- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Soluble Sugars in Arabidopsis thaliana Leaves by Anion Exchange Chromatography
Published: Vol 4, Iss 23, Dec 5, 2014 DOI: 10.21769/BioProtoc.1317 Views: 15163
Reviewed by: Tie LiuXiao-qing Xu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1885 Views

New Approach to Detect and Isolate Rhamnogalacturonan-II in Arabidopsis thaliana Seed Mucilage
Dayan Sanhueza and Susana Saez-Aguayo
Sep 5, 2025 1240 Views

Detailed Method for the Purification of Rhamnogalacturonan-I (RG-I) in Arabidopsis thaliana
Liang Zhang [...] Breeanna R. Urbanowicz
Feb 5, 2026 99 Views
Abstract
Determination of soluble sugars is basic for the study of carbon metabolism in plants. Soluble sugar quantitation can be achieved by enzymatic methods implying different coupled reactions. Here we describe a simple method that allows rapid determination of the most abundant soluble sugars (glucose, fructose and sucrose) in Arabidopsis leaves by anion exchange chromatography. We have applied this method to study the levels of soluble sugars during the photoperiodic transition to flowering (Ortiz-Marchena et al., 2014).
Keywords: Sugar determinationMaterials and Reagents
- Plants grown in soil for 3 weeks
Note: Treatment of samples is explained in the Procedure section.
- Liquid N2
- Absolute ethanol
- HEPES (Sigma-Aldrich, catalog number: H4034-1KG )
- KOH (Panreac Applichem, catalog number: 121515 )
- NaOH 50% (w/v) solution (AppliChem GmbH, catalog number: A3720, 1000 )
- Milli Q grade water
- 100 mM HEPES-KOH (see Recipes)
- Extraction buffer 1 (EB1) (see Recipes)
- 0.1 M NaOH (see Recipes)
- 21.6 mM NaOH (see Recipes)
Equipment
- Small mortar and pestle
- 1.5 ml microfuge tubes
- Automatic pipettes
- Precision scale
- 2 ml microcentrifuge (Eppendorf, model: 5415R )
- Vacufuge concentrator 5301 (Eppendorf)
- Nylon filters (Whatman, catalog number: UN203NPENYL )
- Dionex HPLC system (Dionex ICS 5000)
- CarboPacPA10 column (4 x 250 mm) (Thermo Fisher Scientific, catalog number: 046110 )
- CarboPacPA10 Pre-column (4 x 50 mm) (Thermo Fisher Scientific, catalog number: 046115 )
- Gold electrode (Dionex ICS 5000)
- Computer connected to HPLC system
Software
- Chromeleon software (v.7.0) (Thermo Fisher Scientific)
Procedure
- Collect leaf sample (or plant material) and freeze immediately in liquid N2. Stored at -80 °C until use.
- Grind plant tissue with mortar and pestle in the presence of liquid N2 until the sample is converted to a fine powder.
- Weigh 100-300 mg leaf powder into a microfuge tube and add extraction buffer 1. [The ratio of powered tissue/EB1 should be 1:1 (w/v).]
- Heat the samples at 80 °C for 2 h to extract soluble sugars. Allow them to cool and centrifuge at 15,000 x g (13,000 rpm in a microfuge) 10 min.
- Remove supernatant to a fresh microfuge tube.
- The supernatant is evaporated in vacuum at 45 °C for 2 h.
- Resuspend the dried extract in Milli Q grade water. [The ratio of water/original powdered tissue should be 0.5:1 (v/w).]
- Keep on ice while preparing all samples. Freeze the samples at -20 °C for long storage, when necessary.
- Clean soluble plant extracts by filtering through nylon filters. Suitable extract volumes (typically 50 µl) were made up to 200 µl with Milli Q grade water and filtered through Whatman Mini-UniprepTM nylon filters (0.2 µm pore size).
- Add 250 µl 21.6 mM NaOH to 50 µl filtered extracts and apply into a Dionex HPLC system. Set a 10 µl injection volume.
- Sugars are separated by isocratic elution at 20 °C with 18 mM NaOH as mobile phase at a flow of 1 ml/min [18% (v/v) 0.1 M NaOH and 82% (v/v) Milli Q grade water] through a CarboPac PA10 column and pre-column CarboPacPA10. Detection is carried out by amperometric detection with a gold electrode in nC units.
- The chromatograms are analysed using the Chromeleon software. Peaks are identified by their retention time in comparison with known standard sugars. The peak area is calculated by integrating the area between the start and the end of a peak (nC x min) using the Chromeleon software. A range of sugar mixtures of known concentrations (1, 0.5, 0.1, 0.05 and 0.01 mM; as an example, see the 0.5 mM standard profile in Figure 1A) is applied into the HPLC system, as described above, to generate calibration curves for each sugar, relating peak area (as determined by the software) with concentration. The amount of a certain sugar is calculated comparing its peak area (Figure 1B) with those in the calibration curves. Sugar content in samples is expressed in micromoles of sugar per gram fresh weight.
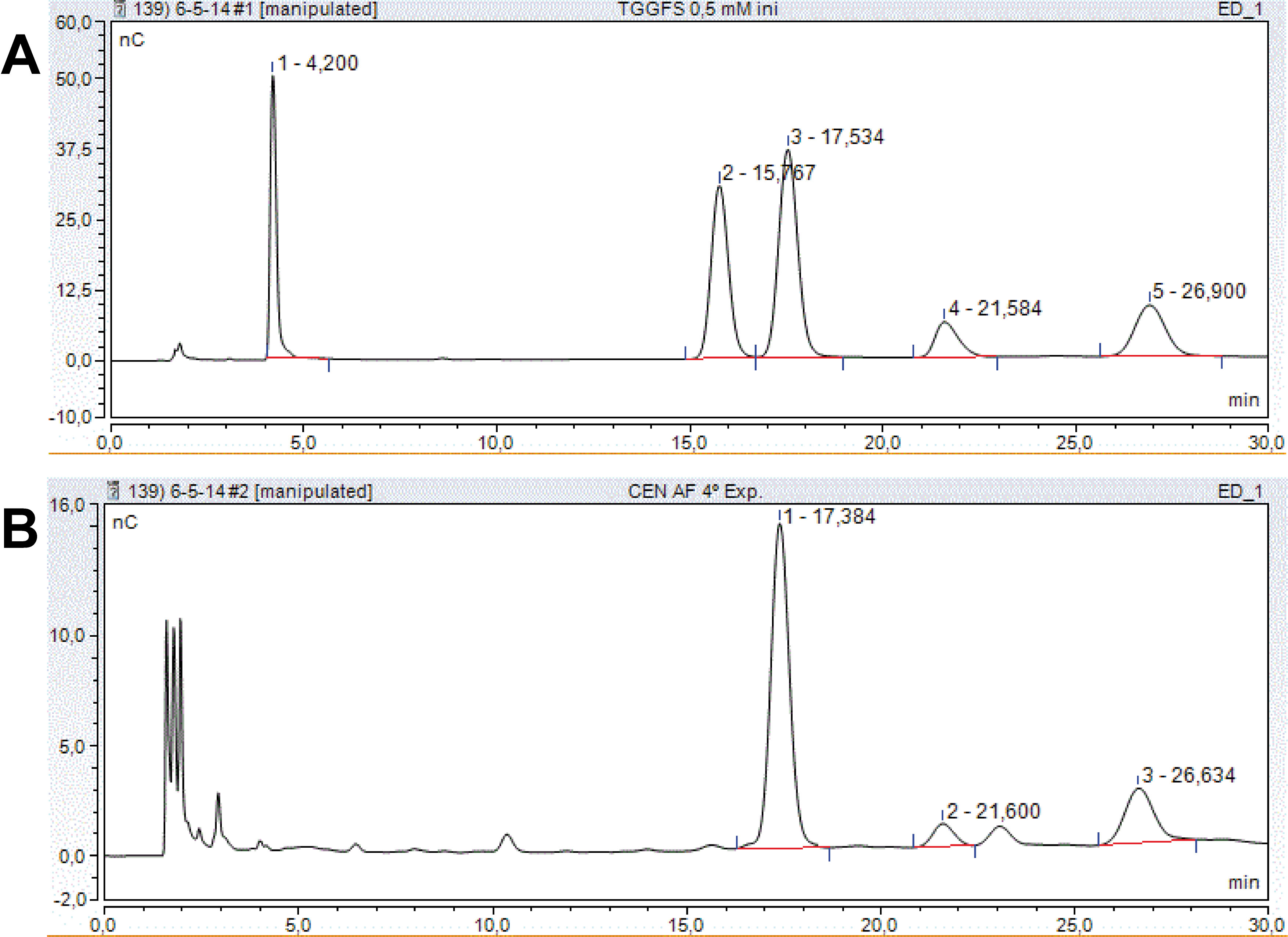
Figure 1. Sugar elution profiles in typical chromatograms. A. Sugar standards: Mixture of trehalose (peak 1), galactose (peak 2), glucose (peak 3), fructose (peak 4) and sucrose (peak 5) at 0.5 mM each. B. Plant extract showing glucose (1), fructose (2) and sucrose (3) peaks. Retention times are indicated for each peak. nC: 10-9 C.
- Add 250 µl 21.6 mM NaOH to 50 µl filtered extracts and apply into a Dionex HPLC system. Set a 10 µl injection volume.
Representative data
- Between working days and during weekends, columns and pre-columns were washed with 0.2 M NaOH at 0.1 ml min-1 flow. After this treatment, they were equilibrated with 18 mM NaOH at 1 ml/min flow for at least one hour prior to sample loading. In order to monitor the reproducibility of the different analysis, aliquots of the 0.5 mM sugar standard mixture were analysed, as mentioned above, at the beginning and at the end of each sample batch.
Table 1. Typical area values for the glucose, fructose and sucrose peaks in the 0.5 mM sugar standard aliquots (n=18)
Area (nC x min)
Glucose
Fructose
Sucrose
Mean
20.5
4.3
8.4
SD
1.2
0.4
0.9
The peak area values from a representative plant extract (i.e. Figure 1B) were 8.3, 0.5 and 2.2 nC x min for glucose, fructose and sucrose, respectively. These values could differ among samples, as expected since they were obtained in different experimental conditions, but they always fell into the measurable range corresponding to the calibration curve.
Notes
- The use of Dionex equipment (or similar chromatographers containing plastic connections and tubing) is mandatory, as regular HPLC systems are not compatible with NaOH solutions.
- A helium stream should be used in order to degasify the mobile phase.
Recipes
- 100 mM HEPES-KOH (pH 7.7)
Weigh 23.83 g HEPES
Adjust pH to 7.7 with KOH
Add Milli Q grade water to 1 L
Stored at 4 °C
- Extraction buffer 1 (EB1)
Mix 20 ml 100 mM HEPES-KOH (pH 7.7) with 80 ml EtOH 100% (v/v)
Stored at RT
- 0.1 M and 0.2 M NaOH
Dilute 5.25 ml or 10.5 ml (respectively) NaOH 50% (w/v) with Milli Q grade water to 1 L
Stored at RT for no longer than 2 weeks due to carbonation
- 21.6 mM NaOH
Dilute 2.16 ml 0.1 M NaOH with Milli Q grade water to 10 ml
Stored at RT for no longer than 2 weeks due to carbonation
Acknowledgments
This work was performed with funding from projects CSD2007-00057, BIO2008-02292, and BIO2011-28847-C02-00 (Spanish Ministry of Economy and Competitiveness, MINECO) and Excellence projects P06-CVI-01450 and P08-AGR-03582 (Junta de Andalucía) partially supported by FEDER funding to F.V. and J.M.R. We also acknowledge the TRANSPLANTA consortium, Project CONSOLIDER 28317 (MINECO).
References
- Ortiz-Marchena, M. I., Albi, T., Lucas-Reina, E., Said, F. E., Romero-Campero, F. J., Cano, B., Ruiz, M. T., Romero, J. M. and Valverde, F. (2014). Photoperiodic control of carbon distribution during the floral transition in Arabidopsis. Plant Cell 26(2): 565-584.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ortiz-Marchena, M. I., Ruiz, M. T., Valverde, F. and Romero, J. M. (2014). Determination of Soluble Sugars in Arabidopsis thaliana Leaves by Anion Exchange Chromatography. Bio-protocol 4(23): e1317. DOI: 10.21769/BioProtoc.1317.
Category
Plant Science > Plant metabolism > Carbohydrate
Plant Science > Plant biochemistry > Carbohydrate
Biochemistry > Carbohydrate > Polysaccharide
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









