- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sex-specific Separation of Plasmodium falciparum Gametocyte Populations
Published: Vol 11, Iss 11, Jun 5, 2021 DOI: 10.21769/BioProtoc.4045 Views: 3889
Reviewed by: Alessandro DidonnaDhaneswar PrustyKarolina Subrtova

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

NBD-lipid Uptake Assay for Mammalian Cell Lines
Sara Abad Herrera [...] Thomas Günther Pomorski
Feb 20, 2022 4699 Views

Babesia duncani in Culture and in Mouse (ICIM) Model for the Advancement of Babesia Biology, Pathogenesis and Therapy
Vandana Kumari [...] Choukri Ben Mamoun
Nov 20, 2022 1929 Views

VAR2CSA Ectodomain Labeling in Plasmodium falciparum Infected Red Blood Cells and Analysis via Flow Cytometry
Olivia M.S. Carmo and Matthew W.A. Dixon
Aug 5, 2023 1309 Views
Abstract
Plasmodium falciparum is a unicellular eukaryotic parasite that causes malaria in humans. The parasite is spread by Anopheles mosquitoes after ingestion of sexual stage parasites known as gametocytes. Malaria transmission depends on parasites switching from the disease-causing asexual blood forms to male and female gametocytes. The current protocol allows the simultaneous isolation of male and female parasites from the same population to study this critical lifecycle stage in a sex-specific manner. We have generated a transgenic P. falciparum cell line that expresses a GFP-tagged parasite protein in female, but not male, parasites. Gametocyte production is stress induced and, through a series of steps, sexual stage parasites are enriched relative to uninfected red blood cells or red blood cells infected with asexual stage parasites. Finally, male and female gametocytes are separated by fluorescence-activated cell sorting. This protocol allows for the separation of up to 12 million live male and female parasites from the same population, which are amenable to further analysis.
Keywords: Plasmodium falciparumBackground
Malaria is still one of the most important infectious diseases of humankind. Each year more than 200 million cases of malaria are reported globally, resulting in over 400,000 deaths (World Health Organization, 2019). The disease is caused by protozoan parasites of the genus Plasmodium, and P. falciparum causes the most severe disease in humans. The parasite is spread between human hosts by Anopheles mosquitoes. Male and female gametocytes (sexual blood stage parasites) are key to the transmission of malaria because only these forms can survive ingestion by the mosquito vector to complete the parasite lifecycle. Hence, understanding the biological makeup of gametocytes holds great promise to identify transmission-blocking intervention strategies.
The analysis of sexual Plasmodium stages in the laboratory poses particular challenges: only a small subset of parasites converts into sexual forms; triggers for gametocyte commitment are numerous but poorly defined; complete development of the sexual stages takes 10-12 days; and the sex ratio is female-biased (3-5:1), meaning studies on total gametocytes tend to overlook the contribution of male parasites (e.g., omics approaches or drug susceptibility studies).
Although male and female parasites can be distinguished by subtle morphological features, the enrichment of sex-specific gametocyte populations remains challenging. Previously, separate cell lines were used to obtain either male or female populations. For example, the proteome of a non-male-gametocyte-producing strain was compared to that of combined male and female gametocytes to predict the sex-specific proteome (Tao et al., 2014). Lasonder et al. (2016) later generated two independent transgenic cell lines, one with a male-specific marker (dynein heavy chain) and the other with a female-specific marker (P47), to determine the sex-specific proteome experimentally. However, when the authors introduced both tags into the same cell line, a substantial subset of the population expressed both tags, revealing that the markers were not sex-specific. Miao et al. (2017) took advantage of alpha-tubulin II (differentially expressed between sexes) to determine another sex-specific proteome. Although each of these studies contributed information on the sex-specific molecular makeup of the parasites, a more reliable and sensitive method for distinguishing male and female gametocytes could accelerate sex-specific gametocyte research.
We previously discovered a protein that is only expressed in female gametocytes (Tran et al., 2014). This molecule belongs to the ATP-binding cassette transporter family (ABCG2) and locates to a single round uncharacterized organelle in the female parasite. This protocol uses a cell line that expresses a chimeric protein consisting of gABCG2 and green fluorescent protein as a female-specific marker.
The method presented here has the advantage of sorting gametocytes from the same population, allowing for paired analysis of male and female gametocytes. The method is not only suitable for sex-specific omics approaches, but is also compatible with live-cell analysis, such as testing sex-specific drug susceptibility.
Materials and Reagents
Sterile blunt end 22G needles (e.g., Livingstone, catalog number: DN22GX1.25B)
25 and 75 cm2 cell culture flasks (Corning, catalog numbers: 430168 and 430720)
175 cm2 cell culture flasks (Sarstedt, catalog number: 83.3912)
Microscope slides (Hurst Scientific, catalog number: WGDFCE90)
15 and 50 ml centrifuge tubes (Corning, catalog numbers: 430791 and 430829)
Disposable 500 ml bottle-top vacuum filters (Corning, catalog number: 431118) and sterile collection bottles
General plastic consumables (serological pipettes, aspiration pipettes, 1.7 ml microcentrifuge tubes, 20 ml syringes, pipette tips; Sarstedt or Corning)
Fresh (less than 10 days old) human red blood cells (RBCs), blood type O+ (Australian Red Cross Lifeblood)
Heat-inactivated human serum pooled from at least 5 donors of the same blood type (Australian Red Cross Lifeblood)
Plasmodium falciparum 3D7-gABCG2-GFP; P. falciparum 3D7 strain parasites expressing a GFP-tagged gametocyte ATP-binding cassette transporter family member 2 (gABCG2) protein (PlasmoDB accession no. PF3D7_1426500) (Tran et al., 2014)
RPMI 1640 Medium, GlutaMAXTM Supplement, HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 72400120)
Gentamicin 10 mg/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15710072)
D-(+)-Glucose (Sigma-Aldrich, catalog number: G7021)
Hypoxanthine (Sigma-Aldrich, catalog number: H9377)
Sodium hydroxide (NaOH, Sigma-Aldrich, catalog number: S5881)
Milli-Q water (fresh from any available purification system)
AlbuMAXTM II Lipid-Rich BSA (Thermo Fisher Scientific, GibcoTM, catalog number: 11021045)
WR99210 (Jacobus Pharmaceuticals)
D-Sorbitol (Sigma-Aldrich, catalog number: S6021)
Methanol (for Giemsa staining) (Merck, catalog number: 1060092511)
Giemsa’s solution (Merck, catalog number: 109240500)
Immersion oil (Sigma-Aldrich, catalog number: 56822)
N-Acetyl-D-glucosamine (GlcNAc; Carbosynth, catalog number: MA00834)
Hoechst 33342 (Sigma-Aldrich, catalog number: B2261)
1× Phosphate Buffered Saline, pH 7.4 (PBS; see Recipes)
45% D-(+)-glucose (w/v) (see Recipes)
1 M NaOH (see Recipes)
200 mM hypoxanthine (see Recipes)
Incomplete culture medium (ICM; see Recipes)
5% AlbuMAX II (w/v) (see Recipes)
Heat-inactivated human serum (see Recipes)
Complete culture medium (CCM; see Recipes)
5% D-sorbitol (w/v) (see Recipes)
Complete culture medium supplemented with 50 mM GlcNAc (see Recipes)
10 mg/ml Hoechst 33342 (see Recipes)
1× PBS with 10 mM glucose (see Recipes)
10 µg/ml Hoechst 33342 in 1× PBS with 10 mM glucose (see Recipes)
Equipment
Biological safety cabinet (Safemate Vision Class II, Edwards Group)
Vacuum pump and liquid aspiration system (single cylinder TC501, Sparmax)
37 °C incubator (S.E.M. bench top incubator)
Malaria gas mixture (1% O2, 5% CO2, 94% N2; BOC, catalog number: CCS402562G2)
Water bath (WB7, Ratek)
Compound microscope with 100× objective (Olympus, model: BX41)
Tabletop centrifuge for 15 and 50 ml tubes (Beckman Coulter, model: Allegra X-15R)
Tabletop centrifuge for microcentrifuge tubes (Thermo Scientific, model: Heraeus Pico 17)
CS columns (Miltenyi Biotec, catalog number: 130-041-305) with a sterile stopcock
SuperMACSTM II Separator (Miltenyi Biotec, catalog number: 130-044-104)
BD FACSAriaTM Cell Sorter (BD Biosciences)
Software
BD FACSDivaTM Software (BD Biosciences)
Procedure
Maintenance of asexual P. falciparum
Maintain asexual P. falciparum transgenic parasites (3D7-gABCG2-GFP) in complete culture medium at 4% hematocrit and less than 5% parasitemia in cell culture flasks. This transgenic strain is maintained in the presence of 2 nM WR99210. Monitor parasitemia every second day by Giemsa-stained thick smear (Maier and Rug, 2013) (Note 1). Count at least 300 cells (ideally using a blood cell counter or tally counter) to determine the culture parasitemia.
Replace culture medium with complete culture medium (CCM) and split cultures to 0.2% parasitemia every second day. Flush the culture flask with malaria gas mixture of 1% O2, 5% CO2, and 94% N2 for 30 s, close the lid tightly, and incubate at 37 °C (standard culture conditions) (Maier and Rug, 2013).
To generate large populations of highly purified male and female gametocytes, expand the cell culture to at least 60 ml and proceed with gametocyte production (Note 2).
Production of male and female gametocytes (Figure 1)
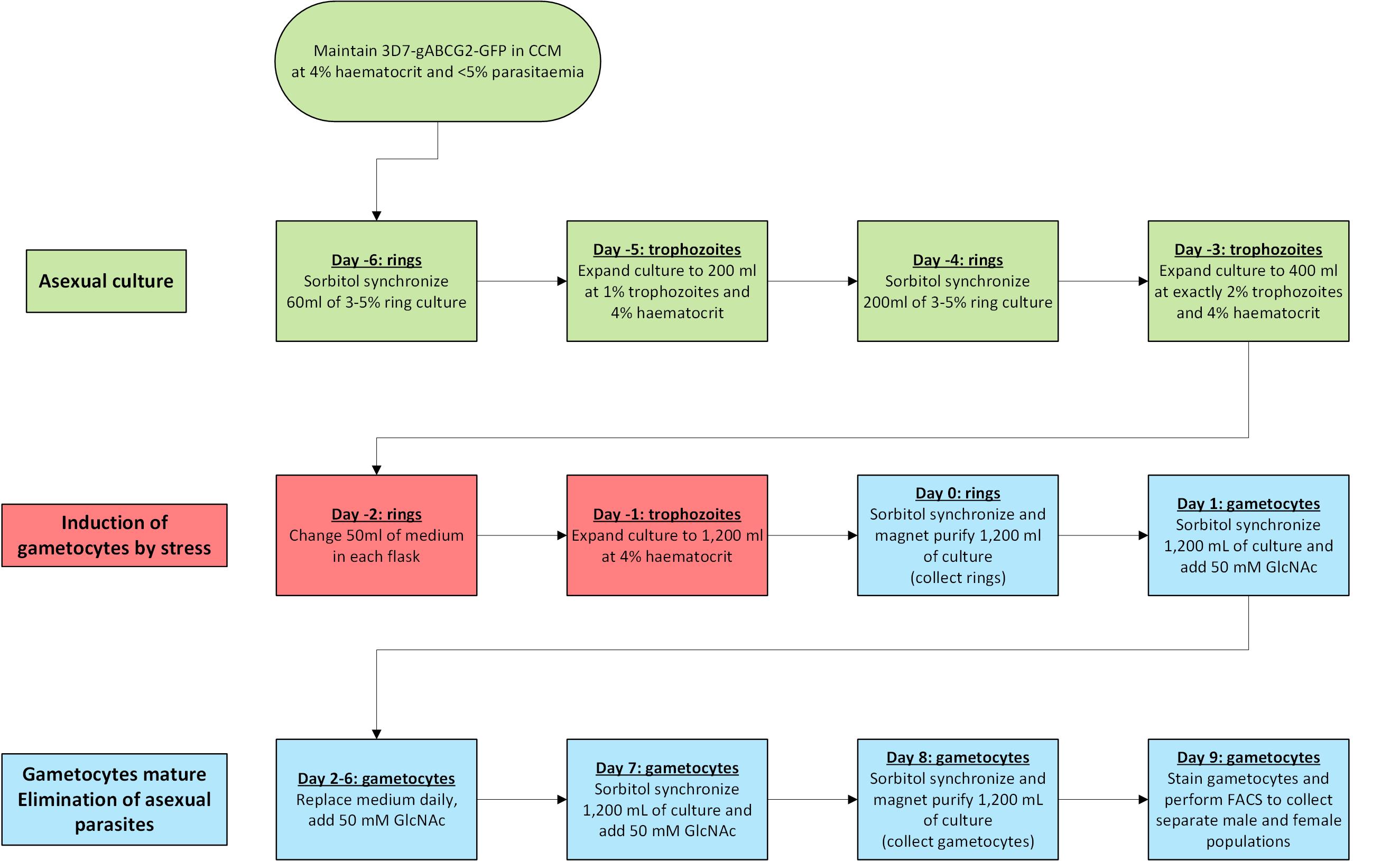
Figure 1. Flow-diagram outlining the timeline of the procedureDay -6. Start with 60 ml of culture at 3-5% predominantly ring parasitemia and 4% hematocrit. Perform a sorbitol synchronization as follows (Lambros and Vanderberg, 1979). Split the culture in half and transfer to two 50 ml centrifuge tubes and centrifuge at 800 × g for 7 min at room temperature (RT). Discard the supernatant and resuspend each cell pellet in 10 ml of 5% D-sorbitol. Incubate for 10 min at 37 °C. Centrifuge at 800 × g for 7 min at RT, discard the supernatant, and resuspend each cell pellet in 30 ml of CCM. Combine the suspensions from both tubes and incubate the culture under standard conditions.
Day -5. Determine the parasitemia early in the day (12-18 h after synchronization) from a Giemsa-stained thick smear (Note 3). Expand the culture to 200 ml at 1% trophozoite parasitemia and 4% hematocrit in large 175 cm2 cell culture flasks. Flush the culture flask with malaria gas mixture and incubate under standard conditions.
Day -4. Determine the parasitemia from a Giemsa-stained thick smear. The culture should be at 3-5% ring parasitemia. If parasitemia is lower, culture parasites for another replication cycle without allowing parasitemia to exceed 5% trophozoites. Perform sorbitol synchronization as follows (Lambros and Vanderberg, 1979). Aspirate medium carefully from the flask without disturbing the RBC layer and resuspend the cells in 30 ml of 5% D-sorbitol. Transfer the suspension into a centrifuge tube. Wash the flask with 10 ml of 5% D-sorbitol and add it to the centrifuge tube to prevent loss of cells. Incubate for 10 min at 37 °C. Centrifuge the culture at 800 × g for 7 min at RT, discard the supernatant, and resuspend the cell pellet in 30 ml of CCM. Return the suspension to the 175 cm2 cell culture flask rinsed with 30 ml of 5% D-sorbitol, top up to 200 ml with CCM, flush with malaria gas mixture, and incubate under standard conditions overnight.
Day -3. Determine parasitemia from a Giemsa-stained thick smear. Expand culture to 400 ml at exactly 2% trophozoite parasitemia and 4% hematocrit in two 175 cm2 cell culture flasks (each flask containing 200 ml of culture), flush with malaria gas mixture, and incubate under standard conditions overnight.
Day -2. Determine parasitemia from a Giemsa-stained thick smear. Ideally, the culture should be at 10-12% ring stage parasitemia (Note 4). Carefully remove 50 ml of medium from each cell culture flask without disturbing the cells and add 50 ml of fresh CCM (only replace 25% of the medium volume to continue to stress the culture). Flush the flasks with malaria gas mixture and incubate the cultures under standard conditions overnight.
Day -1. Determine parasitemia from a Giemsa-stained thick smear. Resuspend the culture and divide equal volumes into six 175 cm2 cell culture flasks. Top up each flask to 200 ml with fresh CCM and adjust hematocrit to 4% with fresh red blood cells (this results in 1,200 ml of cell culture in 6 flasks). Flush the flasks with malaria gas mixture and incubate the cultures under standard conditions.
Day 0.
Perform a sorbitol synchronization (Lambros and Vanderberg, 1979) as follows. Aspirate medium carefully from each flask and resuspend the cells of each flask in 30 ml of 5% D-sorbitol. Transfer the suspension into a centrifuge tube, wash each flask with 10 ml of 5% sorbitol, and add it to the centrifuge tube to prevent loss of cells. Incubate for 10 min at 37 °C. Centrifuge the culture at 800 × g for 7 min at RT, discard the supernatant, and resuspend the cell pellet in 30 ml of CCM (resulting in a cell suspension of about 25% hematocrit).
Remove asynchronous parasites at trophozoite or schizont stages and spontaneously committed gametocytes using a SuperMACS separator and CS column (Note 5).
Assemble the CS column and magnetic separator according to the manufacturer’s instructions in the biological safety cabinet (Figure 2). To avoid accidental needle pricks, use a blunt end needle (22G drawing up needle, Note 6) and clip the end of the needle sheath with pliers instead of removing the sheath.
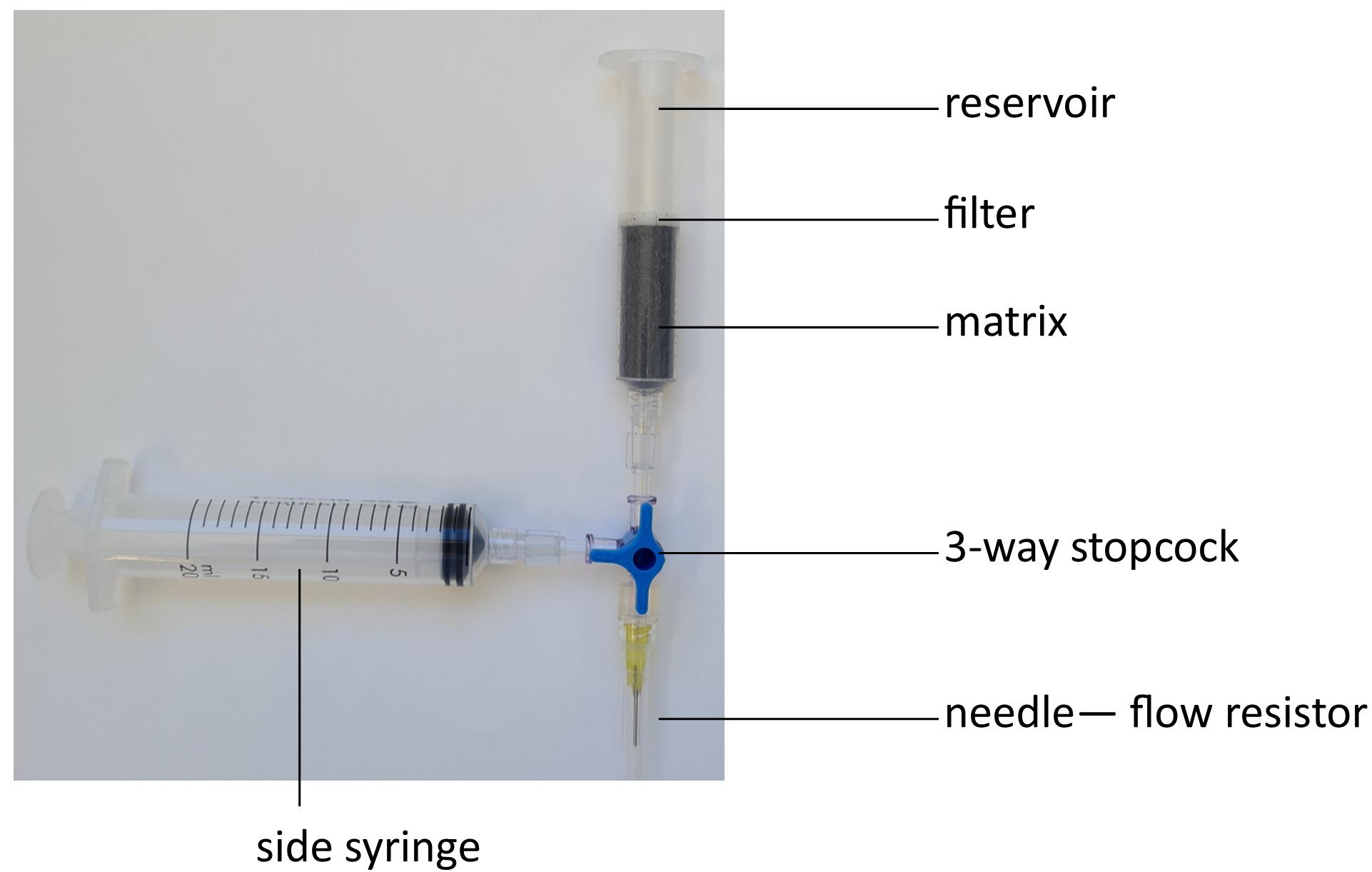
Figure 2. Assembly of the CS column for use in the SuperMACSTM magnetic separatorSterilize the column with 25 ml of 80% ethanol (if it has previously been used) (Note 7), rinse with 25 ml of 1× PBS (pH 7.4, warmed to 37 °C), then prime with 25 ml of ICM (warmed to 37 °C) by filling the reservoir and drawing liquid through the column with the syringe. To remove air bubbles, load the syringe with ICM, reattach it to the stopcock, and flush ICM upward through the column. Draw ICM back into the syringe until the ICM level is just above the filter. Repeat flushing the column until all air bubbles are removed, then drain excess ICM through the needle until the ICM level is just above the filter. Ensure the column never runs dry from here onward (the liquid surface must always stay above the filter) as air bubbles reduce the binding surface of the column.
Place column between the magnets and position a collection tube below the needle (flow resistor). Load the 25% hematocrit RBC suspension of one flask onto the column (Figure 3). Drain through the column drop-wise (adjust the flow rate to approximately 3 ml/min with the stopcock), collecting the effluent that contains uninfected red blood cells and rings committed to gametocytogenesis.
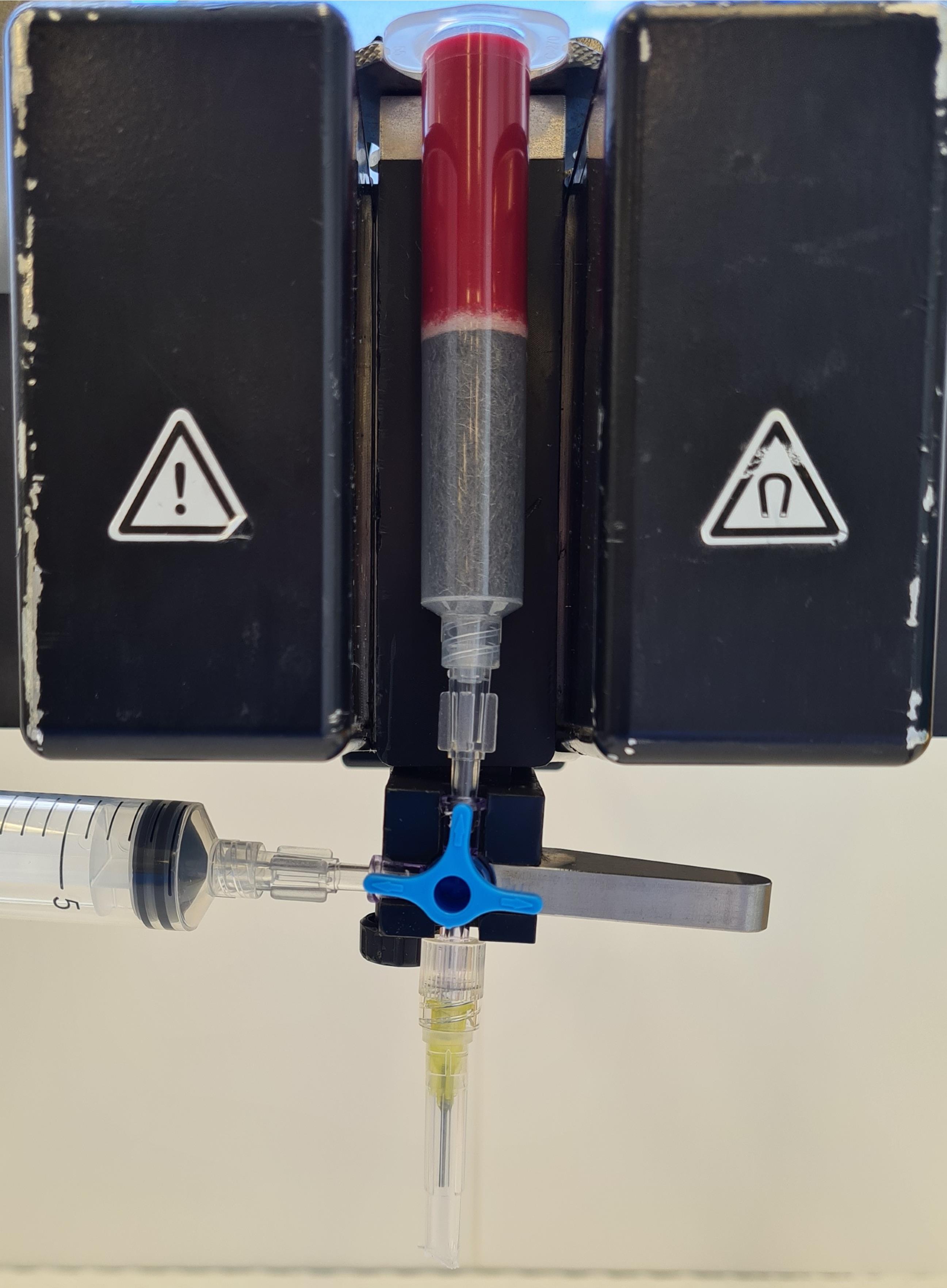
Figure 3. CS column loaded with 25% hematocrit RBC suspension placed in the magnetic field. Stopcock is blocking downward flow through the needle. Gradually turn it clockwise to drain culture slowly.Load the column with 50 ml of CCM (warmed to 37 °C) and drain through the flow resistor into the collection tube until the effluent is no longer red.
Transfer all effluent to a 175 cm2 cell culture flask and top up to 200 ml with CCM. Flush the flask with malaria gas mixture and incubate under standard conditions.
Remove the column from between the magnets and rinse by loading 25 ml of ICM onto the column, drawing the liquid into the syringe, and then discarding. Repeat until the rinse is no longer brown. Fill the reservoir with 6 ml of ICM and allow it to flow through the column and needle.
Repeat Steps B7b (iii-vi) until all culture flasks have been purified.
Rinse the column with 25 ml of 1× PBS, 50 ml of autoclaved Milli-Q water, and 25 ml of 100% ethanol by drawing the liquid into the syringe and discarding it. Fill the reservoir with 6 ml of 100% ethanol and allow it to flow freely through the column and needle. Air-dry the column in the biological safety cabinet. Disassemble the column and stopcock and store in a sealed sterile plastic bag for reuse. Discard the used needle and syringe.
Day 1. Perform a sorbitol synchronization as described on day 0 (Step B7a) but resuspend the RBC pellet of each flask (approximately 8 ml packed cell volume) in 30 ml of CCM containing 50 mM N-Acetyl-D-glucosamine (GlcNAc). Transfer the suspension to a 175 cm2 cell culture flask, top up to 200 ml with CCM containing 50 mM GlcNAc, flush the flask with malaria gas mixture, and incubate under standard conditions [Note 8; Fivelman et al. (2007)].
Days 2-6. Replace the medium in the cultures daily with fresh CCM supplemented with 50 mM GlcNAc, flush the flask with malaria gas mixture, and incubate under standard conditions.
Day 7. Perform a sorbitol synchronization as described on day 1 (Step B8).
Day 8 (Note 9).
Perform a sorbitol synchronization as described on day 0 (Step B7a), creating a cell suspension of about 25% hematocrit in ICM.
Purify mature gametocytes using a SuperMACS separator and CS column. Purify and elute the gametocytes from each flask separately.
Follow the same procedure as in Step B7b (i-iii). To maximize yield, reload the effluent onto the column and drain drop-wise into a waste bottle. Mature gametocytes (mostly stage IV) are retained in the column (Note 10).
Load the column with 50 ml of ICM warmed to 37 °C and drain through the needle into the waste bottle until the effluent is no longer red.
Remove the column from between the magnets and elute the retained gametocytes by loading the column with 25 ml of CCM and draining through the needle drop-wise into a collection tube. Transfer the effluent into a 175 cm2 cell culture flask, flush the flask with malaria gas mixture, and incubate under standard conditions.
Repeat Steps B11b (i-iii) until all cultures have been purified, adding the effluent from all cultures into the same 175 cm2 cell culture flask (resulting in approximately 150 ml of gametocyte suspension).
Set a small aliquot of magnet-purified culture (~1 ml) in a microcentrifuge tube and centrifuge at 2,000 × g for 1 min. Aspirate the supernatant and resuspend the pellet in 5 µl of CCM. Use this to prepare a Giemsa-stained smear to confirm the presence of gametocytes and determine their stage visually.
Rinse, dry, and store the column as in Step B7b (viii).
Fluorescence-activated cell sorting (FACS) of male and female gametocytes (Table 1)
Prepare gametocyte samples for FACS.
Resuspend magnet-enriched gametocytes. Set aside a small aliquot (~1 ml) in a microcentrifuge tube (GFP single color control). Transfer the rest of the cells to 50 ml centrifuge tubes and centrifuge at 800 × g for 10 min at RT.
Discard the supernatant and resuspend all cells in 3 ml of 1× PBS with 10 mM glucose and 10 μg/ml Hoechst 33342.
Incubate the sample at 37 °C for 15 min in the dark and wash twice in 1 ml of 1× PBS, with 1 min spins at 2,000 × g. After the last wash, resuspend the sample for sorting in 3 ml of 1× PBS containing 10 mM glucose.
Prepare single color controls for FACS
Resuspend a culture of asexual 3D7-gABCG2-GFP parasites and transfer 2× 0.5 ml of 4% hematocrit cell suspension to two microcentrifuge tubes.
Set aside a small aliquot (~1 ml) of the resuspended magnet-enriched gametocyte culture.
Centrifuge the above three tubes for 1 min at 2,000 × g. Discard the supernatant from the microcentrifuge tubes.
To prepare the Hoechst single color control, resuspend one aliquot of asexual 3D7-gABCG2-GFP parasites in 1 ml of 1× PBS containing 10 mM glucose and 10 µg/ml Hoechst 33342. Incubate the sample at 37 °C for 15 min in the dark and wash twice in 1 ml 1× PBS with 1 min spins at 2,000 × g. After the last wash, resuspend the control in 1 ml of 1× PBS containing 10 mM glucose.
Resuspend the gametocyte aliquot and the other asexual parasite aliquot in 1 ml of 1× PBS containing 10 mM glucose to prepare the GFP single color control and unstained control, respectively.
Table 1. Single color controls and samples for FACS
Sample Cell culture Staining conditions Unstained control Asexual 3D7-gABCG2 cell culture (~0.5 ml aliquot) No stain GFP control 3D7-gABCG2 gametocytes (~1 ml aliquot) No stain (GFP expressed in female gametocytes) Hoechst 33342 control Asexual 3D7-gABCG2 cell culture (~0.5 ml aliquot) Stain with 10 µg/ml Hoechst 33342 in 1 ml of PBS with 10 mM glucose for 15 min in the dark at 37 °C Sample for sorting 3D7-gABCG2 gametocytes (~150 ml of purified culture) Stain with 10 µg/ml Hoechst 33342 in 3 ml of PBS with 10 mM glucose for 15 min in the dark at 37 °C
Gating strategy and FACS
Create a new experiment in FACS Diva. In the cytometer window, select the following channels: FSC A, H, and W; SSC A, H, and W; Pacific Blue A (or a corresponding channel to record Hoechst 33342) and Alexa Fluor 488 A (or a corresponding channel to record GFP). Create a worksheet with the dot plots (Figure 4), noting that the plots only show the events included in the parent gate.
Load and run the unstained, Hoechst 33342 only, and GFP only controls. Adjust the voltages to bring the events into range (Figure 4). Adjust the single cell gates to your experimental conditions. The Hoechst only control should contain uninfected red blood cells with no staining, ring-stage infected red blood cells with low staining, and highly stained trophozoite- and schizont-infected red blood cells. Set the Hoechst intensity of the gametocyte gates to the middle population in the Hoechst single color control. The GFP single color control will contain cells with two levels of GFP intensity. Set the GFP intensity of the female gametocyte gate to match the high GFP intensity cells. The male gametocyte gate should match the GFP intensity of the unstained control.
Load and run the gametocyte sample. Adjust the gating strategy if required. Prepare collection tubes filled with 2 ml of CCM at 37 °C, then start sorting cells in male and female gates into the respective collection tubes (Note 11). If multiple collection tubes are required (>10 ml), store full collection tubes at 37 °C.
Check the purity of the sorted samples by analyzing a subset of each using the identical gating strategy.
Since gametocytes are collected at a very low cell density, most applications of this protocol will require a final centrifugation of 1,800 × g for 10 min to pellet collected gametocytes. This protocol yields between 2 and 12 million gametocytes of each sex.
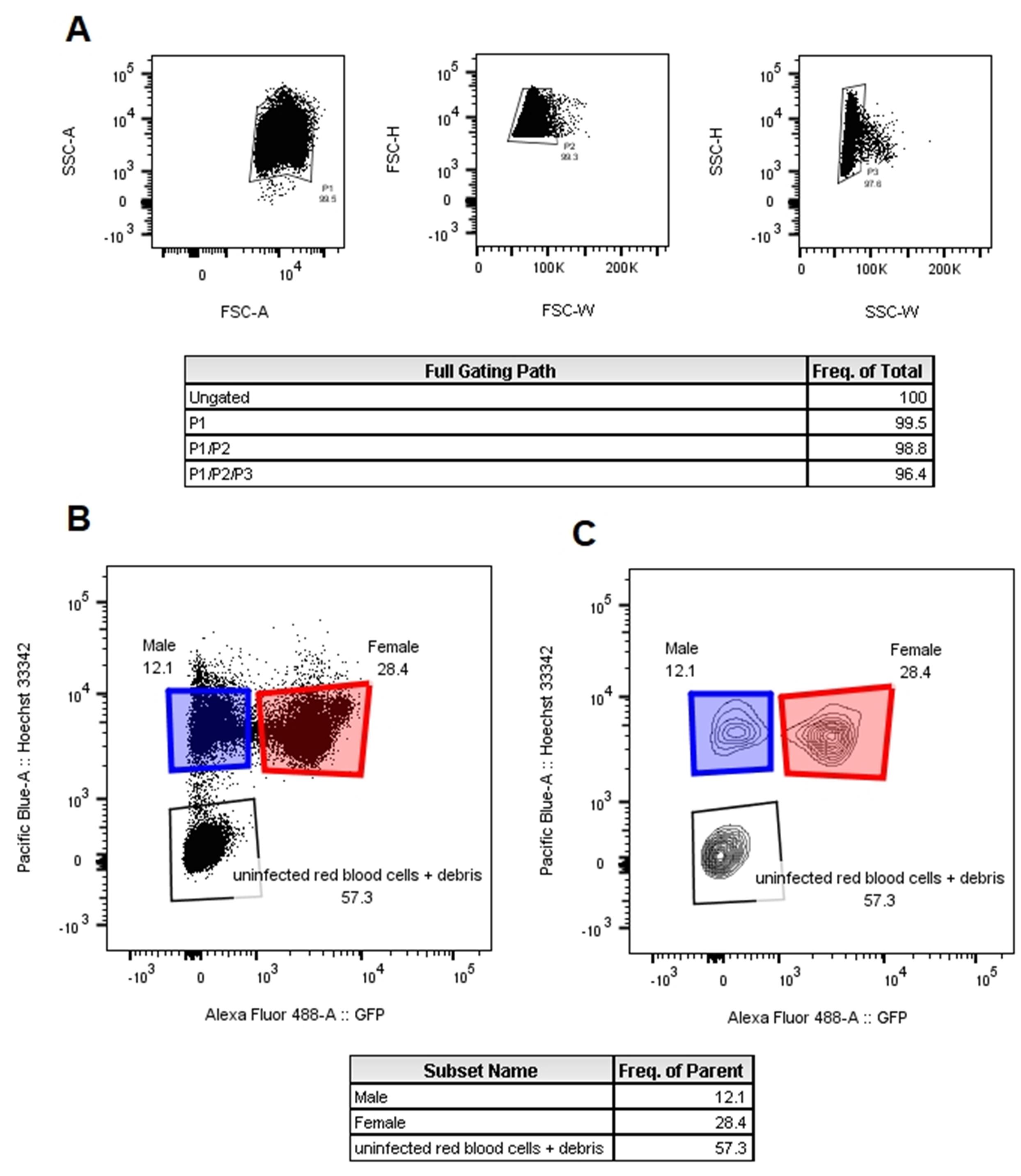
Figure 4. Representative FACS worksheet with the gating strategy for selecting single cells. (A) and sorting gametocytes (B and C). A dot plot (B) and contour plot (C) of male (blue gate) and female (red gate) gametocytes identified by Hoechst 33342 and GFP staining.
Notes
All steps should be performed using standard sterile technique with appropriate biohazard containment procedures and personal protective equipment appropriate for working with live P. falciparum parasites.
Culturing can take place in dishes or flasks. As the culture volume increases, the use of flasks is advisable for ease of handling.
Aim to culture at around the same time every day (within 20-24 h) to keep the availability of nutrients fairly constant.
The high parasitemia induces “stress” by restriction of available nutrients and/or accumulation of metabolic waste products. This acts as a signal for gametocyte commitment.
As asexual parasites and gametocytes mature in the red blood cell and digest the host hemoglobin, free heme is converted to paramagnetic hemozoin crystals. As a result, mature asexual parasites and gametocytes are retained in the column placed in a magnetic field, whereas ring-stage parasites and uninfected red blood cells pass through the magnetic column.
Needle gauge size can be selected by the desired flow rate. The column manufacturer recommends 22G needle for a flow rate of 3 ml/min.
Reuse column only for the same cell line to prevent any potential contamination. The column can be reused up to 5 times for large-scale cultures (1,200 ml) or until magnetic enrichment loses effectiveness.
The addition of GlcNAc to the culture medium at 50 mM prevents the invasion of parasites into red blood cells and can, therefore, be used to prevent asexual parasite proliferation (Fivelman et al., 2007). This step removes all mature asexual stages still present in the culture; gametocytes will not be affected since they are not sensitive to the 5% D-sorbitol solution.
Younger gametocytes at earlier developmental stages can also be harvested by reducing the days the gametocytes are in culture after commitment.
Be careful to maintain the cells at 37 °C as a drop in temperature can activate mature gametocytes, resulting in gamete formation. Pre-warm all solutions to 37 °C and perform sorting at 37 °C to maintain optimal conditions. Use a slide warmer to keep flasks warm in the biosafety cabinet and a centrifuge that can be heated to 37 °C if possible.
Aim for less than 20,000 events/s. If too concentrated, dilute it in PBS.
Recipes
1× PBS
Dissolve 9.1 g of NaCl, 0.144 g of KH2PO4, and 0.42 g of Na2HPO4 in 800 ml of MilliQ water
Adjust pH to 7.4 with HCl and top up to 1 L with water
Sterilize by autoclaving
Store at RT
45% D-(+)-glucose (w/v)
Dissolve 45 g of D-(+)-glucose in 50 ml of warm Milli-Q water and top up to 100 ml
Sterilize using a 0.22 µm filter
Store 2 ml aliquots at -20 °C
1 M NaOH
Dissolve 4 g of NaOH in 80 ml of Milli-Q water and top up to 100 ml
Sterilize using a 0.22 µm filter
Store at RT
200 mM hypoxanthine
Dissolve 2.72 g of hypoxanthine in 80 ml of 1M NaOH and top up to 100 ml
Sterilize using a 0.22 µm filter
Store 1.2 ml aliquots at -20 °C
Incomplete culture medium
Add 2 ml of 45% D-(+)-glucose (w/v), 1.2 ml of 200 mM hypoxanthine, and 1 ml of 10 mg/ml gentamicin to 500 ml of RPMI 1640 Medium, GlutaMAXTM Supplement, HEPES.
Store at 4 °C
5% AlbuMAX II (w/v)
Dissolve 25 g of AlbuMAXTM II Lipid-Rich BSA in 500 ml of RPMI 1640 Medium, GlutaMAXTM Supplement, HEPES while shaking at 37 °C
Sterilize using a 0.22 µm filter and store 37.5 ml aliquots at -20 °C
Heat-inactivated human serum
Pool serum from at least five donors under sterile conditions and heat them to 56 °C in a water bath while shaking occasionally to distribute heat evenly
Keep the serum at 56 °C for at least 1 h
Let it cool to RT. Store 12.5 ml aliquots at -20 °C
Complete culture medium
Add 37.5 ml of 5% Albumax II and 12.5 ml of heat-inactivated human serum to a bottle of incomplete culture medium.
Store at 4 °C
5% D-sorbitol (w/v)
Dissolve 50 g of D-sorbitol in 800 ml of Milli-Q water and top up to 1 L
Sterilize using a 0.22 µm filter
Store at 4 °C
Complete culture medium supplemented with 50 mM GlcNAc
Dissolve 6.1 g of GlcNAc powder in 500 ml of incomplete culture medium
Sterilize using a 0.22 µm filter, then add 37.5 ml of 5% Albumax II and 12.5 ml of heat-inactivated human serum
Store at 4 °C
10 mg/ml Hoechst 33342
Dissolve 100 mg of Hoechst 33342 in 10 ml of Milli-Q water
Sterilize using a 0.22 µm filter
Store 100 µl aliquots at -20 °C
1× PBS with 10 mM glucose
Add 2 ml of 45% D-(+)-glucose (w/v) to 500 ml of 1× PBS.
Store at 4 °C
10 µg/ml Hoechst 33342 in 1× PBS with 10 mM glucose
Add 5 μl of 10 mg/ml Hoechst 33342 to 4,995 µl of 1× PBS with 10 mM glucose immediately prior to use.
Acknowledgments
The authors would like to acknowledge the assistance from Dr. Harpeet Vohra and Mr. Michael Devoy with FACS and from Dr. Phuong Tran, who generated the gABCG2-GFP cell line. We are grateful to the Australian Red Cross for providing human red blood cells and serum. Funding was provided by the Australian Research Council (DP180103212). M.C.R. is supported by the Australian Government Research Training Program Scholarship and The Australian National University. The protocol is based on a method originally described in Ridgway et al. (2020).
Competing interests
The authors declare no financial or non-financial competing interests.
Ethics
The use of human red blood cells and serum was approved by the Human Ethics Committee of the Australian National University, protocol HEC#2017/351.
References
- Fivelman, Q. L., McRobert, L., Sharp, S., Taylor, C. J., Saeed, M., Swales, C. A., Sutherland, C. J. and Baker, D. A. (2007). Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol 154:119-123.
- Lambros, C. and Vanderberg, J. P. (1979). Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65: 418-420.
- Lasonder, E., Rijpma, S. R., van Schaijk, B. C. L., Hoeijmakers, W. A. M., Kensche, P. R., Gresnigt, M. S., Italiaander, A., Vos, M. W., Woestenenk, R., Bousema, T., Mair, G. R., Khan, S. M., Janse, C. J., Bártfai, R. and Sauerwein, R. W. (2016). Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res 44: 6087-6101.
- Maier, A. G. and Rug, M. (2013). In vitro culturing Plasmodium falciparum erythrocytic stages. Methods Mol Biol 923: 3-15.
- Miao, J., Chen, Z., Wang, Z., Shrestha, S., Li, X., Li, R. and Cui, L. (2017). Sex-specific biology of the human malaria parasite revealed from the proteomes of mature male and female gametocytes. Mol Cell Proteomics 16: 537-551.
- Ridgway, M.C., Shea, K.S., Cihalova, D. and Maier, A.G. (2020). Novel method for the separation of male and female gametocytes of the malaria parasite Plasmodium falciparum that enables biological and drug discovery. mSphere 5: e00671-20.
- Tao, D., Ubaida-Mohien, C., Mathias, D. K., King, J. G., Pastrana-Mena, R., Tripathi, A., Goldowitz, I., Graham, D. R., Moss, E., Marti, M. and Dinglasan, R. R. (2014). Sex- partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol Cell Proteomics 13: 2705-2724.
- Tran, P. N., Brown, S. H., Mitchell, T. W., Matuschewski, K., McMillan, P. J., Kirk, K., Dixon, M. W. and Maier, A. G. (2014). A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nat Commun 5(4773): 1-13.
- World Health Organization (2019). World Malaria Report 2019.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ridgway, M. C., Cihalova, D. and Maier, A. G. (2021). Sex-specific Separation of Plasmodium falciparum Gametocyte Populations. Bio-protocol 11(11): e4045. DOI: 10.21769/BioProtoc.4045.
Category
Microbiology > Microbial cell biology > Cell isolation and culture
Microbiology > Antimicrobial assay > Antiparasitic assay
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









