- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Localization and Topology of Thylakoid Membrane Proteins in Land Plants
Published: Vol 4, Iss 24, Dec 20, 2014 DOI: 10.21769/BioProtoc.1363 Views: 13380
Reviewed by: Ru ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Streamlining Protein Fractional Synthesis Rates Using SP3 Beads and Stable Isotope Mass Spectrometry: A Case Study on the Plant Ribosome
Dione Gentry-Torfer [...] Federico Martinez-Seidel
May 5, 2024 2855 Views

An Activity-Based Proteomics with Two-Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE) for Identifying Target Proteases in Arabidopsis Apoplastic Fluid
Sayaka Matsui and Yoshikatsu Matsubayashi
Mar 5, 2025 1975 Views

Advancing 2-DE Techniques: High-Efficiency Protein Extraction From Lupine Roots
Sebastian Burchardt [...] Emilia Wilmowicz
Oct 5, 2025 1763 Views
Abstract
Thylakoids are a formation of flattened membrane vesicles and protein complexes found in cyanobacteria, algae and plants. In the chloroplasts of land plants the thylakoid membrane systems form a network of densely packed stacks called grana lamellae, which are connected by unstacked stroma lamellae. Photosystem II is mainly localized in the appressed grana region, while photosystem I and the ATP synthase complexes are enriched in the stroma lamellae. The cytochrome b6/f complex is distributed laterally throughout both stacked and unstacked membrane regions. The photosynthetic complexes consist of integral and peripheral proteins. The first part of this protocol (A) shows how to fractionate thylakoids into grana and stroma lamellae. The second part of this protocol (B) shows how to distinguish between strong hydrophobic integral membrane associations and weak electrostatic membrane and/or membrane complex associations. As it is necessary to specifically detect the protein of interest in the fractions, a specific antibody raised against the protein of interest or a complemented null mutant of a structural component expressing a tagged fusion protein would be of great advantage. The last part of this protocol (C) shows, how to investigate the topology of integral and peripheral proteins. This method requires a specific antibody for the protein of interest. For integral membrane proteins peptide-specific antibodies or epitope-tagged versions are required. The protocol is suitable for the investigation of low molecular weight proteins (LMW) below 5 kDa (Torabi et al., 2014).
Keywords: StromaMaterials and Reagents
- Freshly isolated thylakoids
- Digitonin (water soluble) (SERVA Electrophoresis GmbH, catalog number: 19551.02 )
- Thermolysin from Bacillus thermoproteolyticus (Calbiochem®, catalog number: 58656 )
- NaBr (Sigma-Aldrich, catalog number: S-9756 )
- NaSCN (Merck KGaA, catalog number: 6627 )
- Na2CO3 (Merck KGaA, catalog number: 6392 )
- NaOH (Roth North America, catalog number: 9097.2 )
- Sucrose (Roth North America, catalog number: 9097.2 )
- EDTA (SERVA Electrophoresis GmbH, catalog number: 11280 )
- HEPES (Roth North America, catalog number: 9105.3 )
- Na2HPO4 (Roth North America, catalog number: 4984.3 )
- NaH2PO4 (Roth North America, catalog number: K300.2 )
- 0.1 M sodium phosphate buffer (see Recipes)
- Fractionation buffer (see Recipes)
- HS buffer (see Recipes)
- 0.4% digitonin solution (see Recipes)
- Thermolysin stock solution (40 mg/ml) in HS buffer (see Recipes)
- Salt containing HS buffers (see Recipes)
Equipment
- Centrifuges (Beckmann Coulter, model: Avanti J-25 ; Eppendorf, model: 5430 R )
- Ultracentrifuge (Beckmann Coulter, model: Optima LE-80K )
- Sonifier (Branson, model: B-12 )
- Photometer (Amersham biosciences, model: UltraspeTM 3100 pro )
Procedure
- Fractionation of thylakoid membranes
- To solubilize the thylakoids at the grana margins mix 5 ml of freshly isolated thylakoids (0.8 mg chlorophyll/ml) in fractionation buffer with 5 ml of the 0.4% digitonin solution and incubate 2 min at RT. Prevent sedimentation of the thylakoid solution by slightly agitating.
- Stop the solubilization with the addition of 90 ml ice cold fractionation buffer.
- Centrifuge the solution for 15 min (10,000 x g, 4 °C). Carefully keep the supernatant for the next step and try to avoid contaminations from the pellet. Dissolve the pellet (grana fraction) in 2 ml fractionation buffer and store on ice.
- Centrifuge the supernatant of step A3 for 30 min (40,000 x g, 4 °C). Carefully remove the upper part of the supernatant for step A5 and leave about 3 cm of the solution to avoid contamination of the stroma lamellae fraction. (Optional) dissolve the pellet in the remaining supernatant and keep the intermittent fraction on ice.
Pellet stroma lamellae by ultracentrifugation of the supernatant of step A4 for 60 min (100,000 x g, 4 °C). Carefully remove the supernatant and dissolve the pellet in fractionation buffer. Try to keep the concentration as high as possible to avoid additional ultracentrifugation steps when adjusting the fractions to the desired chlorophyll concentrations. For storage of native thylakoid fractions (-20 °C or -80 °C) high chlorophyll concentrations around 2-4 mg/ml are recommended. For SDS-PAGE analysis final concentrations from 0.25 to 1 µg Chl/µl are used depending on the protein of interest. As a general rule use a lower final protein concentration for bigger membrane proteins with many cysteins to reduce (e.g. CP47, PsaA). Higher protein concentrations are suitable for low molecular weight proteins to reduce the sample volume and to avoid an expanded loading buffer front which could interfere with the migration of very small proteins. - Measure the chlorophyll contents of the fractions in 80% acetone and adjust to equal amounts of µg chlorophyll/µl.
- Analyze the fractionation by SDS-PAGE (Figure 1).
- To solubilize the thylakoids at the grana margins mix 5 ml of freshly isolated thylakoids (0.8 mg chlorophyll/ml) in fractionation buffer with 5 ml of the 0.4% digitonin solution and incubate 2 min at RT. Prevent sedimentation of the thylakoid solution by slightly agitating.
- Salt treatment of thylakoid membranes
- Dissolve freshly isolated thylakoid membranes in HS buffer (0.5 mg chlorophyll/ml) and in the salt containing HS buffers.
- Incubate the samples for 30 min on ice.
- Dilute the samples with two volumes of HS buffer.
- Separate into pellet and soluble fraction by 10 min centrifugation (20,000 x g, 4°C).
- Analyze the fractions by SDS-PAGE.
- Dissolve freshly isolated thylakoid membranes in HS buffer (0.5 mg chlorophyll/ml) and in the salt containing HS buffers.
- Thermolysin treatment of thylakoid membranes
- Dissolve freshly isolated thylakoid membranes in HS buffer (0.5 mg chlorophyll/ml).
- To produce 50% inside-out and 50% right-side out vesicles apply ultrasonic pulses (10-30 sec, 10-30 times) to the solution on ice. Wait about 20 sec in between the pulses for cooling.
- Add the protease thermolysin to a final concentration of 100 µg/ml to the untreated thylakoid membrane solution and the inside- and right-side out vesicles.
- Take probes at different time points (for example 0, 1, 2, 5 min) and immediately stop the digestion by adding EDTA to a final concentration of 20 mM.
- Wash probes in HS buffer containing 20 mM EDTA.
- Compare the digestion of thylakoids and inside- and right-side out vesicles by SDS-PAGE.
- Dissolve freshly isolated thylakoid membranes in HS buffer (0.5 mg chlorophyll/ml).
Representative data
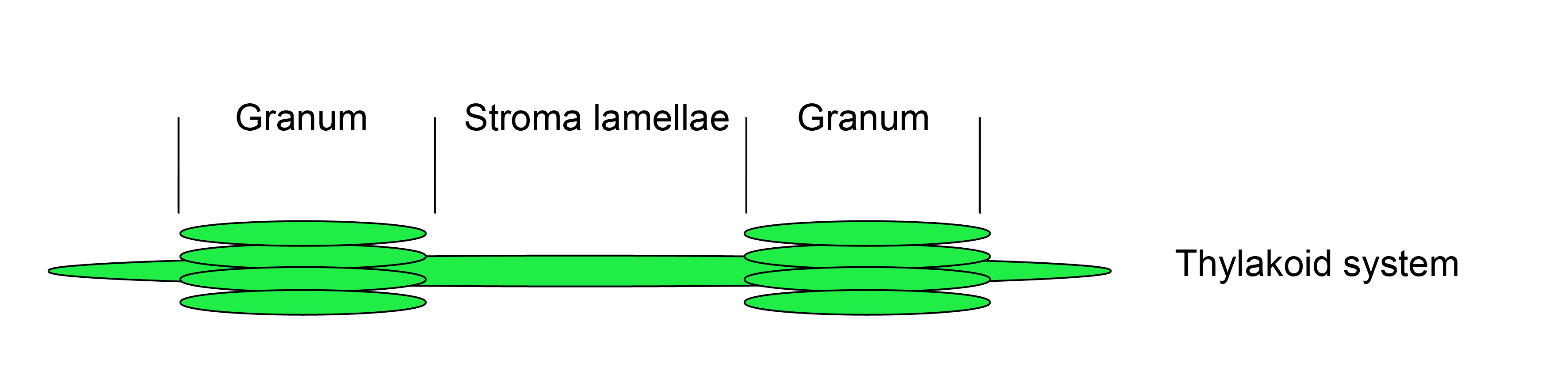
Figure 1. Thylakoid system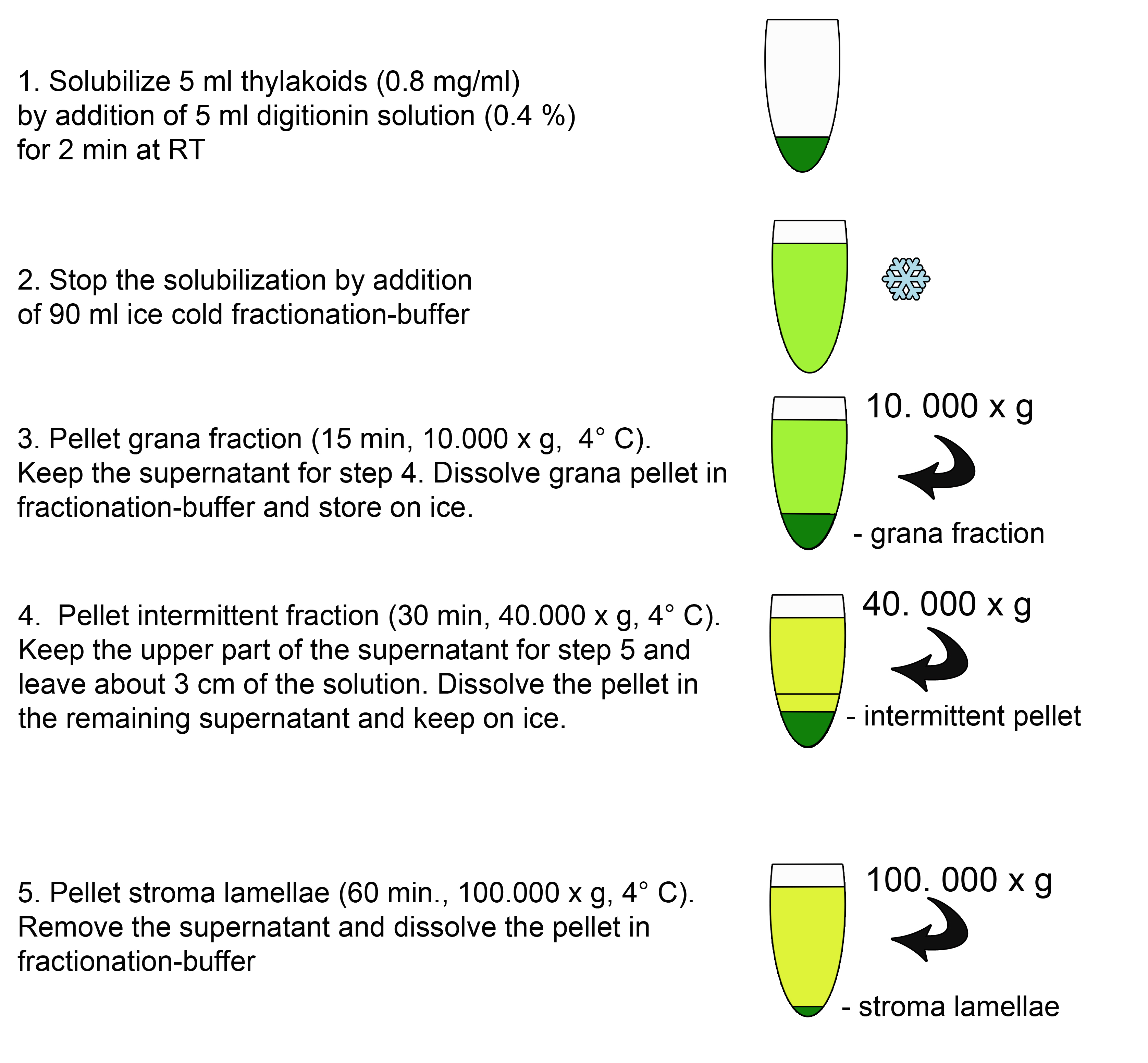
Figure 2. Scheme of the fractionation procedure (A)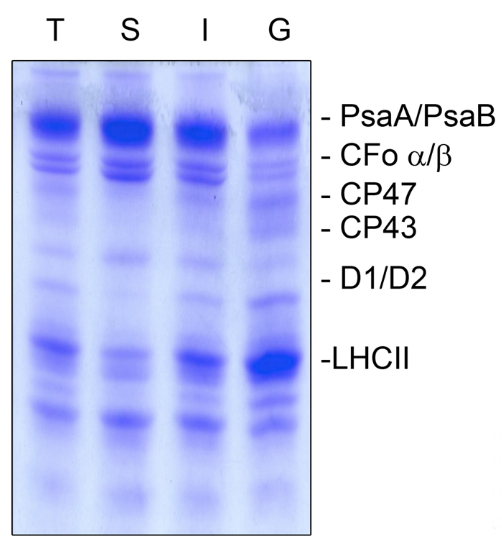
Figure 3. Coomassie staining of the thylakoid fractionation (A). Thylakoid membranes (T) were fractionated into stroma lamellae (S), intermittent fraction (I), and grana lamellae (G), and the separated proteins were subsequently stained with Coomassie to judge the purity of the fractions. PSI proteins PsaA and PsaB as well as the ATP synthase subunits CFo α/β were highly enriched in the stroma lamellae and only small amounts were present in the grana membranes. The PSII proteins CP47, CP43, D1, D2 and the antenna proteins of the light harvesting complex of PSII (LHCII) were predominantly present in grana fractions but almost lacking in the stroma lamellae fraction. The intermittent fraction contained proteins of both photosystems. An equal amount of chlorophyll (5 µg) was loaded.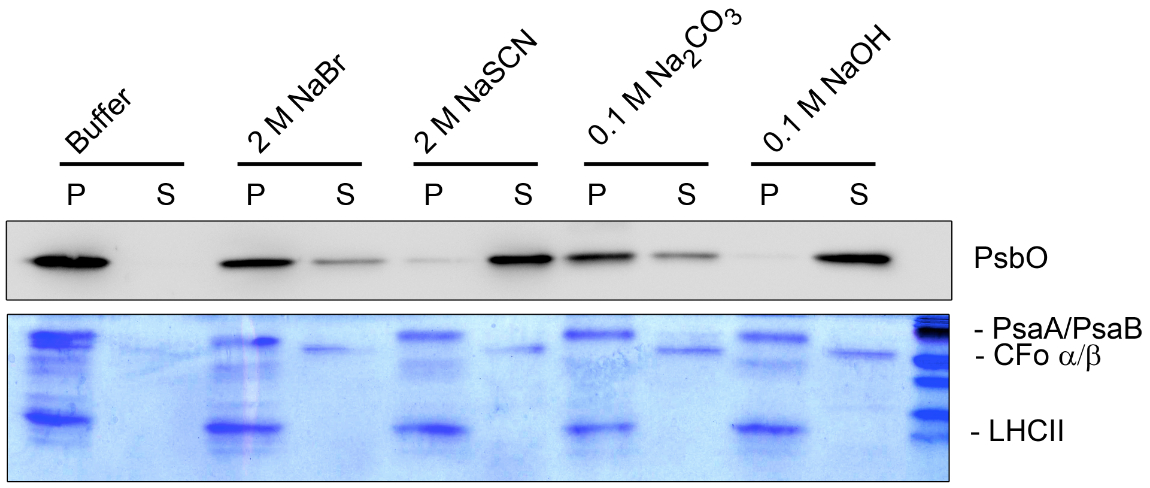
Figure 4. Washing experiment (B). Thylakoid membranes treated with different salt-containing buffers were fractionated into pellet (P) and supernatant (S) and separated by SDS-PAGE. The separated proteins were analyzed by Coomassie staining and immunoblot analysis. The dissociation of the hydrophilic ATP synthase subunits CFo α/β from the membrane can be identified by Coomassie staining under all salt conditions used. In contrast the hydrophobic light harvesting complex of PSII (LHCII) and the PSI core subunits PsaA/B could not be released from the pellet fraction. Specific antisera were used to identify the peripheral lumenal PsbO protein, which is associated with PSII. The PsbO protein could be released completely from the membrane only under stringent salt conditions (2 M NaSCN and 0.1 M NaOH).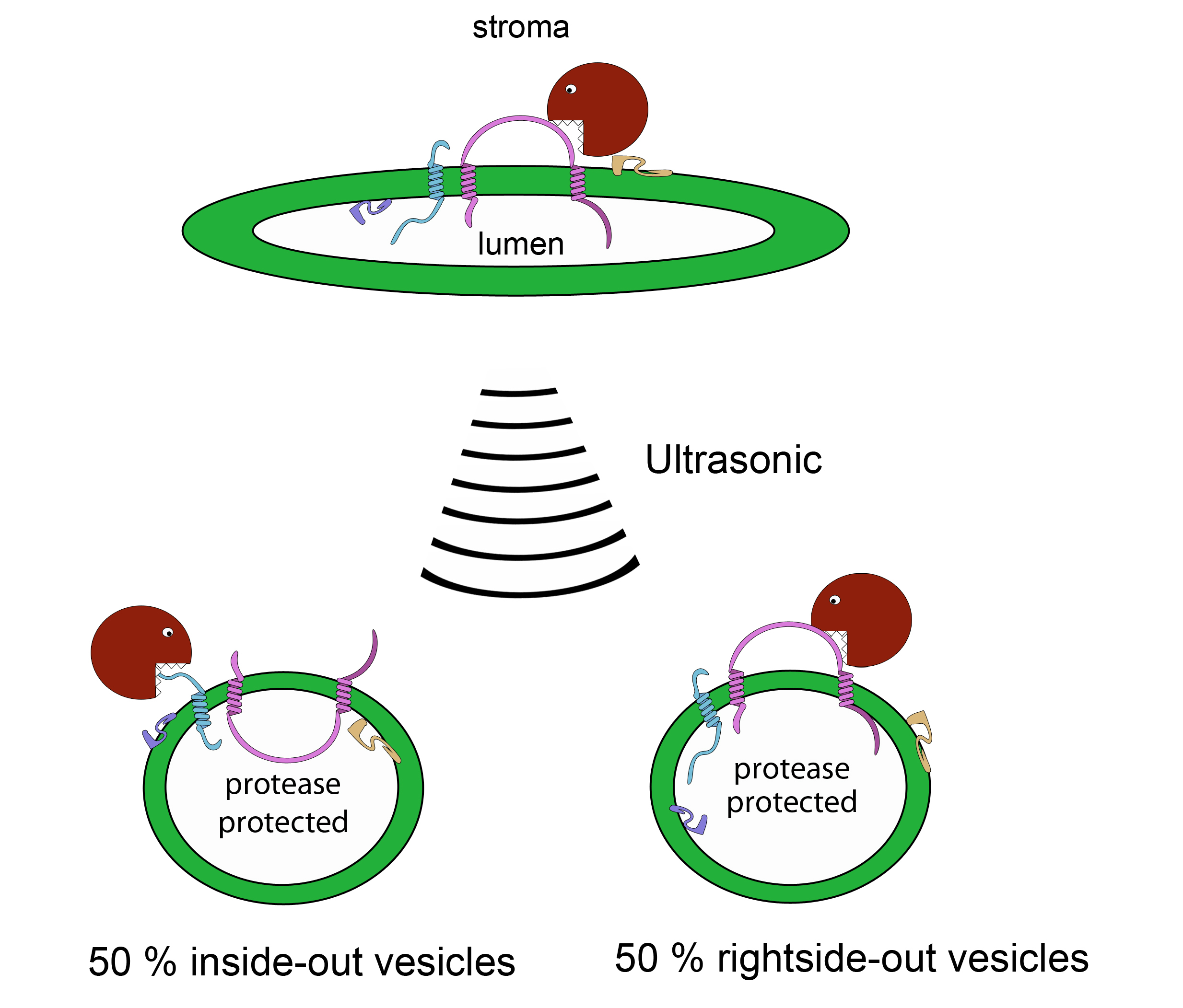
Figure 5. Scheme of the protease treatment (C)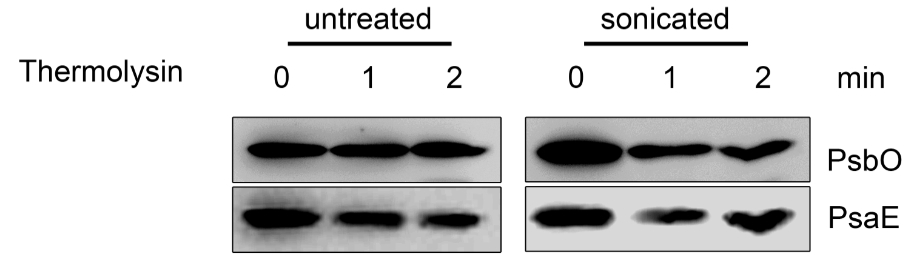
Figure 6. Topology studies (C). Untreated and sonicated thylakoids were incubated with thermolysin and subjected to immunodecoration using PsbO and PsaE antisera. In the untreated thylakoids the lumenal PsbO is protected from thermolysin treatment, while the stromal exposed PsaE is degraded. In the sonicated thylakoids, which form about 50% inside-out vesicles, PsbO is partially degraded, while PsaE is partially protected.
Notes
- To dissolve a large thylakoid pellet as fast and gentle as possible use a fine paint brush.
- For the separation of thylakoid membrane proteins by SDS-PAGE and especially proteins ≤ 10 kDa Tricine-SDS-PAGE is highly recommended for reference (Schägger, 2006).
- For immuno-blot analysis of low molecular weight thylakoid membrane proteins use 0.2 µM PVDF or 0.1 µM nitrocellulose membrane and shorten the transfer time.
Recipes
- 0.1 M sodium phosphate buffer (pH 7.4)
1 M HEPES-NaOH (pH 8.0)
0.5 M EDTA (pH 8) - Fractionation buffer
100 mM sucrose
10 mM sodium-phosphate buffer (pH 7.4)
5 mM MgCl2
5 mM NaCl - HS buffer
0.1 M sucrose
10 mM HEPES-NaOH (pH 8.0) - 0.4% digitonin solution
Dissolve 40 mg digitonin in 10 ml H2O by mixing and heating
Solution can be stored at -20 °C - Thermolysin stock solution (40 mg/ml) in HS buffer
Stock can be stored at -20 °C - Salt containing HS buffers
2 M NaBr in HS buffer
2 M NaSCN in HS buffer
0.1 M Na2CO3 in HS buffer
0.1 mM NaOH in HS buffer A
Acknowledgments
This protocol was adapted or modified from previous work by Karnauchov et al. (1997). This research was supported by the German Science Foundation (Deutsche Forschungsgemeinschaft (ME 1794) to J. M.
References
- Armbruster, U., Zuhlke, J., Rengstl, B., Kreller, R., Makarenko, E., Rühle, T., Schünemann, D., Jahns, P., Weisshaar, B., Nickelsen, J. and Leister, D. (2010). The Arabidopsis thylakoid protein PAM68 is required for efficient D1 biogenesis and photosystem II assembly. Plant Cell 22(10): 3439-3460.
- DalCorso, G., Pesaresi, P., Masiero, S., Aseeva, E., Schünemann, D., Finazzi, G., Joliot, P., Barbato, R. and Leister, D. (2008). A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132(2): 273-285.
- Karnauchov, I., Herrmann, R. G. and Klosgen, R. B. (1997). Transmembrane topology of the Rieske Fe/S protein of the cytochrome b6/f complex from spinach chloroplasts. FEBS Lett 408(2): 206-210.
- Torabi, S., Umate, P., Manavski, N., Plöchinger, M., Kleinknecht, L., Bogireddi, H., Herrmann, R. G., Wanner, G., Schröder, W. P. and Meurer, J. (2014). PsbN is required for assembly of the photosystem II reaction center in Nicotiana tabacum. Plant Cell 26(3): 1183-1199.
- Schagger, H. (2006). Tricine-SDS-PAGE. Nat Protoc 1(1): 16-22.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Torabi, S., Plöchinger, M. and Meurer, J. (2014). Localization and Topology of Thylakoid Membrane Proteins in Land Plants. Bio-protocol 4(24): e1363. DOI: 10.21769/BioProtoc.1363.
Category
Plant Science > Plant biochemistry > Protein > Structure
Plant Science > Plant biochemistry > Protein > Isolation and purification
Plant Science > Plant cell biology > Organelle isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









