- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Collection of Root Exudate from Duckweed
Published: Vol 5, Iss 1, Jan 5, 2015 DOI: 10.21769/BioProtoc.1367 Views: 14331
Reviewed by: Arsalan DaudiSamik BhattacharyaTie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1942 Views

Utilizing FRET-based Biosensors to Measure Cellular Phosphate Levels in Mycorrhizal Roots of Brachypodium distachyon
Shiqi Zhang [...] Maria J. Harrison
Jan 20, 2025 2381 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1886 Views
Abstract
Root exudates play an important role in rhizosphere interactions between plants and microorganisms. However, collection of chemicals from plant root system is difficult due to their low concentrations and high level of contaminants in growth media. The continuous collection method has been described in several terrestrial plants over the past 30 years (Tang and Young, 1982; Kong et al., 2004). Here, we describe a protocol for the collection of sterile root exudate from a floating aquatic plant, duckweed.
Keywords: Root exudateMaterials and Reagents
- Duckweed fronds, Spirodela polyrrhiza (collected from the paddy field drainage in the Tai Lake region of China)
- Sterile deionized water
- Methanol (HPLC grade) (Sigma-Aldrich, catalog number: 179337)
- Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 653799)
- Formaldehyde solution (37 wt.% in H2O) (Sigma-Aldrich, catalog number: 252549)
- Sodium hypochlorite (NaClO) (available chlorine 4.00-4.99 %) (Sigma-Aldrich, catalog number: 239305)
- 0.15% formaldehyde solution (see Recipes)
- 0.5% v/v NaClO (see Recipes)
- Modified Steinberg nutrient solution (see Recipes)
Equipment
- Duckweed root exudate collection device (Figure 1)
- Plexiglass pot (D x H, 16 x 16 cm)
- Glass column (D x H, 23 x 170 mm)
- XAD-4 resin (20-60 mesh) (Sigma-Aldrich, catalog number: 37380-42-0)
- Hydrophobic fluoropore (PTFE) membrane (14 x 14 cm)
- Glass wool (Sigma-Aldrich, catalog number: 18421)
- Teflon stopper
- Aluminum foil
- Silicone tube

Figure 1. Equipment used in root exudate collection
- Plexiglass pot (D x H, 16 x 16 cm)
- Clean bench
- Peristaltic pump
- Rotary evaporator (Tokyo Rikakikai, EYELA, model: N-1100D)
- Freeze dryer (Freezone Plus 2.5) (Labconco)
- Incubation chamber (23 ± 1 °C, under fluorescent lamps at 100 μmol/m/s, 16 h light/8 h dark)
Procedure
- Preparation of the sterile duckweed fronds
- Wash 100 duckweed fronds with deionized water for 5 min. Transfer the fronds to a 500-ml flask with 200 ml of 0.5% v/v NaClO and stir gently for 30 min.
- Rinse the surface-sterilized plants five times with sterile deionized water. Transfer them to a 500-ml flask with 200 ml sterile modified Steinberg nutrient solution.
Note: This should be performed in a clean bench. - The fronds are statically grown in an incubation chamber at 23 ± 1 °C under fluorescent lamps at 100 μmol/m2/s (16 h light/8 h dark) until use (Figure 2).

Figure 2. The incubation condition of the duckweed fronds
Note: The duckweed fronds will gradually lose chlorophyll hours after sterilization (6 hours later). After a day, the growing points which are protected by the capsules remain green, whereas the other parts of the fronds become completely yellow. Three days later, new fronds are grown from the growing points, but smaller. They will recover completely after five days. Therefore, it will take about ten days for duckweed to grow normally after sterilization (Figure 3).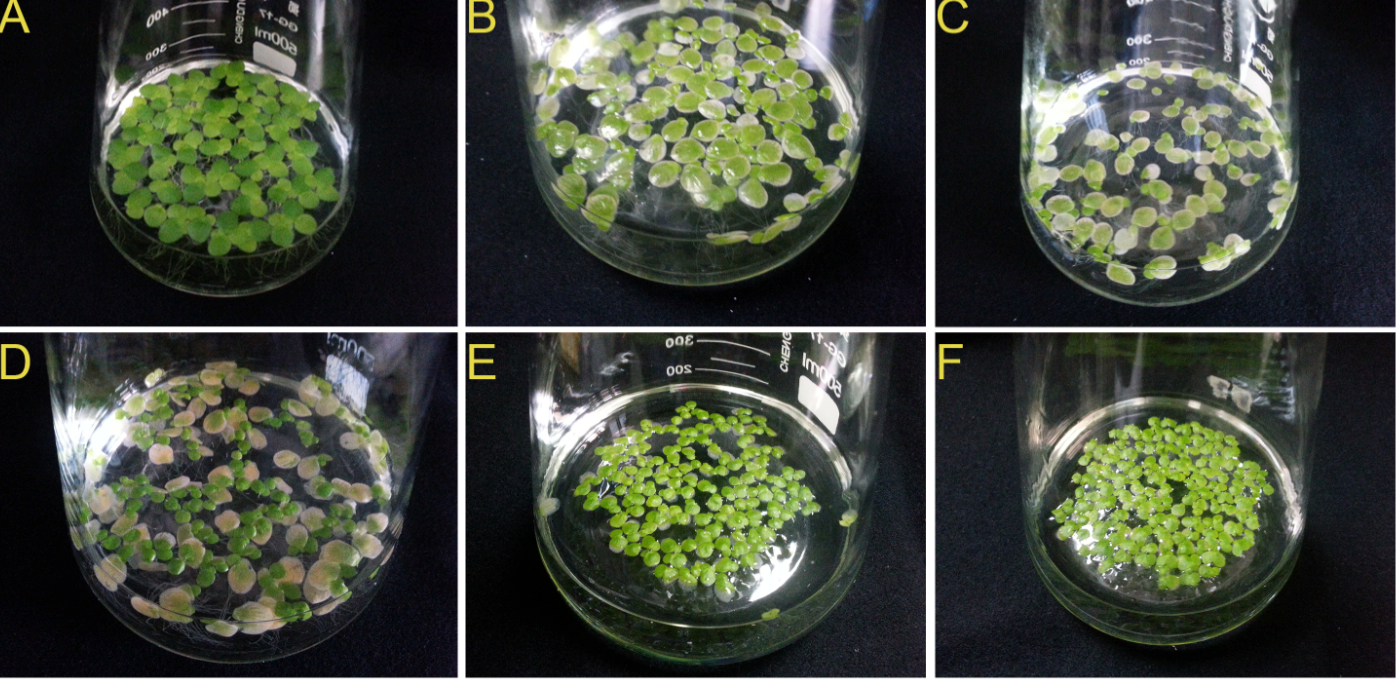
Figure 3. The duckweed fronds at each stage after sterilization. A. Before sterilization. B. After 6 h. C. After 1 day. D. After 3 day. E. After 5 day. F. After 10 day.
- Wash 100 duckweed fronds with deionized water for 5 min. Transfer the fronds to a 500-ml flask with 200 ml of 0.5% v/v NaClO and stir gently for 30 min.
- Preparation of the sterile root exudate collection device
- Soak the plexiglass pot, glass column and silicone tube in the 0.15% formalin solution overnight. Wash them with sterile deionized water.
- Soak the glass wool in the 2 M HCl overnight, wash it using sterile deionized water three times to pH 7.0, then autoclave it.
- Wash the XAD-4 resin with sterile deionized water. Elute the resin with methanol. Store the clean resin in methanol in a dark glass container until use.
- The glass column is fixed on an iron support. Put about 0.2 g of glass wool at the bottom to prevent resin from leaking with a glass rod (Figure 4A). Connect a 5-cm long silicone tube to the bottom of glass column (Figure 4B).
- Stir the XAD-4 resin and pack the column with 60 ml soaked resin (Figure 4C-D). Remove the residual methanol by washing the column with about 1.5 L of sterile deionized water.
Note: Keep the column vertical to avoid bias flow. Pour the resin into column slowly to avoid bubbles. - Fasten the tube with a clip to avoid resin column becoming dry (Figure 4E).
- Keep the water and resin level tangent (Figure 4F). Put about 0.2 g of glass wool on the top of resin to isolate from the air and fix the resin (Figure 4G).
- Connect the column to the top of the pot through a perforated Teflon stopper. Wrap the column with aluminum foil to restrict light and deter algae growth (Figure 4H).
- Connect a peristaltic pump to the bottom of the pot with a silicon tube.
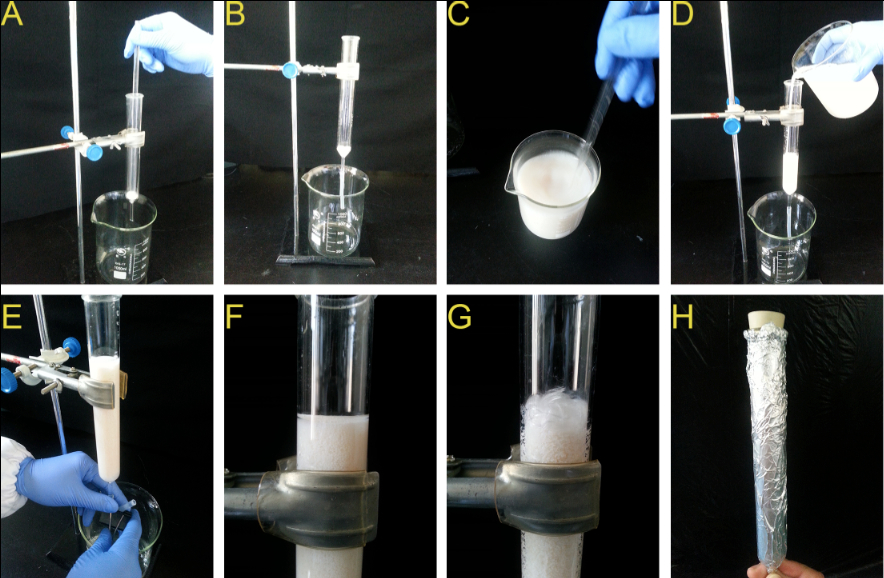
Figure 4. Preparing and packing the column. A, B. Put some glass wool at the bottom and connect with a silicone tube. C, D. Stir the XAD-4 resin and pack the column. E. Fasten the tube with a clip. F, G. Keep the water and resin level tangent and put some glass wool on the top. H. Wrap with aluminum foil.
- Soak the plexiglass pot, glass column and silicone tube in the 0.15% formalin solution overnight. Wash them with sterile deionized water.
- Collection of duckweed root exudate
- Wash 100 cm2 (about 2 g fresh weight, 50% coverage) of the sterile duckweed fronds twice with sterile water. Transplant them into the pot containing 2 L sterile nutrient solution. Cover the pot with an autoclaved hydrophobic fluoropore (PTFE) membrane to maintain a sterile environment.
Note: This should be performed on a clean bench. - Begin the chemical trapping. The nutrient solution circulates at a rate of 10 ml/min by the peristaltic pump. The exudates are continuously attached to the resin.
- After five days, detach the column with 1.5 L of deionized water. Elute it with 150 ml methanol.
- Evaporate the methanol under vacuum at 40 °C. Freeze-dry the remaining aqueous solution, re-dissolve the residue in 1 ml methanol, store at -20 °C.
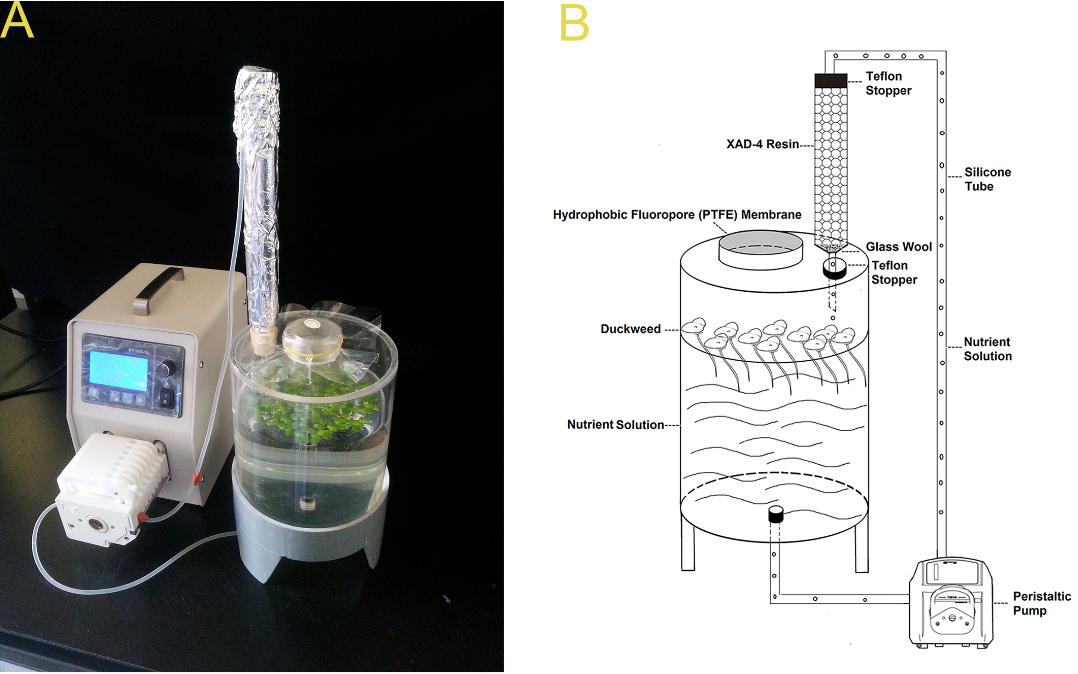
Figure 5. The continuous duckweed root exudate-trapping system (Lu et al., 2014). A. A real picture of the collection system. B. A diagram of the collection system. The nutrient solution was continuously circulated through the root systems of the duckweed plants, eluting the duckweed-derived organic compounds. Hydrophobic or partially hydrophobic exudates were selectively absorbed by the XAD-4 resin while the inorganic nutrients were recycled to sustain duckweed growth.
- Wash 100 cm2 (about 2 g fresh weight, 50% coverage) of the sterile duckweed fronds twice with sterile water. Transplant them into the pot containing 2 L sterile nutrient solution. Cover the pot with an autoclaved hydrophobic fluoropore (PTFE) membrane to maintain a sterile environment.
Recipes
- 0.15% formaldehyde solution
Mix 20 ml of formaldehyde solution in 5 L deionized water - 0.5% v/v NaClO
Mix 1 ml of NaClO in 199 ml deionized water
Stored at 4 °C - Modified Steinberg nutrient solution
0.42 mM Ca (NO3)2.4H2O
0.23 mM NH4Cl
0.41 mM MgSO4.7H2O
12.9 μM KH2PO4
0.63 μM ZnSO4.7H2O
0.91 μM MnCl2.4H2O
1.94 μM H3BO3
0.18 μM NaMoO4.2H2O
2.80 μM FeCl3.6H2O
2.80 μM Na2EDTA.2H2O
Adjusted to pH 6.8 by 1 M NaOH
Autoclave
Stored at room temperature
Acknowledgments
This work was supported by grants from the Strategic Priority Research Program (B)-“Soil-Microbial System Function and Regulation” of the Chinese Academy of Sciences (XDB15030100) and the National Science Technology Program (2012BAD15B03). This protocol was published in our Nature paper (Lu et al., 2014).
References
- Kong, C., Liang, W., Xu, X., Hu, F., Wang, P. and Jiang, Y. (2004). Release and activity of allelochemicals from allelopathic rice seedlings. J Agric Food Chem 52(10): 2861-2865.
- Lu, Y., Zhou, Y., Nakai, S., Hosomi, M., Zhang, H., Kronzucker, H. J. and Shi, W. (2014). Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components. Planta 239(3): 591-603.
- Tang, C. S. and Young, C. C. (1982). Collection and Identification of Allelopathic Compounds from the Undisturbed Root System of Bigalta Limpograss (Hemarthria altissima). Plant Physiol 69(1): 155-160.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lu, Y., Sun, L. and Shi, W. (2015). Collection of Root Exudate from Duckweed. Bio-protocol 5(1): e1367. DOI: 10.21769/BioProtoc.1367.
Category
Plant Science > Plant metabolism > Other compound
Plant Science > Plant physiology > Plant growth
Plant Science > Plant physiology > Tissue analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









