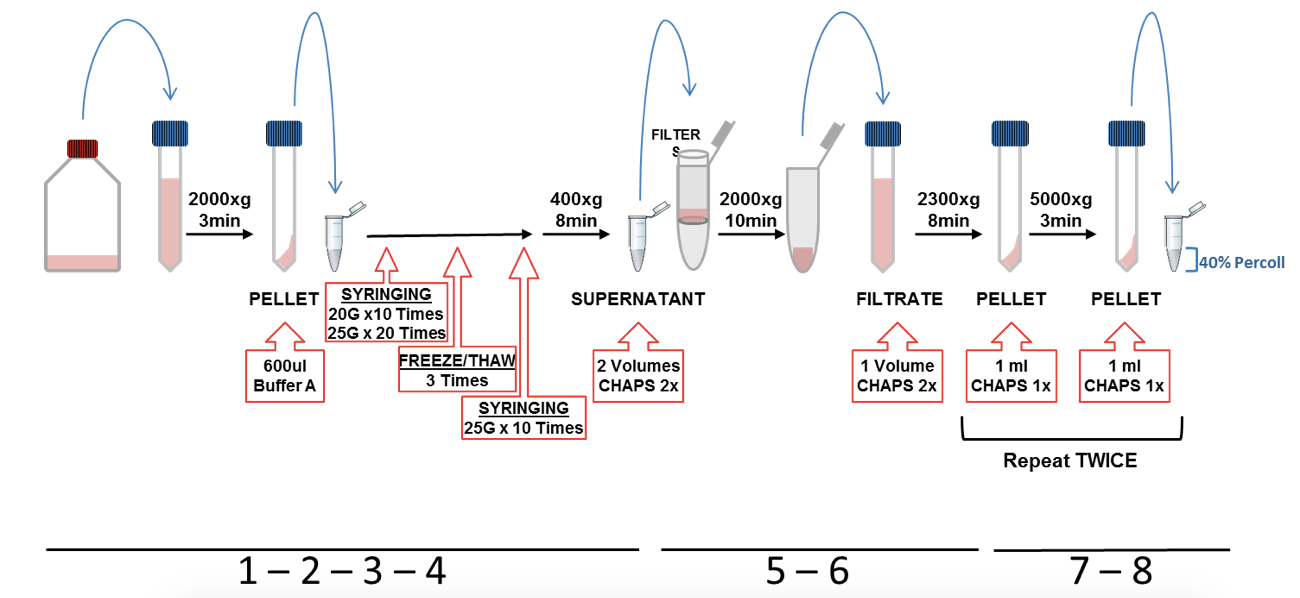
Figure 1. Representative scheme of ASC and NLRP3 (p.D303N) particles purification protocol illustrating steps from 1 to 8 - Percoll was centrifuged at 16,000 x g for 10 min (turn off the break is not necessary). The interface layer containing the inflammasome particles was collected slowly and gently by pipetting and transferred into a new 1.5 ml tube and then washed with 1 ml of CHAPS buffer 1x by centrifugation using the same conditions (Figure 2).
- The supernatant was removed and the pellet was re-suspended in 100 µl of 1x CHAPS buffer (Figure 2).
- Finally, fluorescent particles were quantified in a fluorescence microscope using a Bürker chamber using a 1:10 dilution (this dilution depends on the concentration of recovered ASC or NLRP3 particles). After quantification the volume was adjusted until reach a density of 5 x 105 particles per µl.
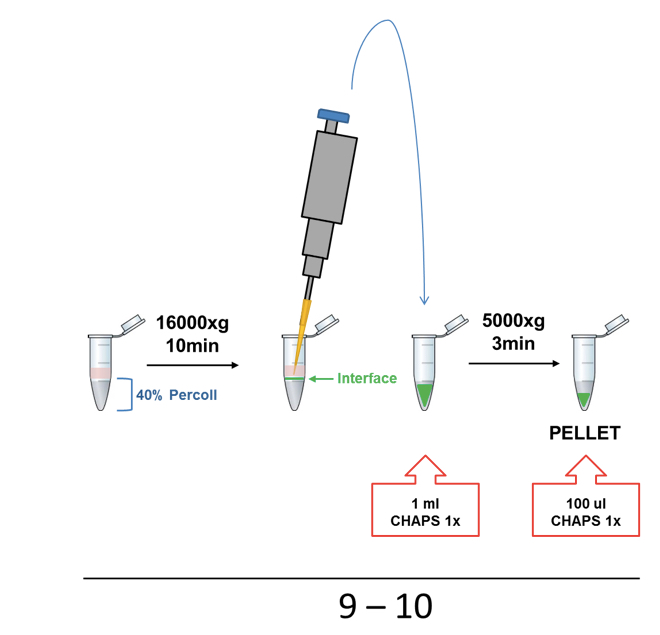
Figure 2. Representative scheme of Percoll step (steps 8-10)
Representative data
Table 1. Example of expected amounts of fluorescent particles recovered after purification protocol relative to 107 HEK293T cells expressing ASC-YFP or NLRP3 (p.D303N)-YFP. The cells were detached 48 h after transfection (cells expressing ASC-YFP) or until reach 90-100% of confluence per flask (cells stably expressing NLRP3(pD303N). The data shown are from experiments performed in different days.
| Experiment | Asc-YFP (106 particles) | NLRP3 (p.D303N)-YFP (106 particles) |
| 1 | 4.70 | 1.50 |
| 2 | 3.81 | 1.54 |
| 3 | 3.03 | 2.25 |
| 4 | 3.9 | 2.15 |
| 5 | 4.92 | 1.15 |
| Average | 4.07 | 1.72 |
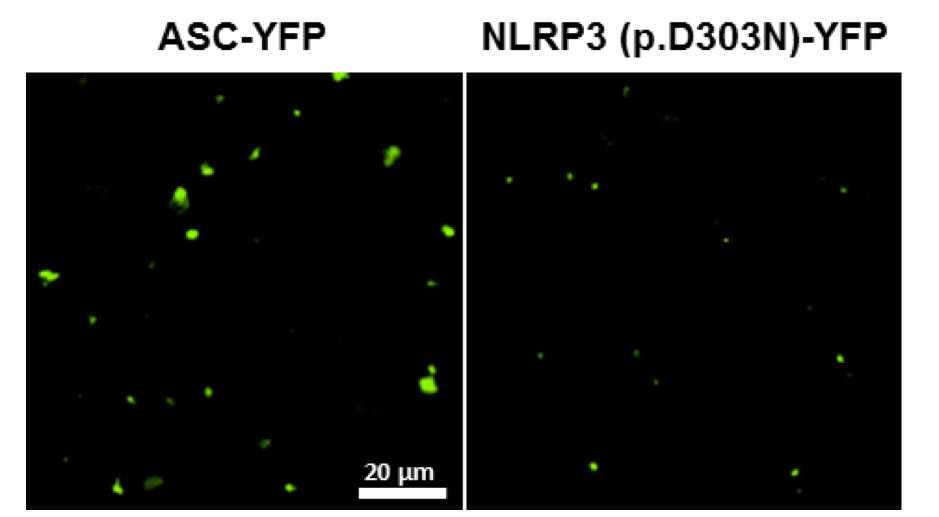
Figure 3. Representative image of ASC and NLRP3 (p.D303N) fluorescent particles after purification protocol
Notes
- In step 2 cell pellet can be frozen at -80 °C (first in liquid nitrogen and then at -80 °C) or proceed with the speck purification protocol.
- Note that the supernatant recovered in step 4 after centrifugation is not clear.
Recipes
- 1x Percoll (to prepare 200 µl)
120 µl 1x CHAPS
80 µl Percoll-Plus - Buffer A
320 mM sucrose
20 mM HEPES-KOH (pH 7.5)
10 mM KCl
1.5 mM MgCl2
1 mM EDTA
1 mM EGTA - 2x CHAPS buffer
40 mM HEPES-KOH (pH 7.5)
10 mM MgCl2
1 mM EGTA
0.2 mM PMSF
0.2 % CHAPS
Note: 1x CHAPS buffer is prepared by diluting the CHAPS buffer 2x in distilled water.
Acknowledgments
This protocol has been adapted from the previously published paper: Baroja-Mazo et al. (2014). This work was supported by grants from PN I+D+I 2008-2011-Instituto Salud Carlos III-FEDER (PI13/00174) and European Research Council (ERC-2013-CoG 614578). The authors declare no conflict of interests.
References
- Baroja-Mazo, A., Martín-Sánchez, F., Gomez, A. I., Martínez, C. M., Amores-Iniesta, J., Compan, V., Barberà-Cremades, M., Yagüe, J., Ruiz-Ortiz, E. and Antón, J. (2014). The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15:738-48.
- Fernandes-Alnemri, T. and Alnemri, E. S. (2008). Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol 442: 251-270.
- Franklin, B. S., Bossaller, L., De Nardo, D., Ratter, J. M., Stutz, A., Engels, G., Brenker, C., Nordhoff, M., Mirandola, S. R. and Al-Amoudi, A. (2014). The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat Immunol 15(8): 727-737.
- Schroder, K. and Tschopp, J. (2010). The inflammasomes. Cell 140(6): 821-832.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Martín-Sánchez, F., Gómez, A. I. and Pelegrín, P. (2015). Isolation of Particles of Recombinant ASC and NLRP3. Bio-protocol 5(10): e1480. DOI: 10.21769/BioProtoc.1480.
Category
Immunology > Host defense > General
Biochemistry > Protein > Isolation and purification
Biochemistry > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









