- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Indole Derivative Feeding Test and Detection of TRP and Indole derivatives by Thin Layer Chromatography
Published: Vol 3, Iss 12, Jun 20, 2013 DOI: 10.21769/BioProtoc.801 Views: 11227
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Salicylic Acid (SA) and SA-glucosides in Arabidopsis thaliana
Valérie Allasia [...] Harald Keller
May 20, 2018 13133 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2634 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1568 Views
Abstract
The mutualistic root endophyte Piriformospora indica colonizes a wide range of plants and the colonization of root cells by this fungus is very often associated with beneficial effects to its host, such as growth promotion and increased biotic and abiotic stress tolerance. These traits could be based on general mechanisms and signaling pathways common to many different plant species. One such mechanism could be the recruitment of phytohormone pathways by P. indica. It is known, that many mutualistic microorganisms are able to synthesize and secrete phytohormones during the interaction with their host plants. This protocol has been successfully utilized to analyze tryptophan (TRP)-dependent biosynthesis of indole-3-acetic acid (IAA) and its indole derivatives by P. indica (Hilbert et al., 2012).
Materials and Reagents
- Indole derivative:
TRP (Sigma-Aldrich, catalog number: T0254-500g )
IAD (indole-3-acetaldehyde) (Sigma-Aldrich, catalog number: I1000-100mg )
IAA (Sigma-Aldrich, catalog number: I5148-2g ) - Standard microscope coverslips
- Standard microscope slides
- 0.002% (v/v) Tween water 20
- 0.9% NaCl
- FeCl3
- HClO4
- NaNO3
- MgSO4.7H2O
- KH2PO4
- Orthophosphoric acid
- Glucose
- Peptone
- Yeast extract
- Casamine acids
- Microelements
- Agar
- Tryptophan (Sigma-Aldrich, catalog number: T0254-500g)
- Ethyl acetate
- Aluminum foil
- p-dimethylaminobenzaldehyde (Sigma-Aldrich)
- Van Urk reagent (see Recipes)
- Salkowski test reagents (see Recipes)
- 10 mM orthophosphoric acid (see Recipes)
- Complete medium (see Recipes)
- 20x salt solution (see Recipes)
- Microelements (see Recipes)
Equipment
- Erlenmeyer flasks (100 ml)
- Neubauer improved counting chamber (Marienfeld-Superior, Lauda, Königshofen, Germany)
- Scalpel (sterile)
- Whatman paper
- Miracloth filter 15 cm x 15 cm (Merck KGaA, catalog number: 475855 )
- Drigalski spatula (sterile)
- Disposable cuvettes
- Preval sprayer
- Thin layer chromatography (TLC) chamber
- Petri dishes (12 mm in diameter)
- 15 ml sterile Falcon tubes
- 2 ml Eppendorf tubes
- Glass test tubes
- Incubator e.g. Certomat BS-1 (B. Braun Biotech International)
- Speedvac sc110 (Savant, Thermo Fisher Scientific)
- Biofuge Primo R (Heraeus) (Rotor, catalog number: 7590 )
- Heraeus Pico 17 centrifuge (Heraeus)
- Spectrophotometer e.g. Ultrospec 3,000 proUV/Visible (GE Healthcare)
- Clean bench Hera Safe (Heraeus)
- Hybridization oven (Hybaid Shake n’ Stack, Thermo Fisher Scientific)
Procedure
- Collect spores from 3 to 4 weeks old Piriformospora indica CM agar plate cultures grown at 28 °C (see Figure 1).
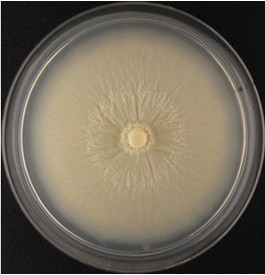
Figure 1. Four-week-old P. indica agar plate
- Pour approximately 5 ml sterile 0.002% Tween water 20 on 3-4 weeks old P. indica plate under sterile condition at room temperature (RT).
- Scratch plate with Drigalski spatula and/or scalpel and mix.
- Pour spore solution through miracloth filter and collect flow through in 50 ml falcon tube.
- Centrifuge for 7 min at 3,500 rpm, discard supernatant.
- Wash pellet with 5-10 ml 0.002% Tween water 20.
- Centrifuge for 7 min at 3,500 rpm, discard supernatant.
- Wash pellet with 5-10 ml 0.002% Tween water 20.
- Centrifuge for 7 min at 3,500 rpm, discard supernatant.
- Resuspend spore pellet in 10 ml 0.002% Tween water 20, count spores with counting chamber (e.g. Neubauer improved) and dilute to requested spore concentration (e.g. 500,000 spores ml-1).
- Pour approximately 5 ml sterile 0.002% Tween water 20 on 3-4 weeks old P. indica plate under sterile condition at room temperature (RT).
- Inoculate 50 ml “complete medium” CM liquid medium (Pham et al., 2004) with 400 μl of 500,000 spores/ml spore solution in 100 ml Erlenmeyer flask.
- Let spores germinate for 1 week at 28 °C and 130 rpm.
- Inoculate with 2.5 mM TRP (prepare the stock 1 day before, dissolve slowly; work in darkness auxin is light sensitive).
- Incubate cultures in darkness (alternatively wrap flasks with aluminium foil) for 3 days.
- Collect 11 ml supernatant through miracloth filter (check the mass of each filter) into 15 ml falcon tube:
- Wash mycelium with 0.9% NaCl and let the whole miracloth filter with fungal biomass dry overnight in oven (85 °C).
- 15 μl aliquot of the culture supernatant can be used for the determination of TRP and indole derivatives by LC-MS/MS (see Hilbert et al., 2013") From the falcon tube take 1 ml into glass test tube for Salkowski test.
- Salkowski test: Mix 1 ml supernatant with 2 ml Salkowski reagent and 50 μl of 10 mM orthophosphoric acid, incubate for 25 min at RT, measure OD530.
- Add 5 ml ethyl acetate to the remaining 10 ml of supernatant and incubate for 3 to 4 h at 200 rpm in darkness (supernatant can be frozen at -20 °C overnight).
- For good phase separation centrifuge 5 min at 4 °C at 3,500 rpm (if needed leave falcons overnight at 4 °C in darkness).
- Transfer 2 ml of organic phase (upper phase) into a 2 ml eppendorf tube and evaporate in SpeedVac concentrator for 30 min with medium heating.
- Add 2 ml of the remaining organic phase to the pellet and repeat the SpeedVac process.
- Resuspend pellet in 60 μl ethyl acetate (Storage in -20 °C).
- Calculate the dry biomass.
- Fill the TLC chamber with Whatman paper.
- Saturate TLC chamber for approximately 1 h with approximately 200 ml of running buffer chloroform: ethanol: water (84:14:1).
- Load extracted samples onto the TLC plate in a volume, that each spot represents the amount of indole derivatives obtained from the samples of equal biomass (use not more than 6 μl for loading on TLC plate).
- Use commercially available indole derivatives as marker control (e.g. TRP, IAD and IAA).
- Let TLC run for 1 to 1.5 h in darkness.
- Dry TLC plate for 5 min at RT for approximately 2 to 5 min.
- Develop TLC plate by spraying on it a mixture of van Urk and Salkowski reagents in the proportion of 1:3 (Ehmann, 1977) and incubate in an incubator at 90 °C up to 10 min.
- Calculate retention factor (Rf) for each spot (the distance travelled by the compound divided by the distance travelled by the solvent) and compare it with Rf calculated for each indole derivative control.
Recipes
- Van Urk reagent
1 g p-dimethylaminobenzaldehyde dissolved in 50 ml concentrated HCl and 50 ml ethanol. This reagent is stable for several months at room temperature when stored in a brown/light protected glass bottle. - Salkowski test reagents
Prepare stock solution of 0.5 M FeCl3 (1.35 g in 10 ml water)
Use 1 ml of this stock to mix with 49 ml of 35 % HClO4 - 10 mM orthophosphoric acid
115 μl (of 85 %) in 100 ml water - Complete medium (CM) 1 L
50 ml 20x salt solution
20 g glucose
2 g peptone
1 g yeast extract
1 g casamine acids
1 ml microelements
15 g agar - 20x salt solution 1 L
120 g NaNO3
10.4 g KCl
10.4 g MgSO4.7H2O
30.4 g KH2PO4 - Microelements 1 L
6 g MnCl2.4H2O
1.5 g H3BO3
2.65 g ZnSO4.7H2O
750 mg KI
2.4 mg Na2MO4.2H2O
130 mg CuSO4.5H2O
Acknowledgments
This protocol is adapted from Hilbert et al. (2012).
References
- Hilbert, M., Voll, L. M., Ding, Y., Hofmann, J., Sharma, M. and Zuccaro, A. (2012). Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol 196(2): 520-534.
- Hilbert, M., Voll, L., Hofmann, J. and Zuccaro, A. (2013). Growth Assay and Detection of TRP and Indole Derivatives in Piriformospora indica Culture Supernatant by LC-MS/MS. Bio-protocol 3(12): e800.
- Ehmann, A. (1977). The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr 132(2): 267-276.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hilbert, M., Voll, L. M., Hofmann, J. and Zuccaro, A. (2013). Indole Derivative Feeding Test and Detection of TRP and Indole derivatives by Thin Layer Chromatography. Bio-protocol 3(12): e801. DOI: 10.21769/BioProtoc.801.
Category
Plant Science > Plant physiology > Endosymbiosis
Microbiology > Microbe-host interactions > Fungus
Biochemistry > Other compound > Plant hormone > Indole
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









