- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Optical Clearing Using SeeDB
Published: Vol 4, Iss 3, Feb 5, 2014 DOI: 10.21769/BioProtoc.1042 Views: 29353
Reviewed by: Xuecai GeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2900 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2626 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 483 Views
Abstract
We describe a water-based optical clearing agent, SeeDB (See Deep Brain), which clears fixed brain samples in a few days without quenching many types of fluorescent dyes, including fluorescent proteins and lipophilic neuronal tracers. SeeDB is a saturated solution of fructose (80.2% w/w) in water with 0.5% α-thioglycerol. In standard SeeDB optical clearing procedure, we treat paraformaldehyde-fixed embryo and brain samples with increasing concentrations of aqueous fructose solutions, and finally equilibrate them in SeeDB. The entire procedure takes approximately three days. Unlike previous methods, this method maintains a constant sample volume during the clearing procedure, an important factor to keep cellular morphology intact. After optical clearing, we can reach > 1,000 μm under confocal microscopy. When combined with two-photon microscopy, SeeDB allows us to image fixed mouse brains at millimeters-scale level. This method facilitates comprehensive and quantitative analyses for understanding neuronal circuitry, both in the adult and developing mouse brain. A SeeDB variant (SeeDB37) and optimized procedures (SeeDBp and SeeDB37ht protocols) are also supplied for specific requirements.
Keywords: Tissue clearingMaterials and Reagents
- Phosphate-buffered saline (PBS)
- 4% paraformaldehyde (PFA) in PBS
- D(-)-Fructose (≥99%) (e.g., Sigma-Aldrich, catalog number: F0127-500G )
- α-thioglycerol (≥95%) (e.g., Sigma-Aldrich, catalog number: M1753-100ML )
- 2,2’-thiodiethanol (≥99%) (e.g., Sigma-Aldrich, catalog number: 166782-500G ) (optional)
- Glycerol (≥99%) (e.g., Sigma-Aldrich, catalog number: G9012-500ML ) (optional)
- Low melting point agarose (e.g., Life Technologies, catalog number: 16520 -100) (optional)
- SeeDB (see Recipes)
- SeeDBp (see Recipes)
- Immersion solution (for commercial objective lenses) (see Recipes)
- Immersion solution (for customized objective lens for SeeDB) (see Recipes)
Equipment
- 50 ml conical centrifuge tube (e.g., BD Biosciences, Falcon®) (for whole- and hemi-brain samples)
- Culture dish (30 or 60 mm diameter) (e.g., BD Biosciences, Falcon®) (for slice preparation and fragile samples)
- Overhead tube rotator (recommended for clearing large samples) (Figure 1A)
- Seesaw shaker (for fragile samples) (Figure 1B)
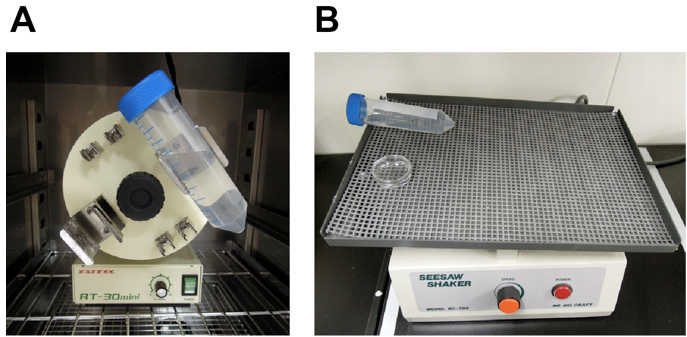
Figure 1. Equipment used for optical clearing. For efficient optical clearing, it is important to shake samples in fructose solutions. We recommend using 50 ml conical centrifuge tube and overhead rotator for clearing large samples (e.g., whole- or hemi-brain samples). Fragile samples (e.g., brain slices) can be cleared in culture dishes and seesaw shaker. A. Overhead tube rotator, B. Seesaw shaker - Air incubator (optional, for SeeDB37 and SeeDB37ht protocols)
- Coverslips (24 mm x 60 mm for small preparation and slices; 50 mm x 70 mm for whole-mount samples) (e.g., Matsunami Glass, catalog number: C050701 )
- Handmade glass bottom Petri dish (100 mm diameter) or a commercial glass bottom dish (MatTek, catalog number: P100G-1.5-30-F )
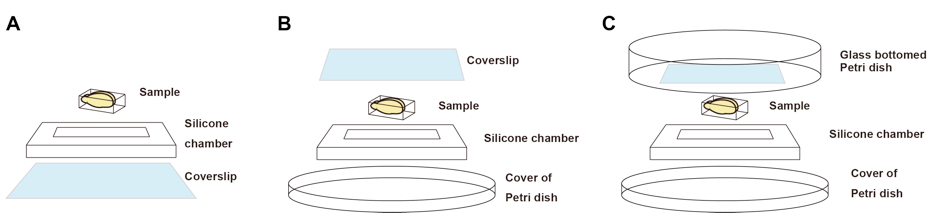
Figure 2. Imaging chambers for fluorescence microscopy. A. An imaging chamber for inverted microscopes. B. An imaging chamber for upright microscopes with short working distance objective lenses. C. An imaging chamber for upright microscopes with long working distance objective lenses. We use glass bottom Petri dish to keep immersion solutions. - Silicone rubber sheet (optimal thickness should be chosen. For example, 6-mm-thick silicone sheet is recommended for 6-mm thick adult mouse brain.) (e.g., TOGAWA RUBBER, catalog number: K-125 )
Note: Silicone rubber sheets adhere to the coverslips and Petri dishes without any adhesives. - Thermo plate (optional, only for samples cleared with SeeDB37 or SeeDB37ht) (e.g., Tokai Hit)
- Fluorescence microscope
Confocal microscope (for imaging up to 1-2 mm depth)
Multiphoton microscope (recommended for deeper imaging) - Objective lenses (examples)
10x air (NA=0.4, WD=3.1 mm) (OLYMPUS, model: UPLSAPO10X2 )
10x water immersion (NA=0.3, WD=3.5 mm) (OLYMPUS, model: UMPLFLN10xW )
25x water immersion (NA=1.05, WD=2 mm) (OLYMPUS, model: XLPLN25XWMP )
25x scale immersion (NA=1.0, WD=4 mm) (OLYMPUS, model: XLPLN25SVMP )
Water-immersion lenses perform much better than air-immersion objective lenses because spherical aberrations are smaller (Figure 5). In the two-photon microscopy, we also use a custom-made objective lens that performs best under the refractive index of SeeDB (~1.49). Please contact Olympus for its availability.
Procedure
Part I. Choice of protocols
- Standard SeeDB and SeeDB37: brain slice, mouse embryo, young mouse whole-brain, and adult mouse hemi-brain
- SeeDBp: embryonic and neonatal mouse brain
- SeeDB37ht: large samples, such as adult mouse whole-brain
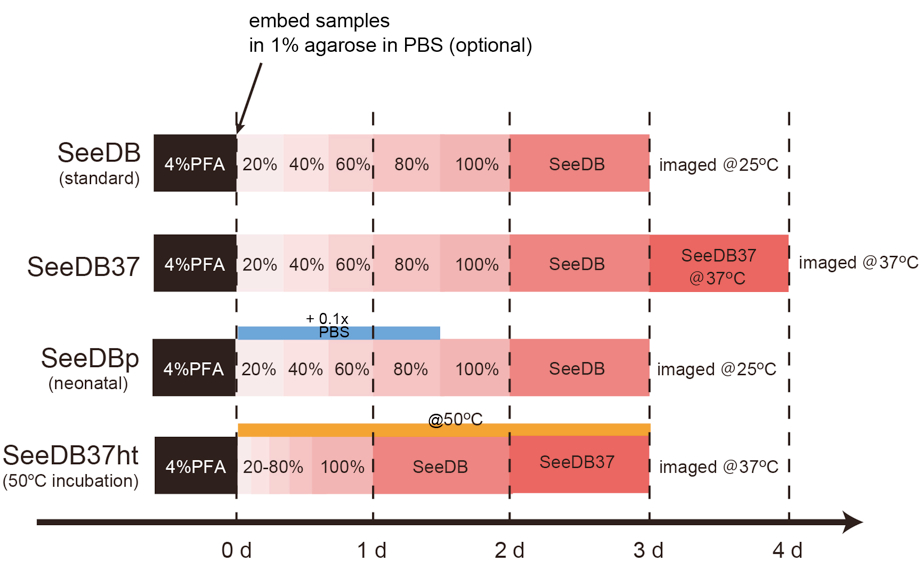
Figure 3. Timing of SeeDB protocols- SeeDB (standard)
- Fix the sample in 4% PFA at 4 °C with gentle shaking overnight.
- Wash the sample in PBS three times (10 min each).
- (Optional step for fragile samples) Embed the sample in 1% low melting point agarose gel in PBS with desired orientation and then trim away extra portion to minimize the sample size. The surface of the sample should be close to the surface of agarose gel, because the working distance of commercially available objective lens is limited. Agarose embedding should not be used for large tissues, because agarose embedding reduces the penetration of SeeDB into the samples.
- Incubate the sample in ~20 ml of 20% (w/v) fructose solution in 50 ml conical tube, and then place the conical tube on a tube rotator (~4 rpm) or a seesaw shaker (~17 rpm) for 4-8 h, respectively. A small piece of sample (e.g., slices) requires less time for optical clearing. Incubation should be performed at 25-37 °C.
- Incubate the sample in 40% (w/v) fructose for 4-8 h as above.
- Incubate the sample in 60% (w/v) fructose for 4-8 h. (Samples may no longer sink in 60% or higher concentrations of fructose.)
- Incubate the sample in 80% (w/v) fructose for 12 h.
- Incubate the sample in 100% (w/v) fructose for 12 h.
- Incubate the sample in ~20 ml SeeDB for 24 h. The incubation time can be extended up to 48 h.
- The transparency can be evaluated by eye at this stage (Figure 4). If the sample is successfully cleared, the adult brain sample should look amber under a light source.
- If the clarity is still not enough, incubate the sample further in ~20 ml SeeDB37 solution at 37 °C (in an air incubator) with gentle rotation for 24 h. We recommend this step for adult brain samples.
- SeeDBp
Incubation schedule for SeeDBp is the same as the standard SeeDB protocol. To prevent sample expansion, 20% ,40%, 60%, and 80% fructose solutions are made in 0.1x PBS instead of distilled water. - SeeDB37ht
Note: Fluorescent proteins may be partially quenched. Some sample expansion may occur.- Fix the sample in 4% PFA at 4 °C with gentle shaking overnight.
- Wash the sample in PBS three times (10 min each).
- Incubate the sample in 20% (w/v) fructose at 50 °C for 2-4 h. To prevent sample expansion, the incubation time should not exceed 4 h.
- Incubate the sample in 40% (w/v) fructose at 50 °C for 2-4 h.
- Incubate the sample in 60% (w/v) fructose at 50 °C for 2-4 h.
- Incubate the sample in 80% (w/v) fructose at 50 °C for 2-4 h.
- Incubate the sample in 100% (w/v) fructose at 50 °C for 12 h.
- Incubate the sample in SeeDB at 50 °C for 24 h.
- Incubate the sample in SeeDB37 at 50 °C for 24 h. Transfer the sample to 37 °C for imaging. Extension of incubation at 50 °C is not recommended.
- SeeDB (standard)
Part II. Choice of objective lenses and imaging
Because refractive index of SeeDB is 1.49, it is important to choose appropriate objective lenses to minimize spherical aberration. In the two-photon microscopy, index-matched and long working distance customized objective lens is most ideal to obtain high-resolution images at millimeter-scale. We used custom-made objective lens from Olympus (it will be commercialized from Olympus). Commercial water or Scale immersion objective lens can be used for imaging up to 2-4 mm depth. In the confocal microscopy, a water-immersion objective lens (refractive index 1.33) works better than an air-immersion objective lens (refractive index 1.0) (Figure 5). Glycerol/Oil immersion lenses are also suitable. Customized objective lens is not necessary for confocal imaging; we did not see obvious differences in resolution within 1 mm depth. When using an air or water-immersion lens, the imaging depth should be calibrated because an apparent axial scale is shortened in the SeeDB solution due to its high refractive index (refractive index 1.49).
- Imaging with upright microscopes
- Prepare an appropriate imaging chamber (Figure 2B-C) for your microscope.
- Fill in the imaging chamber with SeeDB or SeeDB37. To prevent subtle index mismatch between media and sample, we recommend using SeeDB or SeeDB37 that was equilibrated with the sample, rather than freshly prepared solutions.
- Put the sample into the imaging chamber and then place a handmade glass bottom Petri dish (or a coverslip) carefully above the chamber. Air bubble should not remain in the chamber. When samples are cleared by SeeDB37, the chamber should be placed on a heat pad to keep the temperature at 37 °C.
- (Optional for an upright microscope) Pour immersion solution onto the handmade glass bottom Petri dish. SeeDB should not be used for immersion; its viscosity causes an uneven refraction index distribution due to evaporation of water from the surface of SeeDB during imaging and can impair image quality.
- Imaging with inverted microscopes
- Transfer the sample and SeeDB to a custom-made imaging chamber (Figure 2A) or a commercial glass-bottomed dish/chamber (35 mm diameter).
Anticipated Results

Figure 4. Transmittance images of neonatal, P21, and adult mouse brain samples. Transparency can be evaluated by eye under the light source. Typically, adult samples are more difficult to clear than young samples.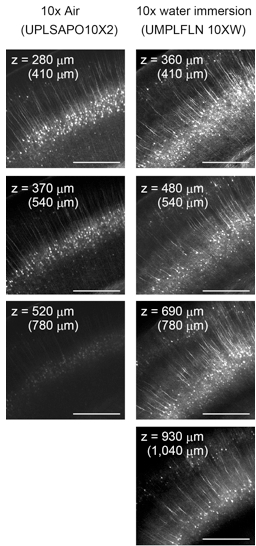
Figure 5. Confocal imaging of adult brain slices (Thy1-YFP-H mouse). Water-immersion objective lenses work better than air lenses because of smaller spherical aberration in SeeDB. The maximum imaging depth possible is ~500 μm at spine resolution, ~1 mm at fiber resolution, and ~2 mm at cellular resolution. In the two-photon microscopy, the maximum imaging depth possible with commercial 25x objective lenses is ~1 mm at spine resolution and 3-4 mm at fiber resolution. When a commercial water- or air-immersion objective lens is used for imaging cleared samples, depth (z) needs to be calibrated. In order to obtain the correct z position in the sample, the real depth is calculated by multiplying the depth by nSeeDB and then dividing by nobjective, where n represents refractive index. nSeeDB = 1.49; nSeeDB37 = 1.50; nair = 1.0; nH2O = 1.33.
Recipes
- Preparation of SeeDB
Dissolve D(-)-fructose completely in distilled water or 0.1x PBS at 65 °C. We recommend using 50 ml conical centrifuge tube. After cooling to 25 °C or 37 °C, add α-thioglycerol to give a final concentration of 0.5% to prevent Maillard reaction.
Notes:
a.20-100% fructose solutions are weight/volume and SeeDB/SeeDB37 are prepared on the basis of percent weight/weight. SeeDB should be freshly prepared.
b.Do not leave SeeDB solutions at 65 °C too long (> 5 h), because fructose will gradually caramelize.Composition Fructose Solvent α-thioglycerol 20% w/v 4 g add distilled water to make a total volume of 20 ml 100 μl 40% w/v 8 g 60% w/v 12 g 80% w/v 16 g 100% w/v 20 g SeeDB 20.25 g add 5 ml distilled water SeeDB37 27 g - Preparation of SeeDBp
Composition Fructose Solvent α-thioglycerol 20% w/v 4 g add 0.1x PBS to make a total volume of 20 ml 100 μl40% w/v 8 g 60% w/v 12 g 80% w/v 16 g 100% w/v 20 g add distilled water to 20 ml SeeDB 20.25 g add 5 ml distilled water SeeDB37 27 g - Immersion solution (for commercial objective lenses)
Water (for water-immersion lens)
30% glycerol (v/v, in distilled water; for Scale-immersion lens) - Immersion solution (for customized objective lens for SeeDB)
80% 2,2’-thiodiethanol – 20% H2O (v/v; for SeeDB)
90% 2,2’-thiodiethanol – 10% H2O (v/v; for SeeDB37)
Troubleshooting
- Poor transparency
Because SeeDB is viscous, it is important to ensure efficient penetration of the solution into the tissues. If possible, trim away unnecessary portion of your sample to increase the penetration. Remove skin and skull, which are typically very difficult to clear. If you need to clear large samples (e.g., whole brains of adult mice), try SeeDB37ht protocol. Shaking of samples, step-wise soaking, timing, and temperature are all important for successful optical clearing. - Autofluorescence
Prolonged incubation in SeeDB will accumulate autofluorescence even with 0.5% α-thioglycerol. It is ideal to image samples soon after the clearing (within a few days). For long-term storage, samples should be recovered in PBS following exactly the reverse of the clearing process. No detergents and salts should be included in SeeDB (except for SeeDBp). PFA should be freshly prepared to minimize autofluorescence. - High temperature protocol (SeeDB37ht)
Incubation of the sample at higher temperature may result in mild sample expansion. To minimize the sample expansion, incubation time for 20-80% fructose should be minimized. In the SeeDB37ht protocol, some fluorescent protein may be partially quenched. - In utero electroporation
Do not use FastGreen dye, because it will react with fructose and result in strong autofluorescence. We instead recommend 0.05% AlexaFluor 647-dextran when you need to visualize DNA solution. - Antibody staining
SeeDB can also be used to clear samples stained with antibodies, although penetration of antibodies is typically limited to 100-250 μm even after permeabilization step with Triton-X100. Because optical clearing with SeeDB is reversible, samples cleared with SeeDB can be restored in PBS and subsequently analyzed by immunohistochemistry in sections.
Acknowledgments
This protocol was adapted from our original publication of the protocol (Ke et al., 2013). We thank J.R. Sanes for providing mouse strains. This work was supported by grants from the PRESTO program of the Japan Science and Technology Agency, the Sumitomo Foundation, the Nakajima Foundation, the Mitsubishi Foundation, the Strategic Programs for R&D (President's Discretionary Fund) of RIKEN, and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (KAKENHI 23680038). The imaging experiments were supported by the RIKEN Center for Developmental Biology Imaging Facility. Animal experiments were supported by the Laboratory for Animal Resources and Genetic Engineering at the RIKEN Center for Developmental Biology.
References
- Ke, M. T., Fujimoto, S. and Imai, T. (2013). SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci 16(8): 1154-1161.
Note: SeeDB Resources (https://sites.google.com/site/seedbresources/) provides updated information from the authors. This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ke, M., Fujimoto, S. and Imai, T. (2014). Optical Clearing Using SeeDB. Bio-protocol 4(3): e1042. DOI: 10.21769/BioProtoc.1042.
Category
Neuroscience > Neuroanatomy and circuitry > Animal model
Cell Biology > Tissue analysis > Tissue isolation
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










