- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Intestinal Permeability in Mice
Published: Vol 4, Iss 22, Nov 20, 2014 DOI: 10.21769/BioProtoc.1289 Views: 47228
Reviewed by: Kate HannanIsabel Cristiane da SilvaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Systematic Analysis of Smooth Muscle and Cartilage Ring Formation during Mouse Tracheal Tubulogenesis
Haoyu Wu [...] Wenguang Yin
Jul 5, 2023 1537 Views

Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model
Dina Gadalla [...] David G. Lott
Sep 20, 2024 1453 Views

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3920 Views
Abstract
The intestinal epithelial layer serves as a barrier against pathogens and ingested toxins, which are present in the lumen of the intestine. The importance of the intestinal epithelial barrier is emphasized by the alterations in paracellular permeability and tight junction functions observed in inflammatory bowel disease (IBD) and colon cancer.
Keywords: epithelial barrierMaterials and Reagents
- 8-10-week old mice (in-house bred mice mostly C57BL/6 background; both male and female)
- Fluorescein isothiocyanate conjugated dextran (FITC-dextran) (Sigma-Aldrich, catalog number: FD4 )
- Sterile 1x phosphate-buffered saline (PBS) (pH 7.4)
- Anesthesia (isoflurane) (ESTEVE, catalog number: 103287025 )
Equipment
- BD Microtainer SST tubes (BD Biosciences, catalog number: 365968 )
- 1 ml syringes (ENFA, catalog number: JS1 )
- Autoclaved oral gavage needles (22 Gauge /25 mm long) (Fine Science Tools, catalog number: 18061-22 )
- Spectrophotofluorometer (BioTek Instruments, catalog number: FLx800 )
- 96-well microplates (flat bottom) (Corning, catalog number: 3650 )
- Dissection equipment (forceps, tweezers, scissors)
- Balance
- Table top anesthesia machine (Parkland Scientific, catalog number: V3000PK )
Procedure
- On day 1, in the evening remove water bottles from the cages to water starve the mice overnight.
- On day 2, in the morning weight the mice.
- Dissolve FITC-dextran in PBS at a concentration of 100 mg/ml and administer FITC-dextran to each mouse (44 mg/100 g body weight) by oral gavage with a needle attached to a 1 ml syringe. The procedure for oral gavage is described elsewhere (Bertola et al., 2013). A gap of 30 min between each mouse is recommended for the FITC-dextran oral gavage (see Notes).
- After 4 h, anesthetize the mice by isoflurane inhalation and collect the blood using a 1 ml syringe with 25 G needle by cardiac puncture, then kill the mice by cervical dislocation. Isoflurane is delivered at a concentration of 4% in oxygen using a precision vaporizer for the initial induction and then is maintained at 3% during the blood collection. Typically, we collect 300-400 μl of blood in order to get enough serum for the next step.
- Immediately, transfer blood to a Microtainer SST tube, mix by inverting the tube 3-4 times and store at 4 °C in in the dark. Once blood has been collected from all the mice, SST tubes are processed to separate the serum following the manufacturer’s instruction.
- Dilute the serum with an equal volume of PBS.
- Add 100 μl of diluted serum to a 96-well microplate in duplicate.
- Determine the concentration of FITC in serum by spectrophoto fluorometry with an excitation of 485 nm (20 nm band width) and an emission wavelength of 528 nm (20 nm band width) using as standard serially diluted FITC-dextran (0, 125, 250, 500, 1,000, 2,000, 4,000, 6,000, 8,000 ng/ml) (Figure 1). Serum from mice not administered with FITC-dextran is used to determine the background.
Representative data
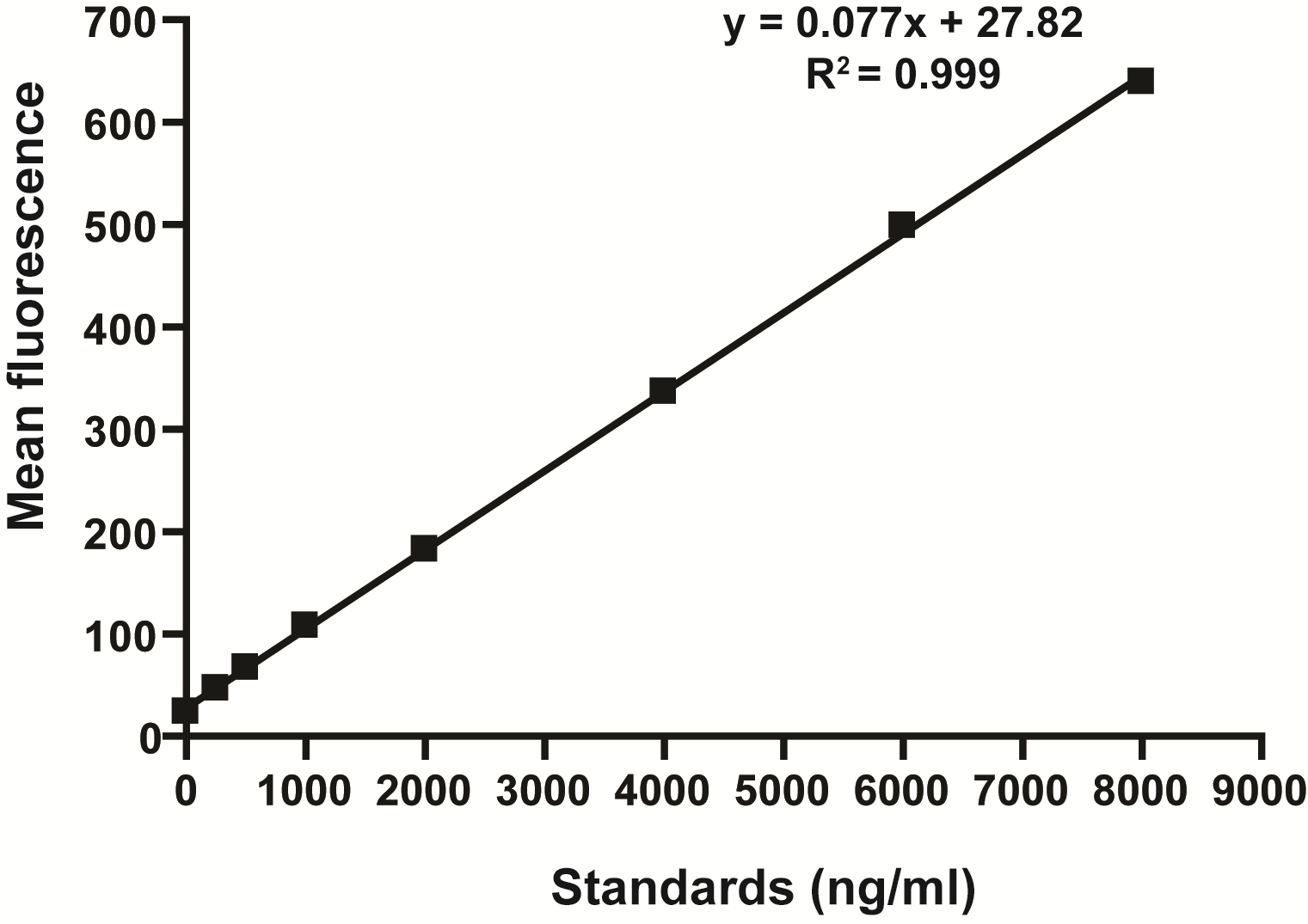
Figure 1. Standard curve for the intestinal permeability assay showing linearity over the range of concentrations tested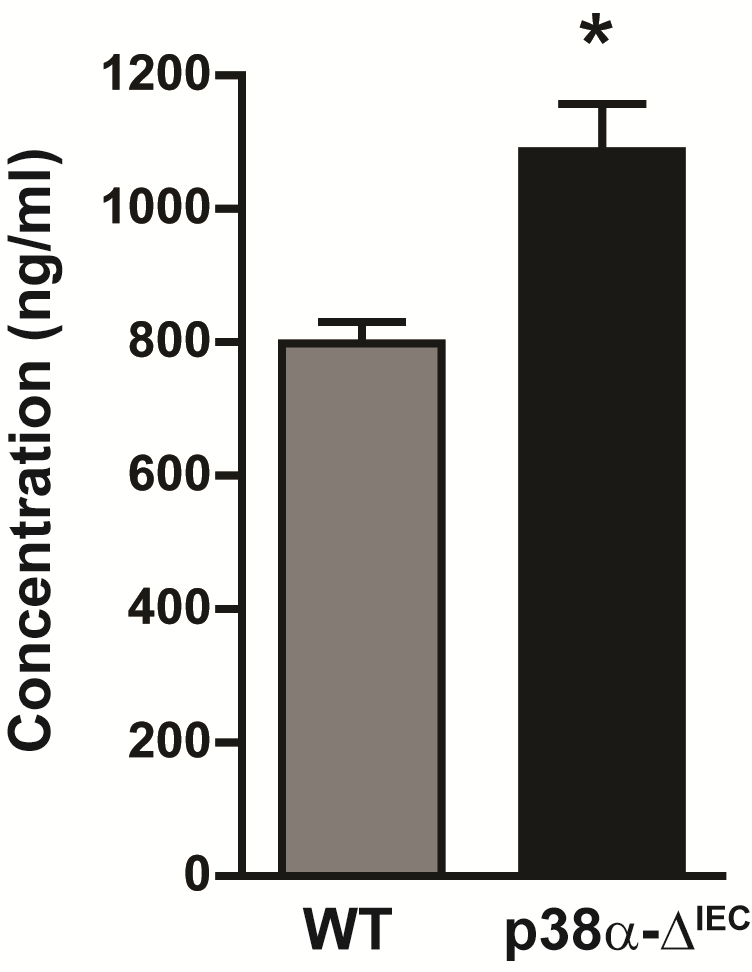
Figure 2. Intestinal permeability measured by determining the concentration of FITC-dextran in the serum of WT and p38α-ΔIEC mice. Data are means ± SEM (n = 4). *p < 0.05
Notes
- The administration of FITC-dextran with a gap of 30 min between animals allows for anesthetization and collection of blood by cardiac puncture from each mouse while maintaining the 4 h interval between FITC-dextran administration and blood collection.
- In different experiments, we observed FITC concentration ranges of 500-800 ng/ml in the serum of WT mice. The mean concentration of FITC in the serum of WT mice administered with FITC-dextran, without any further treatment, should be considered normal. In our case (Gupta et al., 2014), we found that mice with deletion of p38α in intestinal epithelial cells (p38α-ΔIEC) show higher concentration of FITC-dextran in the serum and therefore concluded that intestinal permeability was enhanced in these mice compared to WT mice (Figure 2).
Acknowledgments
Our work is supported by the Fundación BBVA and by grants from the Spanish MICINN (BFU2010-17850) and the European Commission FP7 (INFLA-CARE 223151 and ERC 294665). This protocol is a modification of the protocol published by Calon et al. (2007).
References
- Bertola, A., Mathews, S., Ki, S. H., Wang, H. and Gao, B. (2013). Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8(3): 627-637.
- Calon, A., Gross, I., Lhermitte, B., Martin, E., Beck, F., Duclos, B., Kedinger, M., Duluc, I., Domon-Dell, C. and Freund, J. N. (2007). Different effects of the Cdx1 and Cdx2 homeobox genes in a murine model of intestinal inflammation. Gut 56(12): 1688-1695.
- Gupta, J., del Barco Barrantes, I., Igea, A., Sakellariou, S., Pateras, I. S., Gorgoulis, V. G. and Nebreda, A. R. (2014). Dual function of p38alpha MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 25(4): 484-500.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gupta, J. and Nebreda, A. R. (2014). Analysis of Intestinal Permeability in Mice. Bio-protocol 4(22): e1289. DOI: 10.21769/BioProtoc.1289.
Category
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








