- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and in vitro Activation of Mouse Peyer’s Patch Cells from Small Intestine Tissue
Published: Vol 4, Iss 21, Nov 5, 2014 DOI: 10.21769/BioProtoc.1282 Views: 21338
Reviewed by: Andrea IntroiniKathrin SutterAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Adhesion of Enteroaggregative E. coli Strains to HEK293 Cells
Jorge Luis Ayala-Lujan and Fernando Ruiz-Perez
Apr 20, 2018 6850 Views

Intracellular Invasion and Killing Assay to Investigate the Effects of Binge Alcohol Toxicity in Murine Alveolar Macrophages
Victor Jimenez Jr and Fernando P Monroy
Jan 20, 2019 6735 Views

Model of Chemotherapy-associated Mucositis and Oral Opportunistic Infections
Takanori Sobue [...] Anna Dongari-Bagtzoglou
Nov 5, 2019 5322 Views
Abstract
The lumen of gastrointestinal tract is exposed to several potentially pathogenic microorganisms, thus it is extremely relevant to understand how immunosurveilance can be established. Peyer’s Patches (PPs) are oval or round lymphoid nodules that protrude from the outer wall of the ileum portion of small intestine. PPs contain a high percentage of B and T lymphocytes, macrophages and dendritic cells. Here we summarize a protocol for isolation and culture of mouse PP cells, which can be used to get a better insight into immunopathologies of microbes and to evaluate immune responses elicited by mucosal vaccines.
Keywords: MucosaeMaterials and Reagents
- Mouse (e.g. Balb/c) 8-20 weeks old
- Sterile PBS without Ca/Mg (Euroclone, catalog number: ECB4004L )
- Pen/Strep: 100x solution of penicillin/streptomycin/fungizone (Life Technologies, Gibco®, catalog number: 15240-062 )
- Gentamycin (50 mg/ml solution) (Life Technologies, Gibco®, catalog number: 15750-037 )
- RPMI 1640 (with glutamine) (Lonza, catalog number: BE12-702F )
- Fetal bovine serum (FBS) (heat inactivated) (Lonza, catalog number: DE14-801F )
- 0.4% trypan blue (Sigma-Aldrich, catalog number: T8154 )
- Phorbol 12-Myristate 13-Acetate (PMA) (Sigma-Aldrich, catalog number: P8139 - follow the manufacturer's instructions to prepare stock solutions)
- Ionomycin (Sigma-Aldrich, catalog number: I9657 - follow the manufacturer's instructions to prepare stock solutions)
- Common disinfectant (i.e. 70-90% ethanol, tincture of iodine)
- PBS + 2% Pen/Strep (see Recipes)
- RPMI + 2% Pen/Strep (see Recipes)
- Complete medium (see Recipes)
Equipment
- Soft wood tablet and pins
- Aluminium foils (or another smooth, non-absorbent surface)
- Three sterile small thin surgical scissors
Note: In particular one for cutting the skin, the second one for cutting the muscular wall and the intestine, and the last one for removing PPs. - Four sterile small thin surgical tweezers, two straight and two curved (straight ones for cutting the skin, the muscular wall and the intestine; curved ones to expel faeces and to remove PPs)
- 70 µm cell strainer (BD Biosciences, Falcon®, catalog number: 352350 )
- 2.5 ml syringes
- 15 and 50 ml centrifuge tubes
- 24-well cell culture plates
- Refrigerated centrifuge
- Sterile flow hood
- Thermostatic water bath
- Inverted microscope for cell cultures
- 37 °C, 5% CO2 cell culture incubator
Procedure
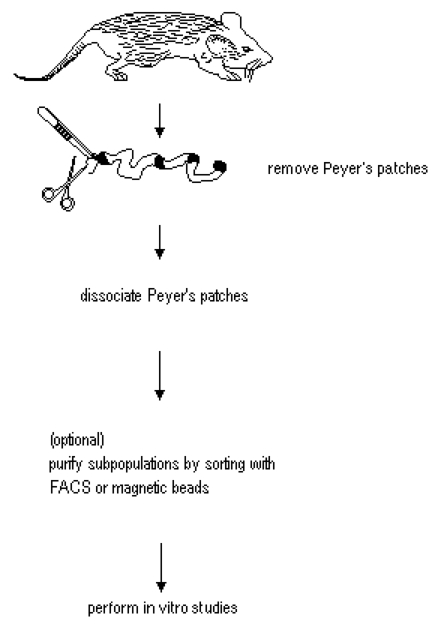
Figure 1. Diagram for the isolation of PP cells from small intestine. Modified from Lefrancois and Lycke (2001).
- Removal of small intestine and excision of Peyer’s patches
- Euthanize mouse by CO2 asphyxiation, following guide lines approved by your Institutional Animal Care and Use Committee. Proceed as soon as possible to the aseptic intestine explantation and PP dissociation to obtain maximum cell viability.
Note: All subsequent steps should be performed in a sterile flow hood. - Gently lay down the mouse on its back, on a soft wood surface, stretch the limbs and fix the four paws with pins.
- Clean the abdomen with the disinfectant.
- A pair of scissors and a straight tweezers are used to perform a midline incision and retract the skin, then open the muscular wall with another cutting along median axis with a new pair of scissors and tweezers.
- Cut small intestine approximately 0.5 cm below the stomach, draw out of peritoneal cavity unfolding it. Cut intestine about 1 cm above the caecum, then remove fat, mesenteric lymph nodes and adjacent tissues.
Note: You can cut the intestine with the same scissors and tweezers used for the muscular wall. - Place the intestine on an aluminium foil and continuously moisten with cold PBS + 2% Pen/Strep to prevent drying.
- Expel faeces by placing a curved tweezers flat on the surface of the intestine and press down along the entire length. Press gently to avoid breaking the intestine.
- Remove PPs by tightening the patch with a new curved tweezers and cutting it with scissors, avoiding as much as possible the surrounding intestinal wall. The patches appear as small protruding whitish/greyish nodules (diameter 1-2 mm), similar to the lymph nodes, embedded in the outer intestinal wall, which appears to be yellow-brown. In general, five to ten PPs can be obtained from a single mouse, depending of age and strain.
Notes:- The presence of an excessive amount of surrounding epithelium reduces the yield of recovered cells, as it could prevent a good mechanical dissociation.
- The removal of the surrounding tissue is crucial if the enzymatic dissociation is carried out; in this last case the intraepithelial cells could contaminate the resulting PP-single cells.
- The presence of an excessive amount of surrounding epithelium reduces the yield of recovered cells, as it could prevent a good mechanical dissociation.
- Immediately after excision, save all PPs in 1 ml cold RPMI + 2% Pen/Strep.
- Euthanize mouse by CO2 asphyxiation, following guide lines approved by your Institutional Animal Care and Use Committee. Proceed as soon as possible to the aseptic intestine explantation and PP dissociation to obtain maximum cell viability.
- Isolation of PP cells by mechanical dissociation
- Transfer all PPs in a 70 µm cell strainer placed over a 50 ml tube.
- Dissociate PPs using the plunger of a 2.5 ml syringe and gently crush the patches forcing through the filter. During dissociation wash the filter with 5 ml of cold RPMI + 2% Pen/Strep (medium flow through the filter contains the single cells of PPs).
- Repeat step 2 twice more, until they are no longer visible pieces of tissue. At this point, the tube contain PP-single cells flowed through the filter and suspended in 15 ml of RPMI + 2% Pen/Strep.
- Centrifuge at 400 x g, 4 °C for 10 min. Discard the supernatant, resuspend the cells and add 10 ml of cold RPMI + 2% Pen/Strep.
- Repeat step 4 two more times. After the last centrifugation resuspend the cells in 1-2 ml of cold RPMI + 2% Pen/Strep.
- Count viable cells using trypan blue dye exclusion.
Notes:- The variability in the number of cells recovered from a single mouse is very high, mainly due to the mouse (strain and age), the time elapsed between the euthanization and dissociation of PP, and the accuracy in removing every single PP.
- The mechanical dissociation allows to isolate mainly non-adherent cells, such as B and T lymphocytes. Adherent cells including monocytes, dendritic and non-immune epithelial cells appear to be present in small quantities since they require an enzymatic treatment to be isolated. The phenotype of recovered PP cells can be checked by FACS analysis.
- If needed, PP cells can be further purified by sorting with magnetic beads or FACS [see Guo et al. (2008) and Thornton (2003) in the reference section].
- The variability in the number of cells recovered from a single mouse is very high, mainly due to the mouse (strain and age), the time elapsed between the euthanization and dissociation of PP, and the accuracy in removing every single PP.
- Transfer all PPs in a 70 µm cell strainer placed over a 50 ml tube.
- In vitro activation of PP cells
- Centrifuge an appropriate amount of cells at 400 x g, at 4 °C for 10 min.
- Resuspend the pellet in complete medium at a concentration of 2 x 106 cells/ml and culture the PP cells in 24-well plate, 1 ml/well, in presence of 10 ng/ml PMA and 500 ng/ml Ionomycin for at least 24 h, in a 37 °C and 5% CO2 incubator.
Note: Other activation protocols, specific for B or T lymphocytes, can be used [see Li et al. (2006) and Muul et al. (2011) in the references section].
Optionals:- To eliminate adherent cells, PP cells can be cultured for 2-4 h in Complete Medium (see recipes) with PMA and Ionomycin without antibiotics. The presence of adherent cells (monocytes and/or dendritic cells) may differentially modulate the activation of B and T lymphocytes with a consequent alterations of the immune responses.
- To avoid as much as possible a bacterial contamination, PP cells are washed and cultured for 24 h as describe above, in Complete Medium + PMA/Ionomycin, with 100 μg/ml Gentamycin instead of Penicillin/Streptomycin. The use of a different antibiotic ensures the elimination of all kinds of bacteria.
- To eliminate adherent cells, PP cells can be cultured for 2-4 h in Complete Medium (see recipes) with PMA and Ionomycin without antibiotics. The presence of adherent cells (monocytes and/or dendritic cells) may differentially modulate the activation of B and T lymphocytes with a consequent alterations of the immune responses.
- After 24 h (or more) the supernatants of cultures can be collected, centrifuged at 400 x g, at 4 °C for 10 min to eliminate the cells, and used to test the presence of specific antibodies or cytokines. Activated cells can be harvested, washed by centrifugation at 4 °C for 10 min and used in flow cytometry assays and sorting.
- Centrifuge an appropriate amount of cells at 400 x g, at 4 °C for 10 min.
Representative data
FACS analysis of in vitro activated PP cells.
PPs were obtained from 6 Balb/c mice as previously published (Pastori et al., 2014). In detail, two different immunization protocols were used: 3 mice received one intranasal (i. n.), while the other 3 mice received one intraperitoneal (i. p.) immunization. Aluminium hydroxide was used as adjuvant in i. p. immunization. For immunization Flock House Virus (FHV) was used in which an external loop of CCR5 (a seven transmembrane protein, belonging to chemokine receptor family and coreceptor for HIV-1) was introduced. The detailed immunization protocol has been described in Pastori et al. (2014).
After excision, PPs were mechanically dissociated and PP cells were in vitro activated with PMA and Ionomycin, as describe above. After 24 h cells were harvested and stained with antibodies against some markers of B cell activation.
As shown in Figure 2, CD138 and CD40/CD19 expression indicate a B cell activated phenotype for both protocols.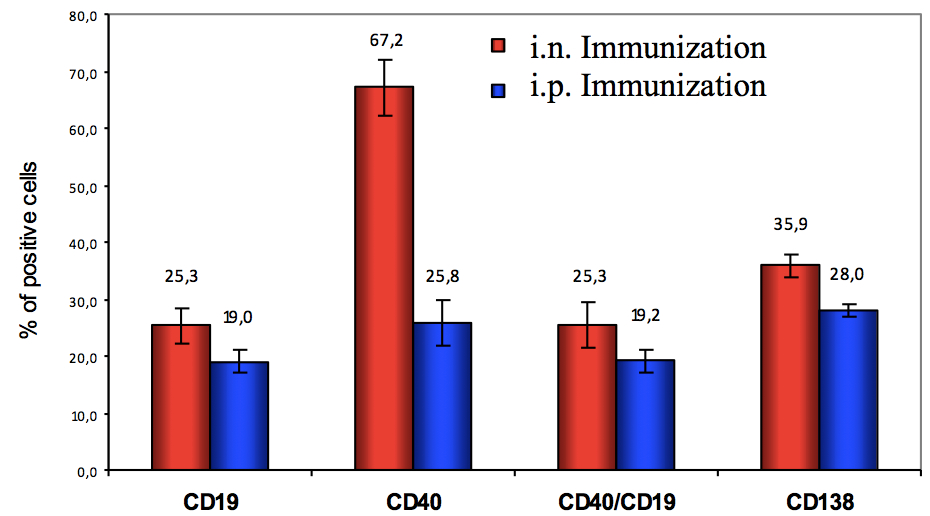
Figure 2. Expression of CD19, CD40, CD138 and co-expression of CD19/CD40 on PP cells after 24 h of PMA/Ionomycin activation. Percentage of positive cells refers to lymphocytes (gated on FSC vs SSC plot). Mean and standard deviation are shown.
Notes
- Images and more detailed information about mouse necropsy, intestine explantation and Peyer’ patches can be found in Scudamore (2013).
- The yield of cells isolated from PPs or intestinal lymph nodes is time sensitive. Proceed with the isolation as quickly as possible to obtain better cell viability.
- The mechanical dissociation of PPs gives a lower yield compared to the enzymatic one, but it does not alter the expression of surface antigens, thus, it is better in the case of subsequent FACS analysis or in vitro assays. The mechanical dissociation also leads to a relative loss of adherent accessory cells, such monocytes and/or dendritic cells, therefore, for the isolation of these cell types the enzymatic dissociation is preferable.
Recipes
Note: Prepare and keep all solutions in a sterile condition.
- PBS + 2% Pen/Strep
For 100 ml combine 98 ml of PBS and 2 ml of Pen/Strep
Refrigerate at 4 °C before use - RPMI + 2% Pen/Strep
For 100 ml combine 98 ml of RPMI and 2 ml of Pen/Strep
Refrigerate at 4 °C before use - Complete medium
For 100 ml combine 89 ml of RPMI (with Glutamine), 10 ml of heat inactivated FBS and 1 ml of Pen/Strep
Warm at 37 °C in a water bath before use
Acknowledgments
We thank Maria Rescigno for her help in mouse PPs isolation. This work was supported by Italian Ministry of Health, grant 40H15.
References
- Guo, Z., Jang, M. H., Otani, K., Bai, Z., Umemoto, E., Matsumoto, M., Nishiyama, M., Yamasaki, M., Ueha, S., Matsushima, K., Hirata, T. and Miyasaka, M. (2008). CD4+CD25+ regulatory T cells in the small intestinal lamina propria show an effector/memory phenotype. Int Immunol 20(3): 307-315.
- Lefrancois, L. and Lycke, N. (2001). Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol Chapter 3: Unit 3 19.
- Li, Y. Y. Y., Yang, Y., Bao, M., Edwards III, C. K. and Parnes, J. R. (2006). Mouse splenic B lymphocyte activation using different activation stimuli induces in vitro splicing of tumor necrosis factor-α nuclear pre-mRNA. Mol Immunol 43(6): 613-622.
- Muul, L. M., Heine, G., Silvin, C., James, S. P., Candotti, F., Radbruch, A. and Worm, M. (2011). Measurement of proliferative responses of cultured lymphocytes. Curr Protoc Immunol Chapter 7: Unit7 10.
- Pastori, C., Diomede, L., Venuti, A., Fisher, G., Jarvik, J., Bomsel, M., Sanvito, F. and Lopalco, L. (2014). Induction of HIV-Blocking Anti-CCR5 IgA in Peyers's Patches without Histopathological Alterations. J Virol 88(7): 3623-3635.
- Scudamore, C. L. (2013). A Practical Guide to the Histology of the Mouse, John Wiley & Sons.
- Sheridan, B. S. and Lefrancois, L. (2012). Isolation of mouse lymphocytes from small intestine tissues. Curr Protoc Immunol Chapter 3: Unit 3 19.
- Thornton, A. M. (2003). Fractionation of T and B cells using magnetic beads. Curr Protoc Immunol 3.5 A. 1-3.5 A. 11.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pastori, C. and Lopalco, L. (2014). Isolation and in vitro Activation of Mouse Peyer’s Patch Cells from Small Intestine Tissue. Bio-protocol 4(21): e1282. DOI: 10.21769/BioProtoc.1282.
Category
Immunology > Immune cell isolation > Lymphocyte
Immunology > Immune cell isolation > Macrophage
Microbiology > Microbe-host interactions > In vitro model > Cell line
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









