- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protocol to Induce Follicular T Helper Cells, Germinal Centers, and Skin Lesions in Mouse Models for Skin Blistering Diseases
Published: Vol 12, Iss 10, May 20, 2022 DOI: 10.21769/BioProtoc.4414 Views: 2945
Reviewed by: Chiara AmbrogioLaura Molina-GarcíaMarieta Ruseva

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
Jeremy R. Pearson [...] Eduardo Rosa-Molinar
Apr 20, 2025 2500 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views

Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
Mi Rim Lee [...] Yun-Hee Kim
Oct 5, 2025 1703 Views
Abstract
Autoreactive T cells in autoantibody-mediated autoimmune diseases can be divided into two major subsets: (i) follicular T helper cells (Tfh) that provide T cell help in germinal centers (GC) and (ii) effector T (Teff) cells that immigrate into peripheral tissue sites such as the skin and mediate local inflammation. To study the sequence of events leading to the loss of tolerance in autoantibody-mediated autoimmune diseases it is required to investigate both T cell subsets simultaneously. This approach is hampered mainly because the appearance of skin inflammation in mouse models is a random process, which makes it difficult to define the location of inflammation at the right time point. To overcome this problem, we developed a scratching technique for ear skins that leads to the establishment of chronic autoimmune wounds in the mouse model for the pemphigoid-like disease epidermolysis bullosa acquisita. By defining the exact place where the skin wounds should form, this protocol enables a detailed analysis of skin-immigrating Teff cells. Of note, this protocol induces GC in draining lymph nodes in parallel so that Tfh cells in GC can be investigated concurrently. This protocol is not restricted to T cells and can be used for any other skin-immigrating inflammatory cells.
Keywords: Germinal centersBackground
Chronic tissue inflammation in autoantibody-mediated immune diseases is induced by the binding of IgG autoantibodies to their target structure (Ludwig et al., 2017). One main effect of bound autoantibodies is the formation of immune complexes that lead to uncontrolled recruitment of inflammatory cell infiltrates and subsequent tissue destruction. Typical examples are autoimmune skin diseases such as bullous pemphigoid or epidermolysis bullosa acquisita, in which autoantibodies specific for structural hemidesmosomal proteins of the skin such as type XVII or type VII collagen have been described, respectively (Tull and Benton, 2021). During the formation of these high-affinity autoreactive antibodies, autoantigen-specific Tfh cells, and B cells, closely interact with each other in GC within secondary lymphoid tissues (Vinuesa et al., 2009; Crotty, 2019). Furthermore, autoantigen-specific Teff cells get activated in T cell zones of the draining lymphoid organ, enter the circulation and infiltrate into the autoantibody-mediated inflamed peripheral tissues site (Chemin et al., 2019; Niebuhr et al., 2020). The presence of Teff cells in autoimmune wounds is well established, but their role is ill-defined. Especially, the assessment of autoantigen-specific T cells in tissues requires analysis even before wounds become clearly visible due to the dynamic migratory behavior of Teff cells (Ghani et al., 2009). It is therefore required to define the position of a developing autoimmune wound for detailed analysis. In this protocol, we describe a mouse model of type VII collagen-mediated skin inflammation (epidermolysis bullosa acquisita), in which (i) Tfh cells are induced in GC of the activated draining lymph nodes and (ii) the position of the autoimmune skin wounds is defined by slight scratching of the ear skin (Figure 1A–1C). This protocol has been applied successfully to study Tfh cells in GC and Teff cells in skin wounds in parallel (Niebuhr et al., 2020, 2021). Furthermore, we provide the details of subsequent immunohistochemical staining.

Figure 1. Induction of germinal centers and skin lesions in mice. A. Experimental setup is shown. Autoantigen emulsified in TiterMax were injected into both footpads of one mouse. The inner side of the ears was slightly scratched with an electric nail fail. Germinal centers containing Tfh cells will develop in footpad-draining lymph nodes. B. Picture shows a skin wound early after scratching (white arrow). C. Germinal centers (black arrows) are visualized by immunohistological staining with anti-mouse Ki67 (red) and anti-mouse B220 (blue). Figure adapted from Niebuhr et al. (2021).
Materials and Reagents
Female SJL/J mice (8 to 12 weeks old) were obtained from Charles River Laboratories (Sulzfeld, Germany)
Reaction tubes, 1.5 mL, safe seal (Sarstedt, catalog number: 72.706.400)
Coverslips thickness 1, 24 × 60 mm Gerhard Menzel GmbH (Carl Roth, catalog number: H878.2)
600 mL plastic beaker (BRAND® ETFE beaker with spout, low form (Merk, catalog number: BR87618)
Aldrich® microscale syringe 1 mL (Sigma-Aldrich, catalog number: Z684309)
Dako Pen (DAKO, catalog number: 5200230-2)
Superfrost plus microscope slides (Thermo Scientific, catalog number: J1800AMNZ)
TiterMax classic (Sigma-Aldrich, catalog number: H4397), store at 2–8°C
Potassium chloride (KCl) (Roth, catalog number: 6781.1), store at room temperature
Sodium dihydrogen phosphate monohydrate (NaH2PO4·H2O) (Merck, catalog number: 1.06346.1000), store at room temperature
Sodium chloride (NaCl) (Roth, catalog number: 3957.1), store at room temperature
Di-Sodiumhydrogenphosphate dodecahydrate (Na2HPO4·12H2O) (Merck, catalog number: 1.06579.1000), store at room temperature
Sodium azide (NaN3) (Sigma-Aldrich, catalog number: S8032), store at room temperature in a dark and well-ventilated place
Tween 20 (Merck, catalog number: 8.22184.0500), store at room temperature
Tris base (Sigma-Aldrich, catalog number: T1503), store at room temperature
Ketanest S (25 mg/mL) (Pfizer, catalog number: PZN 08707288), store at 2–8°C
Rompun (Xylazine) (20 mg/mL) (Bayer Vital GmbH, catalog number: PZN 1320422), store at 4–30°C
Bepanthen, eye and nose ointment (Bayer Ag, catalog number: PZN 01580241), store at room temperature
Trichlormethan/Chloroform 99% (Roth, catalog number: Y015.1), store at room temperature in a ventilated place
Acetone 99.8% (Roth, catalog number: 9372.5), store at room temperature in a ventilated place
Paraformaldehyde (AppliChem, catalog number: A3813,1000), store at 2–8°C
ExtrAvidin Alkaline Phosphatase (Sigma-Aldrich, catalog number: E2636), store in the dark at 2–8°C
Fast Red TR Salt (Sigma-Aldrich, catalog number: 368881), store at room temperature
Fast Blue BB Salt (Sigma-Aldrich, catalog number: F3378), store at -20°C
Aquatex (Merck, catalog number: 1.08562.0050), store at 15–25°C
Methanol 99.9% (Roth, catalog number: 4627.5), store at room temperature in a ventilated place
ExtrAvidin Peroxidase (Sigma, catalog number: E2886), store in the dark at 2–8°C
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153), store at 2–8°C
Liquid DAB+ Substrate Chromogen System (Agilent Technologies, catalog number: K3468), store in the dark at 2–8°C
Purified anti-mouse Ki-67 (clone 16A8) (BioLegend, catalog number: 652402), store at 2–8°C
Naphthol AS-MX phosphate (Sigma-Aldrich, catalog number: N4875), store at -20°C
Dimethylformamide (SERVA, catalog number: 20270), store at room temperature
(-)-Tetramisole hydrochloride (Levamisole) (Sigma-Aldrich, catalog number: L9756), store at 2–8°C
Purified Rat Anti-Mouse CD45R/B220 (clone RA3-6B2) (BD Biosciences, catalog number: 553084), store in the dark at 2–8°C
Biotin hamster anti-mouse TCRβ chain (clone H57-597) (BD Biosciences, catalog number:553169), store undiluted at 4°C
Rabbit Anti-Rat IgG(H+L), Human ads-BIOT (Southern Biotech, catalog number: 6185-08), store at 2–8°C
Normal mouse serum (Invitrogen, catalog number: 10410), store at -20°C
Recombinant mCOL7c-GST (see Recipes)
5 × PBS (phosphate-buffered saline) (see Recipes)
Anesthetic solution (see Recipes)
TBS-Tween (Tris base saline-tween) (see Recipes)
Tris Buffer (0.1 M) (see Recipes)
PFA 4% (paraformaldehyde) (see Recipes)
Alkaline Phosphatase - Anti-Alkaline Phosphatase (APAAP) substrate (see Recipes)
Antibody solution (see Recipes)
Fast Red staining solution (see Recipes)
Fast Blue staining solution (see Recipes)
Equipment
Electrical nail file with a round grinding cone device and a micromotor providing a rotating speed of 2,000 rpm (Tchibo, TCM, Article number 66075, Germany)
Vortex-Genie 2 (Scientific Industries INC, USA)
MAULalpha 2000G, weight range 2 kg Readability (JAKOB MAUL GmbH, model·16420-90)
LEICA® manual microtome (LEICA, model: CM3050S)
pH211 Microprocessor pH Meter (HANNA Instruments)
Refrigerator and freezer
Nitrogen Tank
Procedure
Induction of germinal centers and Tfh cells in mice
Preparation of the injection solution and injections into mice0.02 mM (20 µM) of the protein should be injected. It is important to quantify the protein by its molar concentration because only a small fraction of the entire protein is immunogenic. The molar concentration versus mass concentration can be calculated by equation 1.
Equation 1:[M]=Ci/MW
Where M is the molar concentration (μM), Ci is the mass concentration (μg/mL), and MW is molecular weight (kDa).
Here, in this case, the molecular weight of mCOL7c-GST is 49.5 kDa. Thus, the mass concentration would be 0.02 mM × 49.5 kDa = 0.990 µg/µL; simplified = 1 µg/µL. Because this solution will be diluted at 1:2, a sterile stock solution of 2 µg/mL mCOL7c-GST in 1× PBS should be prepared shortly before usage and kept one ice.
Pipette the adjuvant TiterMax into a sterile Eppendorf tube. Add an equal volume of the protein solution to get a final concentration of 0.01 mM (in case of mCOL7c-GST 1 µg/µL), in the proportion of 1:2 (for example, 25% 2 µg/µL mCOL7c-GST, 25% PBS 1× and 50% Titermax for the autoimmune group or 50% PBS 1× and 50% TiterMax for the control group).
Vortex vigorously for 30–40 min to prepare a protein-TiterMax or PBS 1×-TiterMax emulsion. Keep the emulsions sterile at 4°C.
Weigh the animals by putting them into a 600 mL plastic beaker that stands on a digital letter scale. Inject 100 µL anesthetic solution per 20 g body weight intraperitoneally (i.p.). Anesthesia should last 20–30 min. To prevent dryness, cover the eyes of anesthetized animals with Bepanthen.
Hold the footpad for injection of the prepared emulsions. Inject 60 μL into the left and 60 µL right hind footpad subcutaneously slowly and carefully by using the entire footpad for injection (in case of mCOL7c-GST 120 µL (120 µg) per mouse).
Sacrifice the mice 2–4 weeks after injection. Carefully open the skin to harvest activated popliteal and inguinal lymph nodes and shock freeze them immediately for immunohistological staining (by (i) quickly placing the sample into liquid nitrogen before (ii) storing it in a -80°C refrigerator) or place lymph nodes in cold PBS 1× on ice for isolation of cells for flow cytometric analysis.
Note: GC reaction will induce the production of high affinity autoantibodies directed against mCOL7c-GST. These autoantibodies will bind to type VII collagen in the skin and induce skin wounds, which appear 4–5 weeks after injection. Mice in the control group, which receive only TiterMax, will develop GC reactions in activated lymph nodes but no skin pathology.
Determine the place of a skin wound formation by scratching
To induce skin wounds, scratch mouse ear skin slightly 1 week before wounds would usually appear by the binding of autoantibodies.
Note: In our experiment, after injection of mCOL7c-GST, bound mCOL7c-specific autoantibodies and skin wounds can be observed at 4–5 weeks post-injection. Therefore, ear skin was scratched 3 weeks post-injection.
Before scratching, mice must be anesthetized as described above (step A4). Attach the round grinding cone to the electrical nail drill machine (Figure 2A and B), switch it on, and adjust it to a rotational at speed of 2,000 rpm. Take the inner side of the ear on top of one of your fingers. Slightly and briefly touch the epidermis with the tip of the round grinding cone. This approach removes the epidermis, whereas the dermis remains intact (Figures 2C–2E).
Note: A non-bleeding wound with a standardized size should appear (Figure 2C). This skin wound will not heal in mice injected with the autoantigen (here: mCOL7c-GST) in contrast to the control group that received vehicle only, in which the healing process becomes visible 14 days after scratching (Figure 3).
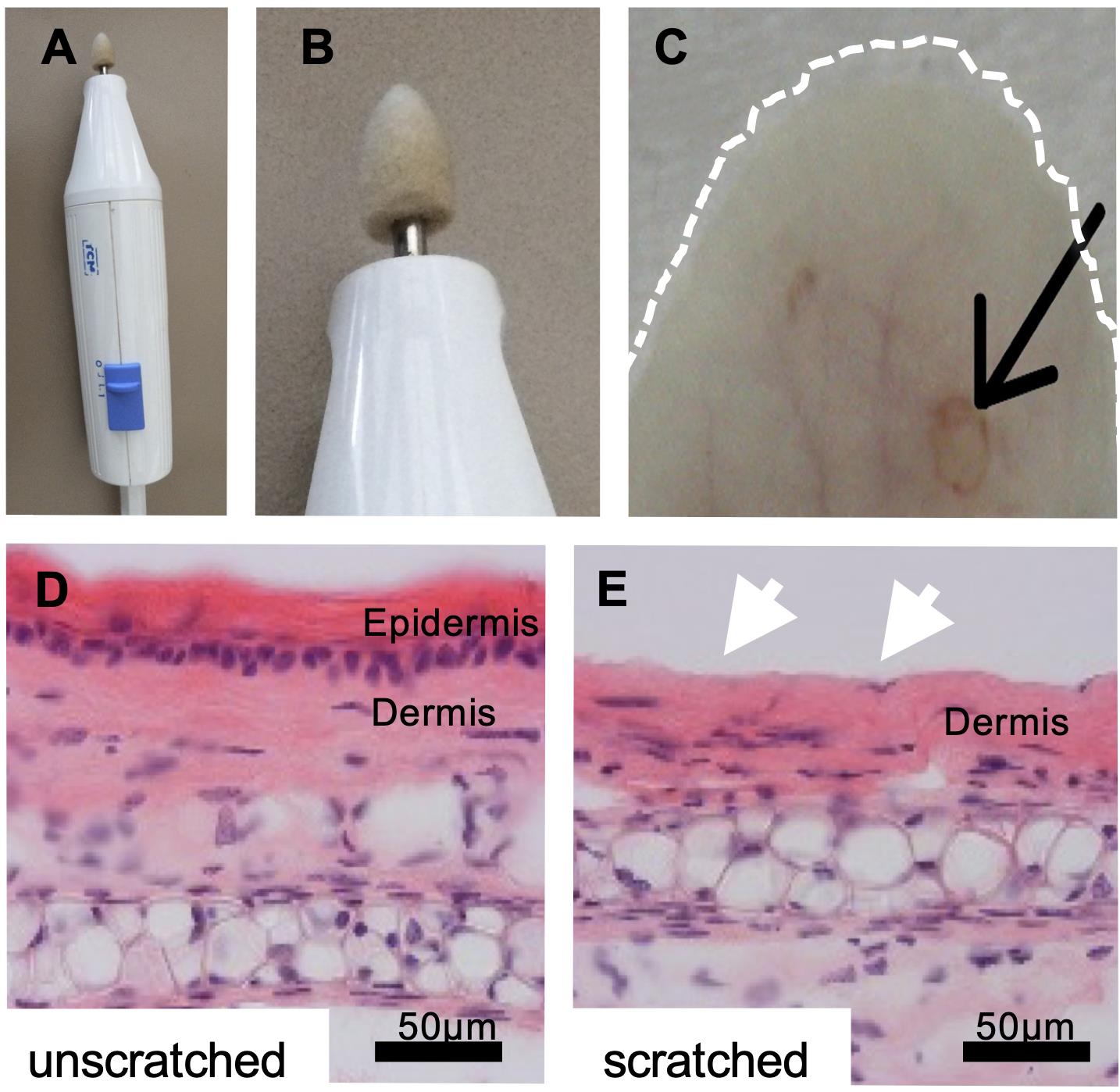
Figure 2. Removal of the epidermal layer with an electric nail file. A. Image shows the electric nail drill machine and B. the grinding attachment at a higher magnification. C. The ear skin was briefly and gently touched with the grinding attachment, which removed the epidermal layer at one spot of approximately 1-mm diameter per ear. The dotted white line marks the ear. The black arrow points to the induced wound spot. D-E. Hematoxylin and eosin staining of skin sections show that only the epidermal layer was removed (white arrows in E). Bar 50 µm. Figure adapted from Niebuhr et al. (2020).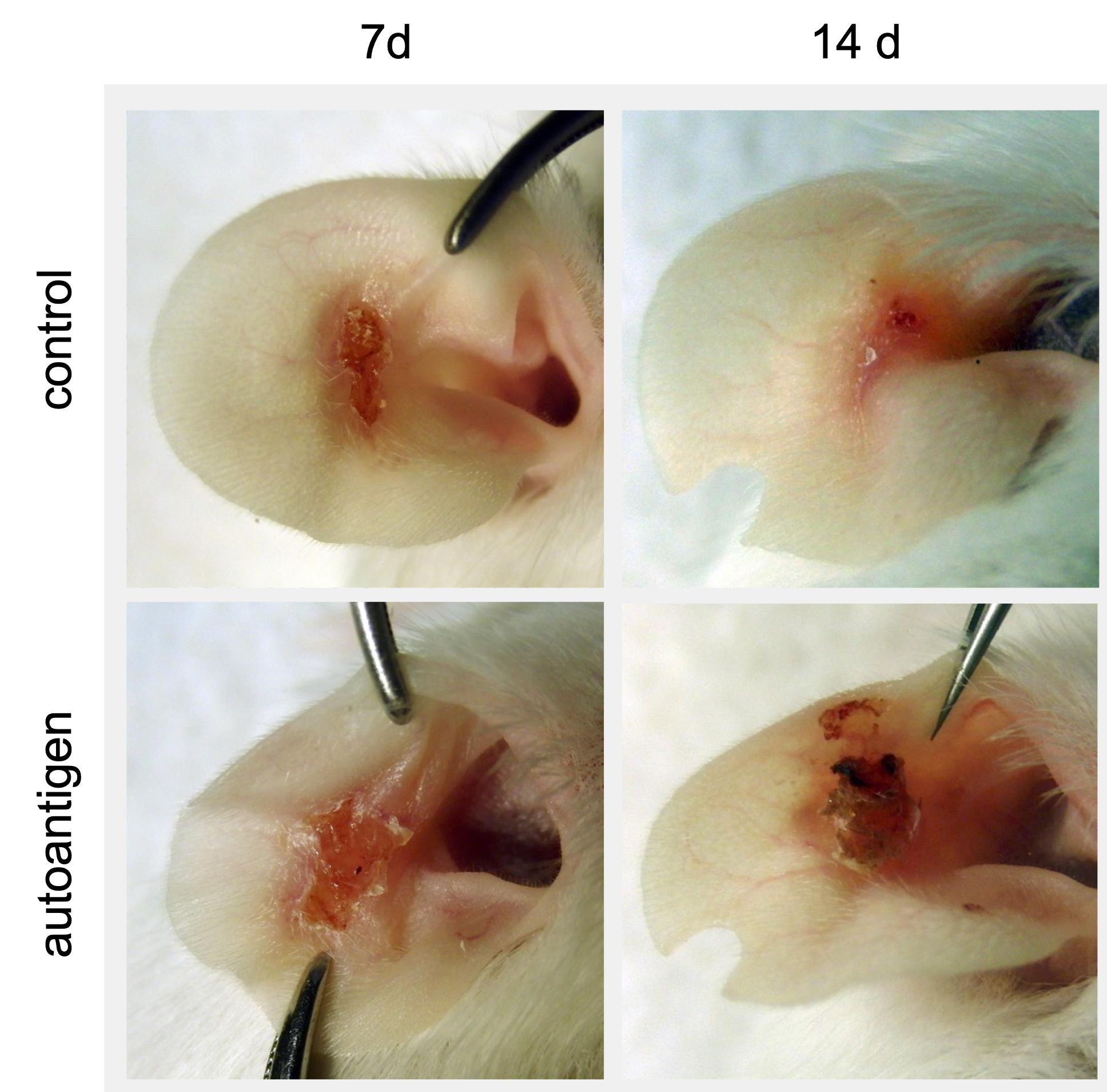
Figure 3. Chronic wounds appear after removal of the epidermal layer. Representative pictures of ear wounds 7 days and 14 days after scratching are shown. Scratched-induced wounds become chronic in the autoimmune group. In contrast, the wound sizes decline in the control group. Figure adapted from Niebuhr et al. (2020).Sacrifice the mice one week after scratching (4 weeks post-injection of the autoantigen) and remove ears. Carefully isolate wounded skin areas from healthy unscratched skin, place wounded and unwounded skin in separate cryotubes and immediately snap freeze tissues using liquid nitrogen or store it short-term in 1× PBS at 4°C. For long-term storage, transfer cryotubes at -80°C. Extract skin pieces under clean and sterilized conditions.
Note: This time point of 1 week was chosen to investigate migrated T eff. To characterize other infiltrating cells, adjust time points accordingly. For example, neutrophils will be found within hours.
Visualization of Tfh cells and GC
Tfh cells localize within the light zone of GC. To stain GC, T, and B cells for traditional light microscopy, prepare serial sections of the collected lymph nodes on two glass slides. Take the first slide and visualize GC by staining proliferating cells with mouse anti-Ki67 antibody and B cells with anti-mouse B220 antibody. Take the second slide and stain T cells and B cells with a primary biotinylated anti-mouse TCRβ antibody and a primary biotinylated anti-mouse B220 antibody, respectively (Figure 4A).
Staining of proliferating cells
Embed collected frozen lymph nodes in tissue freezing medium and cut until a plain cut-level is present. Cut 12 µm-sections of lymph nodes using a cryomicrotom. Distribute 6–10 adjacent sections onto two glass slides. Mount the 1st, 3rd and 5th, ... section on slide 1 and the 2nd, 4th and 6th, ... sections on slide 2 (Figure 4A). Dry slides at RT for at least 1 h.
To fix tissues, incubate glass slide 1 in chloroform for 10 min and acetone for 10 min. Afterwards rinse tissues in TBS-Tween for 15 min and cover lymph node section with 4% PFA and incubate them at 4°C for 45 min.
Rinse glass slide in TBS-Tween for 15 min.
Draw a circle around tissue sections with the Dako Pen to confine the liquids during staining and incubate sections with the first primary antibody anti-mouse Ki-67 at room temperature overnight (1:100 dilution in antibody solution).
Wash unbound antibodies away by incubation with TBS-Tween for 15 min and add the first secondary biotinylated anti-rat IgG antibody (1:500 dilution in PBS 1× with 5% normal mouse serum) for 30 min.
Rinse with TBS-Tween for 30 min and cover tissue sections with ExtrAvidin Alkaline Phosphatase (1:100 in TBS-Tween) for 30 min.
Rinse with TBS-Tween for 15 min and apply Fast Red staining solution for 25 min.
Staining of B cells
Wash glass slide with TBS-Tween for 10 min and add the second primary anti-mouse B220 antibody (1:200 dilution in antibody solution) for 1 h at RT.
Rinse slide in TBS-Tween for 15 min to remove unbound antibody and add the second secondary antibody, the biotinylated anti-rat IgG antibody for 30 min.
Wash slide in TBS-Tween for 30 min and add 200 µL ExtrAvidin Alkaline Phosphatase 1:100 for 30 min.
Wash with TBS-Tween for 15 min and add Fast Blue staining solution for 10 min to visualize B cells.
After rinsing in TBS-Tween for 15 min, mount sections using 150 µL of Aquatex and coverslips.
Staining for T and B cells
Take glass slide 2 with the mounted and dried lymph node sections (numbers 2, 4 and 6).
To fix tissues, incubate glass slide in methanol:acetone (1:2) for 10 min at 20°C. Afterwards rinse tissues in TBS-Tween for 15 min.
Incubate sections with the first primary biotinylated anti-mouse TCRβ antibody 1:50 for 1h and rinse glass slides in TBS-Tween for 30 min.
Add 200 µL ExtrAvidin Peroxidase (1:100 in TBS-Tween) for 30 min and wash slide in TBS-Tween for 15 min.
Incubate sections with Liquid DAB+ Substrate for 5 min to visualize the T cells.
Wash slide with TBS-Tween for 15 min.
Add the second primary anti-mouse B220 antibody (1:200 in antibody solution) for 1 h and proceed as described above (2b–e).
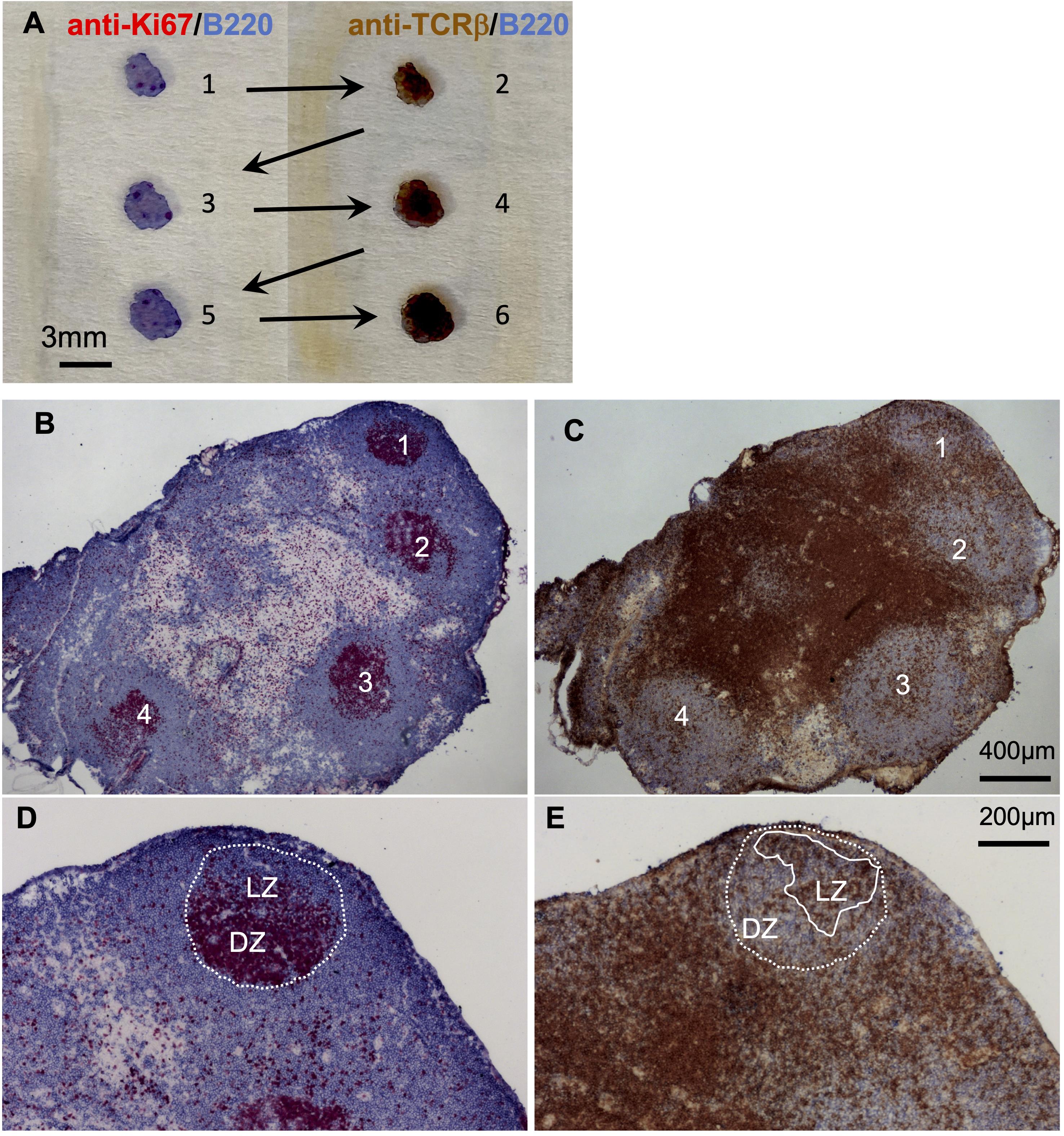
Figure 4. Induction of germinal centers and Tfh cells after injection of antigen in TiterMax. A. Serial cryosections of draining popliteal lymph nodes were distributed on two slides, which were either stained for proliferating cells and B cells (left slide) or T and B cells (right slide). B–E. One example of two adjacent sections is shown. B. GC were visualized by staining the proliferating cells with anti-Ki67 (red) and B cells with anti-B220 (blue). C. Tfh cells were identified as T cells that locate within the light zone of the GC by staining with anti-TCRβ for T cells (brown) and anti-B220 (blue). Four GC were clearly visible (marked with numbers). D. The magnification shows the dark zone (DZ) and light zone (LZ) of the first GC depicted in B. E. Tfh cells can be identified as within the LZ of the GC (brown-colored cells within the area marked with a white line).
Recipes
Recombinant mCOL7c-GST
The recombinant protein mCOL7c-GST was generated as described by Sitaru et al. (2005). Prior utilization, the concentration, and purity of mCOL7c-GST were validated via denaturing polyacrylamide gel electrophoresis. The protein stock was stored in PBS 1× at -80°C. For immunization, an emulsion of mCOL7c-GST in the adjuvant TiterMax was prepared under sterile conditions. Each immunization batch was prepared immediately prior to application by vortexing for 30-40 min at 4°C until complete emulsification.
5× PBS (phosphate-buffered saline)
The composition of 5× PBS is as follows: 90 g NaCl, 2.704 g NaH2PO4 monohydrate, 28.794 g Na2HPO4·12H2O, were diluted in laboratory-graded H2O with the final volume of 2 L. The pH was adjusted to 7.4. 1× PBS was used fresh after diluting 200 mL of 5× PBS in 800 mL of laboratory-graded H2O.
Anesthetic solution
10 mL of anesthetic solution was prepared by adding 3.5 mL Ketanest (25 mg/mL) and 2 mL Rompun (20 mg/mL) to 4.5 mL 0.9% NaCl solution to yield final concentrations of 8.75 mg/ml or 4 mg/mL, respectively. Inject 100 µL i.p. per 20 g body weight for a short-acting anesthesia (43.75 mg/kg Ketanest, 20 mg/kg Rompun).
TBS-Tween (Tris base saline-tween)
The TBS-Tween is prepared using a 10× TBS stock and a Tween-20 (5%) stock. The 10× TBS is prepared using 242.28 g Tris (hydroxymethylaminomethan) and 344.40 g NaCl, dissolved in laboratory grade H2O with a final volume of 4 L, the pH should be adjusted to 7.6 and the solution must be kept at 4°C. The Tween-20 (5%) is prepared by adding 190 mL laboratory-grade H2O to 10 mL of Tween-20. 1 L of 1× TBS-Tween is prepared by adding 890 mL laboratory-grade H2O to 100 mL of 10× TBS and 10 mL of Tween-20 (5%).
Tris Buffer (0.1 M)
The composition of the Tris Buffer (0.1 M) is as follows: 12.1 g Tris base is diluted in laboratory grade H2O with a final volume of 1 L, pH should be adjusted to 8.2 with HCl.
PFA 4% (paraformaldehyde) solution
The composition of the PFA solution is as follows: 8 g of paraformaldehyde in 200 mL of 1× PBS solution.
Alkaline Phosphatase - Anti-Alkaline Phosphatase (APAAP) substrate
The composition of the APAAP substrate is as follows: 20 mg Naphthol AS-MX phosphate, 2 mL N,N-Dimethylformamide, 100 µL Levamisole (0.24 g/mL), 98 mL Tris-buffer 0.1 M. The APAAP substrate is stable maximum for 40 days.
Antibody solution
The composition of antibody solution is as follows: 1× PBS +1% BSA + 0.1% NaN3.
Fast Red staining solution
The composition of the Fast Red staining solution is as follows: 0.01 g of Fast RedSalt in 3 mL of APAAP-substrate. After the dilution, the solution should be shaken gently for 5 min and then should be kept stand steal for an additional 5 min. When added to the samples, the Fast Red staining solution should be subjected to gentle shaking for 25 min. The waste should be considered as hazardous.
Fast Blue staining solution
The composition of the Fast Blue staining solution is as follows: 0.002 g of Fast Blue BB Salt in 4 mL of APAAP-substrate. The solution should be subjected to gentle shaking for 10 min. Prior to staining samples with the Fast Blue staining solution, the solution should be subjected to filtration. After treating the sample with the filtered solution, it should be subjected to gentle shaking for 10 min.
The waste should be considered as hazardous.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG) within the framework of the Schleswig-Holstein Excellence Cluster I and I (EXC 306, Inflammation at Interfaces, project XTP4), the graduate school GRK 1727/2, GRK2633/1 and the TR-SFB654 project C4 at the University of Luebeck to KK and JW. Part of figures are adapted and modified from the studies of Niebuhr et al. (2020 and 2021).
Competing interests
The authors indicate no potential conflicts of interest.
Ethics
All experiments were approved by the Animal Care and Use Committee of the state Schleswig-Holstein (Ministerium fuer Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung), proposals: V312-72241.122-1 (19-2/08), V312-72241.122-1 (92-7/09), V 312-72241.122-1 (104-10), V 242-45884/2016 (90-7/16) and 23/A11/05. All animal experiments were conducted by certified personnel.
References
- Chemin, K., Gerstner, C. and Malmstrom, V. (2019). Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis. Front Immunol 10: 353.
- Crotty, S. (2019). T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50(5): 1132-1148.
- Ghani, S., Feuerer, M., Doebis, C., Lauer, U., Loddenkemper, C., Huehn, J., Hamann, A. and Syrbe, U. (2009). T cells as pioneers: antigen-specific T cells condition inflamed sites for high-rate antigen-non-specific effector cell recruitment. Immunology 128(1 Suppl): e870-880.
- Ludwig, R. J., Vanhoorelbeke, K., Leypoldt, F., Kaya, Z., Bieber, K., McLachlan, S. M., Komorowski, L., Luo, J., Cabral-Marques, O., Hammers, C. M., et al. (2017). Mechanisms of Autoantibody-Induced Pathology. Front Immunol 8: 603.
- Niebuhr, M., Belde, J., Fahnrich, A., Serge, A., Irla, M., Ellebrecht, C. T., Hammers, C. M., Bieber, K., Westermann, J. and Kalies, K. (2021). Receptor repertoires of murine follicular T helper cells reveal a high clonal overlap in separate lymph nodes in autoimmunity. Elife 10: e70053.
- Niebuhr, M., Bieber, K., Banczyk, D., Maass, S., Klein, S., Becker, M., Ludwig, R., Zillikens, D., Westermann, J. and Kalies, K. (2020). Epidermal Damage Induces Th1 Polarization and Defines the Site of Inflammation in Murine Epidermolysis Bullosa Acquisita. J Invest Dermatol 140(9): 1713-1722 e1719.
- Sitaru, C., Mihai, S., Otto, C., Chiriac, M. T., Hausser, I., Dotterweich, B., Saito, H., Rose, C., Ishiko, A. and Zillikens, D. (2005). Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest 115(4): 870-878.
- Tull, T. J. and Benton, E. (2021). Immunobullous disease.Clin Med (Lond) 21(3): 162-165.
- Vinuesa, C. G., Sanz, I. and Cook, M. C. (2009). Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 9(12): 845-857.
Article Information
Copyright
Bahreini et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Bahreini, F., Niebuhr, M., Belde, J., Bieber, K., Westermann, J. and Kalies, K. (2022). Protocol to Induce Follicular T Helper Cells, Germinal Centers, and Skin Lesions in Mouse Models for Skin Blistering Diseases. Bio-protocol 12(10): e4414. DOI: 10.21769/BioProtoc.4414.
- Niebuhr, M., Belde, J., Fahnrich, A., Serge, A., Irla, M., Ellebrecht, C. T., Hammers, C. M., Bieber, K., Westermann, J. and Kalies, K. (2021). Receptor repertoires of murine follicular T helper cells reveal a high clonal overlap in separate lymph nodes in autoimmunity. Elife 10: e70053.
Category
Immunology > Animal model > Mouse
Immunology > Immune cell staining > Immunodetection
Cell Biology > Tissue analysis > Histomorphology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









