- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Studying the Role of Microglia in Neurodegeneration and Axonal Regeneration in the Murine Visual System
Published: Vol 8, Iss 16, Aug 20, 2018 DOI: 10.21769/BioProtoc.2979 Views: 12978
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Flow Cytometry Analysis of Microglial Phenotypes in the Murine Brain During Aging and Disease
Jillian E. J. Cox [...] Sarah R. Ocañas
Jun 20, 2024 3568 Views

Streamlined Quantification of Microglial Morphology in Mouse Brains Using 3D Immunofluorescence Analysis
Maria Helena de Donato [...] Emma Puighermanal
Feb 20, 2025 3520 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views
Abstract
Microglia reside in the central nervous system (CNS) and are involved in the maintenance of the physiologic state. They constantly survey their environment for pathologic alterations associated with injury or diseases. For decades, researchers have investigated the role of microglia under different pathologic conditions, using approaches aiming to inhibit or eliminate these phagocytic cells. However, until recently, methods have failed to achieve complete depletion. Moreover, treatments often affected other cells, making unequivocal conclusions from these studies difficult. Recently, we have shown that inhibition of colony stimulating factor 1 receptor (CSF1R) by oral treatment with PLX5622 containing chow enables complete depletion of retinal microglia and almost complete microglia depletion in the optic nerve without affecting peripheral macrophages or other cells. Using this approach, we investigated the role of microglia in neuroprotection in the retina and axon regeneration in the injured optic nerve under different conditions. Thus, this efficient, reliable and easy to use protocol presented here will enable researchers to unequivocally study the contribution of microglia on neurodegeneration and axon regeneration. This protocol can be also easily expanded to other paradigms of acute and chronic injury or diseases in the visual system.
Keywords: Axon regenerationBackground
Microglia are resident immune cells of the central nervous system (CNS), which continuously scan their environment for pathologic alterations (Nimmerjahn et al., 2005). Upon detection of such conditions, microglia transform into an activated state and migrate towards the source where they perform different tasks (Kreutzberg, 1996; Davalos et al., 2005). Pathologic alterations, such as neurodegenerative diseases or acute injuries, are associated with neuronal loss and phagocytosis of these dying cells by activated microglia. Therefore, the overall assumption is that microglia are involved in this process (Thanos, 1991; Davalos et al., 2005; Hanisch and Kettenmann, 2007). Besides a possible effect of microglia on dying neurons, microglia are also part of the glial scar, which is formed at the lesion site and impairs axon regeneration in the CNS (Silver, 2004; Kitayama et al., 2011). However, as a clear discrimination of infiltrated blood born macrophages and microglia in the injured nerve was not feasible, the specific role of microglia in glial scar formation and axonal regeneration remained elusive (Silver, 2004; Hanisch and Kettenmann, 2007; Prinz and Priller, 2014; Hilla et al., 2017).
A suitable model to address the role of microglia in CNS degeneration and axonal regeneration is the visual pathway with its retinal ganglion cells (RGCs), whose axons project through the optic nerve (Leibinger et al., 2009; Fischer and Leibinger, 2012). Upon axonal damage, axons normally fail to regenerate (Thanos, 1991; Fischer et al., 2000). The degenerative and regenerative processes, following acute injury, can be easily visualized in retinal wholemounts and optic nerves, respectively as presented in the current protocol. Furthermore, the visual system and particularly RGCs are a suitable model to study neurodegenerative processes in chronic diseases such as multiple sclerosis or glaucoma (Kerrison et al., 1994; Howell et al., 2007; Diekmann and Fischer, 2013; Dietrich et al., 2018). In addition, the optic nerve is widely used as a classical model to study general regenerative failure in the CNS and strategies to facilitate axon growth (Fischer and Leibinger, 2012).
Several studies have been published, suggesting detrimental effects of microglia on RGC survival and axon regeneration whereas other studies indicate a beneficial, neuroprotective role (Thanos et al., 1993; Levkovitch-Verbin et al., 2006; Bosco et al., 2008; Chen et al., 2012; Rice et al., 2015). However, due to a paucity of depletion methods, many studies initially analyzed the contribution of microglia by altering their activation state with pharmacologic approaches (Thanos et al., 1993; Levkovitch-Verbin et al., 2006; Bosco et al., 2008; Jiao et al., 2014). In recent years, genetic methods were developed to achieve microglia depletion (Heppner et al., 2005; Bruttger et al., 2015; Waisman et al., 2015). However, apart from the requirement of transgenic animals, these methods have potential drawbacks such as the development of astrogliosis, secretion of pro-inflammatory cytokines and blood-brain-barrier damage, which complicate data interpretation. Only recently, pharmacologic colony stimulating factor 1 receptor (CSF1R) inhibitors PLX3397 and PLX5622 have been developed, which allow near complete depletion of microglia in the CNS independent of the animals genetic background (Elmore et al., 2014; Spangenberg et al., 2016; Hilla et al., 2017). In fact, we have recently demonstrated that an oral PLX5622 treatment for 21 days leads to a complete depletion of microglia in the retina and almost a total elimination in the optic nerve (Hilla et al., 2017). In accordance with previous studies, this approach selectively induces apoptosis of microglia by CSF1R inhibition, while other phagocytic cells such as peripheral macrophages are not affected (Elmore et al., 2014; Hilla et al., 2017). Although some studies suggested that this treatment causes minor astrocyte activation in the brain, we did not find any in the optic nerve or retina (Elmore et al., 2014; Hilla et al., 2017). Furthermore, as the inhibitor is orally bioavailable and capable of passing the blood brain barrier, PLX5622 can be formulated in standard rodent chow preventing the necessity of regular injections. Thus, this approach allows scientists to unequivocally address the role of microglia for different pathologic CNS paradigms, such as injury or chronic diseases, not only in the visual system, but also in other prominent CNS tissues such as brain and spinal cord. Using this approach in the visual pathway, we found that microglia neither affect the degeneration process of RGCs nor their capability to regenerate injured axons into the optic nerve upon an acute injury (Hilla et al., 2017). The current protocol describes the method for depleting microglia in the visual system and how effects on acute degeneration of RGCs and axon regeneration in the optic nerve can be quantified to identify the contribution of microglia in this context. This protocol can be easily expanded to investigate the involvement of microglia in other paradigms of acute and chronic injury or diseases in the visual system.
Materials and Reagents
- Glass capillary (World Precision Instruments, catalog number: 1B100F-6 )
- Glass slide (VWR, catalog number: 631-0108 )
- Surgical filament (Ethicon, catalog number: EH7790 )
- Scalpel blades (B. Braun Melsungen, catalog number: BB511 )
- Swabs (Lohmann & Rauscher, catalog number: 13356 )
- Nitrocellulose membrane (GE Healthcare, catalog number: RPN303E )
- Male and female mice aged between 6-10 weeks
- CSF1R-inhibitor PLX5622 (PLX, Plexxikon, commercially not available)
- AIN-76A standard rodent chow (Research Diets)
- Ketamine (Grovet, Alfasan, Medistar, catalog number: 10002 )
- Xylazine (Bayer, Rompun® 2% Injection 25 ml)
- Cholera toxin subunit B, Alexa FluorTM 555 (CTB) (Thermo Fisher Scientific, InvitrogenTM, catalog number: C22843 )
- Phosphate buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 14190-094 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 252549 )
- Sucrose (Sigma-Aldrich, catalog number: S9378 )
- KP-CryoCompound (KLINIPATH, catalog number: 1620-C )
- Double distilled H2O (Carl Roth, catalog number: 3478.2 )
- Eye ointment (DR. WINZER, Gent-Ophtal®)
- Methanol (Sigma-Aldrich, catalog number: 179957 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- Iba1 antibody (Wako Pure Chemical Industries, catalog number: 019-19741 , RRID: AB_839504, LOT-specific concentration: 1 mg/ml)
- βIII-tubulin antibody (BioLegend, catalog number: 801202 , RRID: AB_2313773, LOT-specific concentration: 1 mg/ml)
- RNA-binding protein with multiple splicing (RBPMS) antibody (Abcam, catalog number: ab194213 , LOT-specific concentration: 0.25 mg/ml)
- Secondary antibodies
- Donkey serum (Bio-Rad Laboratories, catalog number: C06SB )
- Bovine serum albumin (Sigma-Aldrich, catalog number: A3294 )
- Tween 20 (Sigma-Aldrich, catalog number: P1379 )
- Paraformaldehyde (PFA) solution (see Recipes)
- Sucrose solution (see Recipes)
- Blocking solution (sections) (see Recipes)
- Blocking solution (wholemount) (see Recipes)
Equipment
- Jeweler's forceps (Fine Science Tools, catalog number: 11254-20 )
- Capsulotomy scissor (Hermle, catalog number: 564 )
- Mouse head holder (KOPF INSTRUMENTS, catalog number: 921-E )
- Cryostat (Leica Biosystems, model: CM3050 S )
- Fluorescence microscope (ZEISS, model: Axio Observer.D1 )
- Confocal laser scanning microscope (Leica Microsystems, model: Leica TCS SP8 )
- Binocular (ZEISS, model: Stemi DV4 SPOT )
- Cold light source (SCHOTT, model: KL 1600 LED )
Software
- Excel 2016 (Microsoft)
- SigmaStat 3.1 (Systat)
- Photoshop CS6 (Adobe)
Procedure
- Microglia depletion
Notes:- The CSF1R-inhibitor PLX5622 was kindly provided by Plexxikon Inc. formulated in standard AIN-76A rodent chow at 1,200 mg/kg.
- All experimental procedures should be approved by the local animal care committee.
- Keep male and female mice aged between 6-10 weeks on a 12 h light/dark cycle with ad libitum access to food and water.
- Exchange rodent chow with PLX5622-containing chow and provide mice ad libitum access for at least 21 days to assure maximum depletion efficiency (Figures 1A, 2A and 3D). Respective control mice receive standard AIN-76A rodent chow without the CSF1R-inhibitor PLX5622.
- After 21 days, respective tissue can be harvested to analyze depletion efficiency (Figures 3A and 3B). Otherwise, keep mice on PLX5622-chow for the duration of the experiment to assure continuous microglia depletion. Mice do not show any observable phenotypes when treated with the inhibitor for at least up to 8 weeks.
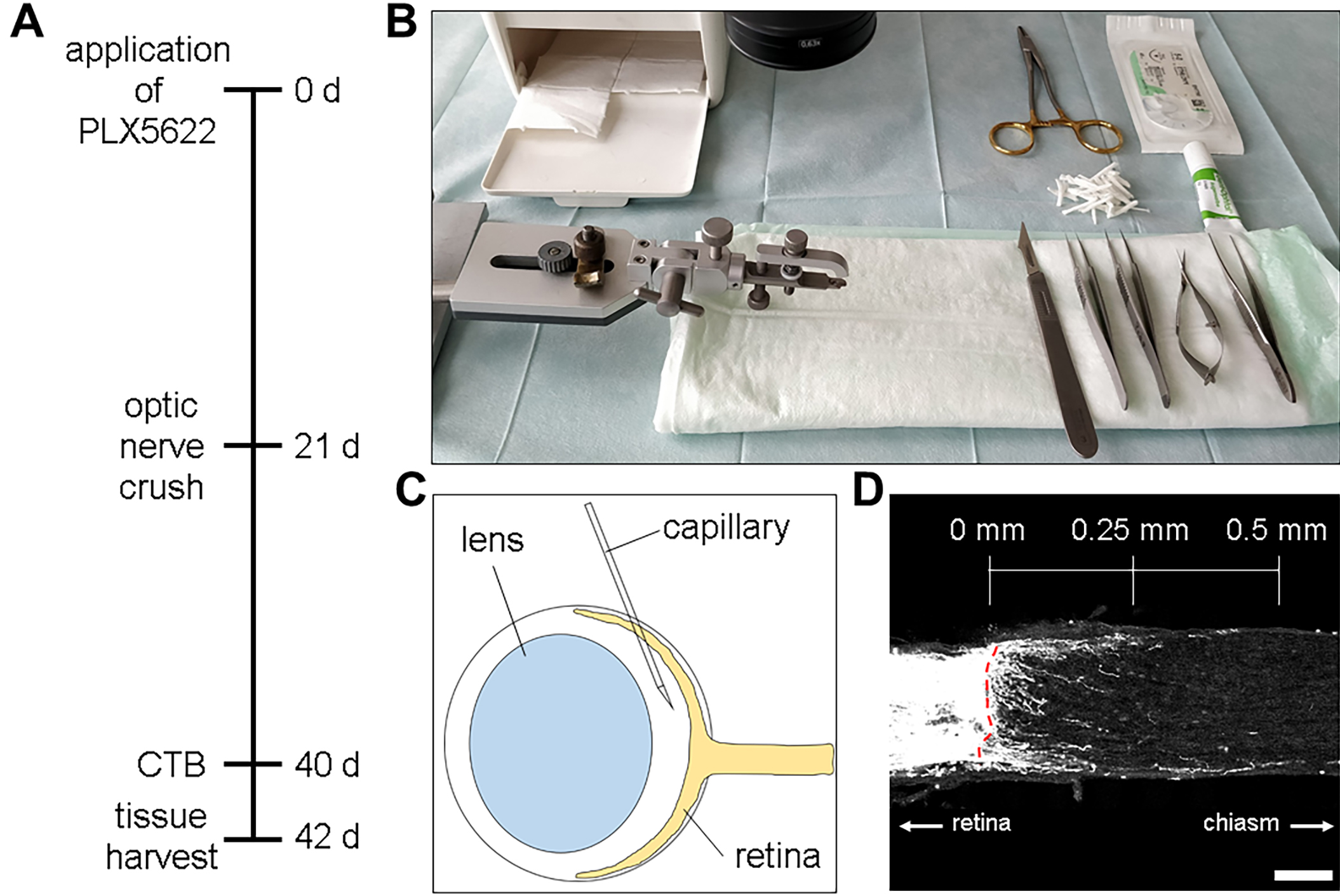
Figure 1. Experimental setup for optic nerve injury and axonal regeneration. A. Timeline of the experiment starting by administration of PLX5622 (CSF1R-inhibitor). Afterwards mice were subjected to surgery, and two days prior to sacrifice retinal ganglion cell axons were anterogradely labeled by intravitreal cholera toxin subunit B (CTB) injection. B. Setup for the optic nerve crush surgery. The workplace, as well as the instruments should be sterile. C. Injection scheme for intravitreal CTB injections. A pulled glass capillary containing CTB is inserted retrolentally at an approximately 45°-50° angle into the vitreous body without touching or damaging the ocular lens. D. Confocal image of an optic nerve 21 d after injury showing some CTB-positive regenerating axons crossing the lesion site (red dotted line). The scale above shows exemplary distances where regenerated axons can be counted and divided by the width of the optic nerve at this distance to extrapolate the number of regenerating axons per width of the optic nerve. Scale bar = 100 μm.
- Intraorbital optic nerve crush
- Make sure that the workplace and the instruments are kept clean and sterile throughout the surgical procedures to minimize the risk of infection (Figure 1B).
- Anesthetize mice by intraperitoneal injections of xylazine (16 mg/kg) and ketamine (120 mg/kg).
- Check for reflexes (paw withdrawal and corneal reflex) to assure full anesthesia before proceeding with the surgery.
- Shave the head using e.g., an electric shaver and perform a sagittal midline incision on the mouse head before fixing it in a mouse head holder.
- Fix the left side of the skin with surgical suture to allow undisturbed access to the orbit throughout the surgery.
- Use a #11 scalpel to cut along the orbit cavity to get access to the intraorbital space.
- In case of excessive bleeding use swabs to absorb the blood, which otherwise would impair your sight.
- Gently move the lacrimal gland aside using jeweler’s forceps leaving the tissue intact to get better access to the eye.
- Transect the superior oblique, superior rectus and the retractor bulbi using a capsulotomy scissor to gain access to the optic nerve. Take care to avoid accidental optic nerve injury.
- Once the optic nerve is accessible, use jeweler’s forceps and crush the optic nerve approximately 1 mm behind the eyeball with consistent pressure for 10 sec. Take care to crush over the entire width as otherwise the injury might remain incomplete (Fischer et al., 2017).
- Position the lacrimal gland back and suture the wound using sterile surgical sutures.
- Apply eye ointment to prevent infection and desiccation of the eyes.
- Afterwards proceed either with the surgery to study axonal regeneration (variant 1) or prepare retinal wholemounts for the analysis of RGC survival (variant 2).
- Variant 1: Anterograde tracing for the analysis of neuronal regeneration
- Nineteen days after optic nerve crush repeat Steps B1-B3 to assure optimal surgical conditions before intravitreally injecting 2 μl of the anterograde tracer CTB (5 μg/μl).
- Fix the eye with jeweler’s forceps and gently pinch through the cornea with a pulled glass capillary to drain approximately 2 μl of aqueous humor from the anterior chamber.
- Fill a pulled glass capillary with 2 μl of the concentrated CTB-solution.
- Gently pull out the eye to gain better access to the retrobulbar surface using a pair of jeweler’s forceps. Access the eye retrolentally at a 45°-50° angle to avoid accidental lens injury and insert the glass capillary into the vitreous body (Figure 1C). After injection, quickly remove the needle from the eyeball.
- Two days later, anesthetize the mouse as described before and intracardially perfuse the mouse with ice-cold PFA solution (Hilla et al., 2017; Leibinger et al., 2017).
- Prepare the optic nerve, embed it in embedding medium for frozen tissue and perform 14 μm cryosections as described previously (Hilla et al., 2017; Leibinger et al., 2017).
- These sections can now be directly analyzed under a fluorescence microscope (Figure 1D).
- Variant 2: Preparing retinal wholemount for the analysis of neurodegeneration
- To analyze RGC-degeneration at a given time point after optic nerve crush, euthanize the mouse according to local animal care guidelines. Perfusion with PFA solution is not required.
- Enucleate the eye and place it in a Petri dish filled with PBS. Remove the cornea by cutting along the ora serrata before removing the lens.
- Make four radial incisions in the eye cup approximately 90° apart from each other (Figure 2B) leaving the optic disk intact.
- Detach the retina from the sclera by cutting the connection between optic disc and sclera and transfer the free-floating retina with vitreous body onto a nitrocellulose membrane with the ganglion cell layer facing up.
- Transfer the membrane onto a dry tissue to let the retina suck onto the membrane. Return into PBS filled Petri dish and repeat this step for at least four times.
- Carefully detach the vitreous body from the retina using jeweler’s forceps while trying to avoid injury to the retina.
- Incubate for 30 min in PFA solution before proceeding to the staining protocol.
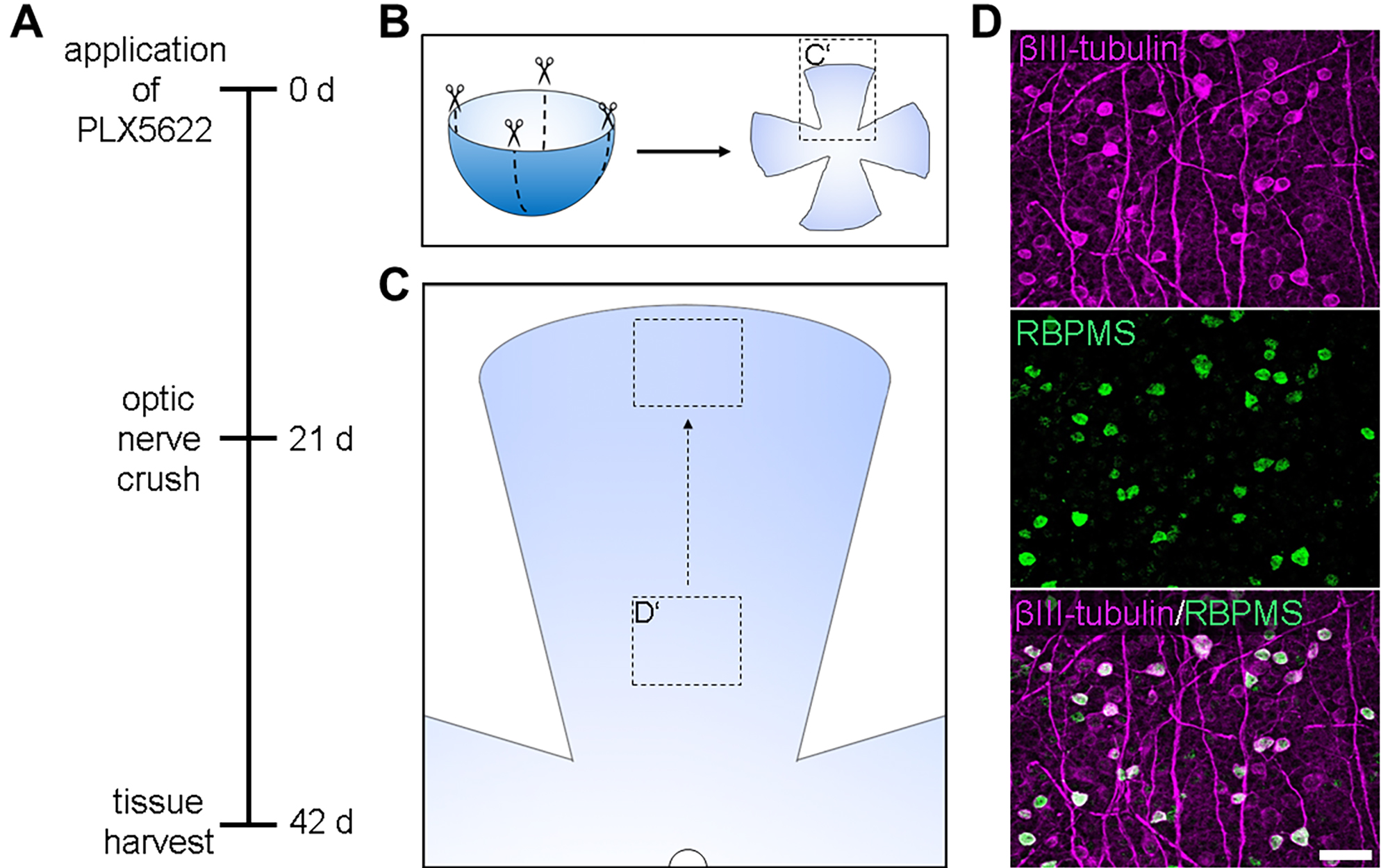
Figure 2. Retinal wholemount preparation and analysis of neuronal survival upon optic nerve injury. A. Timeline of the experiment starting by administration of PLX5622 (CSF1R-inhibitor). Afterwards, mice were subjected to surgery and 21 days later sacrificed to quantify retinal ganglion cell survival after optic nerve injury. B. Schematic drawing of an enucleated eye without the cornea and lens shows the positions of the radial incisions in the retina to achieve a cloverleaf-shape. C. Magnification of a single retinal quadrant explains image acquisition to quantify neuronal survival. Image recording starts approximately one millimeter away from the papilla (semicircle) and progresses to the outer retina without overlap in between the pictures. D. Exemplary confocal image of the ganglion cell layer of a retina 21 days after optic nerve injury. RBPMS-staining (green) shows RGC somata, whereas βIII-tubulin (magenta) additionally highlights RGC axons. The merged picture at the bottom indicates a high overlap between the two RGC markers. Scale bar = 50 μm.
- Retinal wholemount staining for the quantification of RGC survival
- To analyze RGC survival, retinal wholemounts need to be stained against a suitable neuronal marker.
- Permeabilize the retina in 2% Triton X-100 in PBS in a humified chamber for 1 h at room temperature.
- Block the tissue using the blocking solution for wholemounts (see Recipes) in a humified chamber for 1 h at room temperature.
- Incubate the retinae with the primary antibody (either βIII-tubulin [1:1,000] or RNA-binding protein with multiple splicing [RBPMS, 1:500]) diluted in blocking solution in a humified chamber overnight at 4 °C.
- Wash off the nonspecifically bound primary antibodies with PBS three times each for 10 min.
- Incubate retinae with secondary antibodies (diluted 1:1,000 in blocking solution) in a humified chamber for 1 h at room temperature.
- Wash off the nonspecifically bound secondary antibody with PBS three times each for 10 min.
- To visualize Iba1-positive microglia and depletion efficiency one may co-stain the retinae with 1:1,000 Iba1 primary antibody diluted in blocking solution according to the protocol described (Video 1 and Video 2).Video 1. Preparation of a retinal wholemount. This video shows the preparation of a retinal wholemount starting with the already enucleated eye in a PBS filled Petri dish. The detailed protocol is described in Procedure D. (All experimental procedures have been approved by the local animal care committee (LANUV Recklinghausen)).Video 2. Depletion efficiency in the retina. This video shows a z-scan performed on a confocal laser scanning microscope through representative retinae, which were either treated with the CSF1R-inhibitor PLX5622 (right panel) or control chow (left panel) for 21 d. Microglia (Iba1, red) are only detectable in the control retina. There, they are evenly distributed throughout the retina and mainly localized in the ganglion cell layer (GCL) as well as inner (IPL) and outer plexiform layer (OPL). PLX5622-treated retinae show no microglia in any layer. Staining with the neuronal marker βIII-tubulin (green) shows retinal ganglion cells in the GCL and binding of the secondary antibody to IgG in the non-perfused retina shows positively stained blood vessels, thereby indicating the IPL and OPL.
- Optic nerve staining to analyze depletion efficiency
- To analyze depletion efficiency, respective tissue may be immunohistochemically stained against microglia/macrophage markers such as Iba1.
- Fix respective sections with 100% methanol for 10 min at room temperature.
- Block epitopes using the blocking solution for sections (see Recipes) in a humidified chamber for 30 min at room temperature.
- Incubate the sections with the Iba1 primary antibody (diluted 1:1,000 in blocking solution) in a humidified chamber overnight at 4 °C.
- Wash off the nonspecifically bound primary antibody three times each for 10 min with PBS.
- Incubate sections with the secondary antibody (diluted 1:1,000 in blocking solution) in a humidified chamber for 1 h at room temperature.
- Wash off the nonspecifically bound secondary antibody three times each for 10 min with PBS.
- Note that an optic nerve crush leads to an infiltration of blood-borne macrophages at the lesion site (Figure 3C), which can also be detected by most antibodies for microglia.
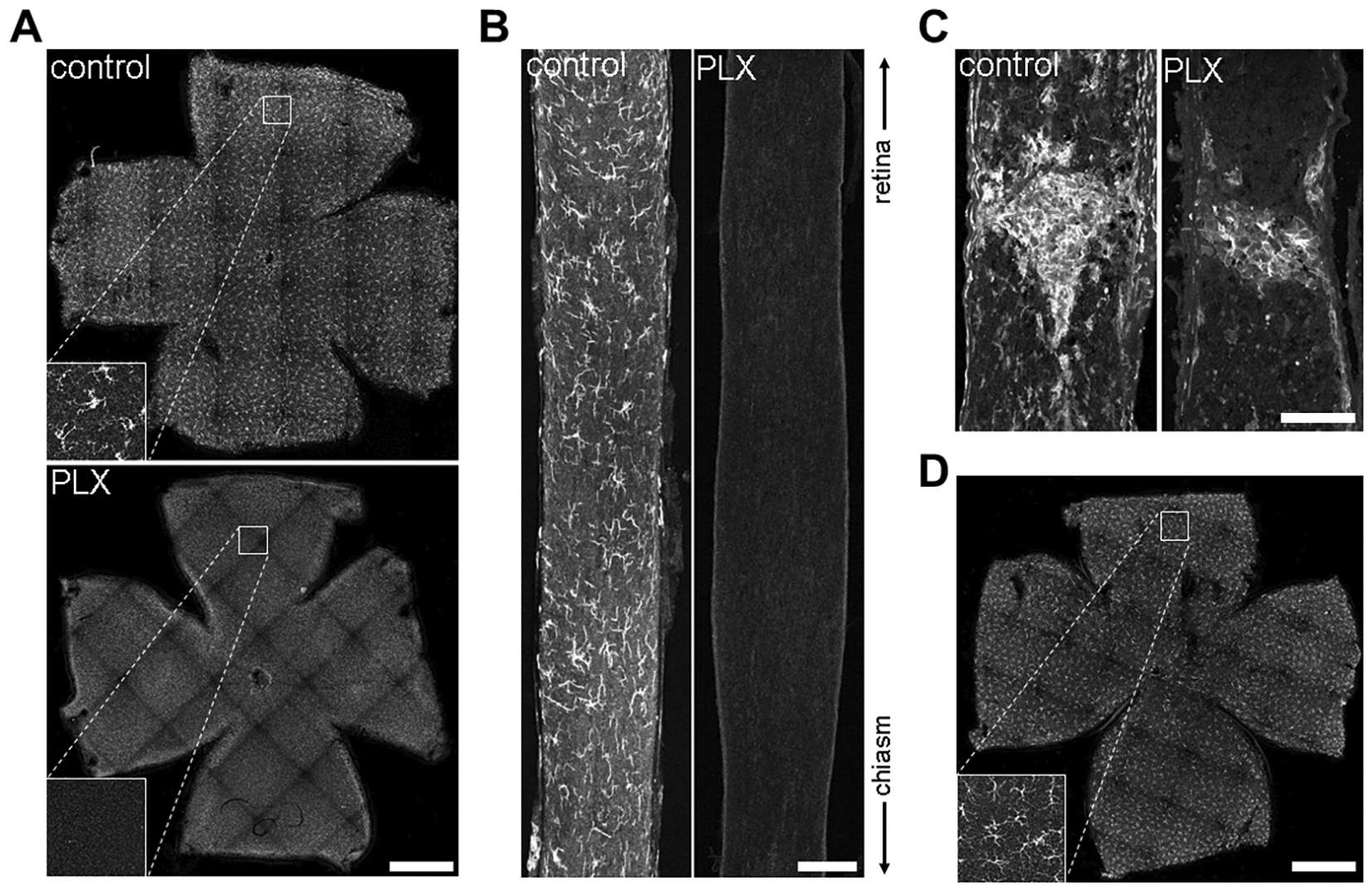
Figure 3. Depletion efficiency of PLX on Iba1-positive microglia in CNS tissue. A. Maximum intensity projected confocal image of naïve retina wholemount from a control mouse shows Iba1-positive microglia throughout the retina and its different layers. Magnification at the bottom left displays microglia in the ganglion cell layer. In contrast, PLX-treated mice lack microglia throughout all retinal layers resulting in a 100% depletion efficiency (also see Video 2). Scale bar = 1 mm. B. Iba1-positive microglia are equally distributed throughout the naïve optic nerve whereas PLX-application caused massive microglia depletion. In contrast to the retina, some microglia were occasionally visible in the optic nerves (Hilla et al., 2017). Scale bar = 100 μm. C. Twenty-one days after optic nerve crush Iba1-positive cells accumulate at the lesion site in control mice as well as microglia-depleted mice (PLX) whereas the rest of PLX optic nerves remain devoid of signal. These remaining cells are most likely infiltrated hematopoietic macrophages as analyzed elsewhere (Hilla et al., 2017). Scale bar = 100 μm. D. Confocal images show that deprivation of PLX caused Iba1-positive microglia to repopulate the retina underlining the necessity of continuous PLX-treatment for efficient microglia depletion. The box in the bottom left shows a magnification of the ganglion cell layer of the indicated area in the overview. Scale bar = 1 mm.
Data analysis
- Variant 1: RGC axon regeneration
- Take images of the entire optic nerve section. Start at the lesion site and progress towards the chiasm.
- Arrange and merge single images to a complete optic nerve using a suitable image processing program (e.g., Photoshop).
- Create a ruler marking the distances to the lesion site you wish to analyze. Overlay it with the optic nerve and use the lesion site as a starting point (Figure 1D).
- Quantify axons that cross the defined distances and measure the width of the optic nerve at this distance.
- Divide number of regenerating axons crossing the indicated distance by the width of the optic nerve:
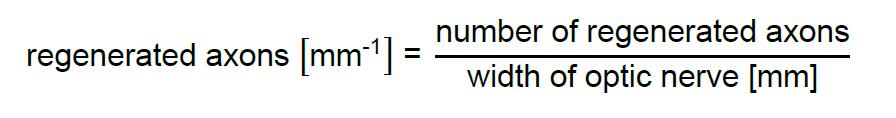
- Only include sections from complete optic nerves where the tissue between the lesion site and 1 mm after the longest regenerated axon is not disrupted.
- To analyze data between two different groups, use a two-way ANOVA with the individual group as the first variable and the distance to the lesion site as the second variable.
- A suitable post-hoc test provides information about significant variations between groups in general and, additionally, significant differences for single distances to the lesion site.
- Variant 2: RGC survival
- Acquire images of the surviving RGCs by starting approximately 1 mm distal to the papilla with a 400x objective (Figure 2C).
- Proceed towards the outer retina and avoid taking images with overlapping areas. Depending on the preparation, four to six images can be recorded (Figures 2C and 2D).
- Repeat the above steps (Data analysis, Steps B1-B2) with the other three quadrants.
- Using suitable software, quantify RGC numbers per image and divide them by the area of the recorded field to obtain RGC density:
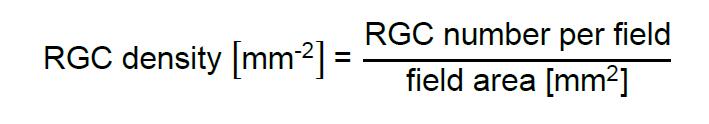
- As the RGC density decreases towards the outer retina, calculate the mean density value for one quadrant and then the mean over all four quadrants.
- To analyze data between two groups, use Student’s t-test or one-way ANOVA with a suitable post-hoc test for more than two groups.
Notes
- To avoid degradation of PLX5622, it is essential to keep chow dry and add only small portions (ca. 20 g/animal) in the cage.
- Depletion efficiency in the retina is 100% after a diet on PLX5622 for 14-21 days and highly reproducible. However, in other CNS tissues, some microglia may remain. If also other CNS tissues are analyzed for depletion efficiency, the retina may be used as a positive control.
- In case of incomplete anesthesia, mice should receive additional isoflurane anesthesia at 1% isoflurane/99% O2 for the duration of the surgery.
- RGC axons normally display only a very limited capacity of regeneration, however it is possible to improve the regenerative outcome by manipulating the regenerative state of RGCs for example by inflammatory stimulation or providing cytokines (Fischer et al., 2000; Leibinger et al., 2016).
- In general, high exposure times might be needed to clearly visualize single regenerating CTB-positive axons in the distal part of the optic nerve. As the number of axons is considerably higher at the lesion site, high exposure times in a widefield microscope might cause the fluorescent signal to scatter into the distal part of the optic nerve. This might thereby shift the assumed location of the lesion site into the distal part of the optic nerve. As this would result in incorrect quantification of regenerated axons at different distances to the lesion site, an image of the lesion site at a lower exposure time might be needed to clearly identify lesion site location.
- More detailed insights into intraorbital optic nerve crush and intravitreal injections can be found elsewhere (Chiu et al., 2007; Magharious et al., 2011).
Recipes
- Paraformaldehyde (PFA) solution
108 ml Paraformaldehyde (37%)
100 ml 10x PBS
ad 1,000 ml ddH2O - Sucrose solution
30 g Sucrose
ad 100 ml ddH2O - Blocking solution (sections)
0.2 g BSA
0.5 ml serum from secondary antibody host (e.g., donkey serum)
5 μl Tween 20
ad 10 ml PBS - Blocking solution (wholemount)
0.2 g BSA
1 ml serum from secondary antibody host (e.g., donkey serum)
5 μl Tween 20
ad 10 ml PBS
Acknowledgments
This work was supported by the German Research Foundation. We thank Plexxikon Inc. for providing PLX5622 chow. Furthermore, we thank Anastasia Andreadaki for technical support. This protocol was adapted from previous work (Elmore et al., 2014; Hilla et al., 2017).
Competing interests
The authors declare no conflict of interest.
Ethics
All experimental procedures have been approved by the local animal care committee (LANUV Recklinghausen).
References
- Bosco, A., Inman, D. M., Steele, M. R., Wu, G., Soto, I., Marsh-Armstrong, N., Hubbard, W. C., Calkins, D. J., Horner, P. J. and Vetter, M. L. (2008). Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 49(4): 1437-1446.
- Bruttger, J., Karram, K., Wortge, S., Regen, T., Marini, F., Hoppmann, N., Klein, M., Blank, T., Yona, S., Wolf, Y., Mack, M., Pinteaux, E., Muller, W., Zipp, F., Binder, H., Bopp, T., Prinz, M., Jung, S. and Waisman, A. (2015). Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43(1): 92-106.
- Chen, Z., Jalabi, W., Shpargel, K. B., Farabaugh, K. T., Dutta, R., Yin, X., Kidd, G. J., Bergmann, C. C., Stohlman, S. A. and Trapp, B. D. (2012). Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci 32(34): 11706-11715.
- Chiu, K., Chang, R. C. and So, K. F. (2007). Intravitreous injection for establishing ocular diseases model. J Vis Exp (8): 313.
- Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., Littman, D. R., Dustin, M. L. and Gan, W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8(6): 752-758.
- Diekmann, H. and Fischer, D. (2013). Glaucoma and optic nerve repair. Cell Tissue Res 353(2): 327-337.
- Dietrich, M., Helling, N., Hilla, A., Heskamp, A., Issberner, A., Hildebrandt, T., Kohne, Z., Kury, P., Berndt, C., Aktas, O., Fischer, D., Hartung, H. P. and Albrecht, P. (2018). Early alpha-lipoic acid therapy protects from degeneration of the inner retinal layers and vision loss in an experimental autoimmune encephalomyelitis-optic neuritis model. J Neuroinflammation 15(1): 71.
- Elmore, M. R., Najafi, A. R., Koike, M. A., Dagher, N. N., Spangenberg, E. E., Rice, R. A., Kitazawa, M., Matusow, B., Nguyen, H., West, B. L. and Green, K. N. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82(2): 380-397.
- Fischer, D. and Leibinger, M. (2012). Promoting optic nerve regeneration. Prog Retin Eye Res 31(6): 688-701.
- Fischer, D., Harvey, A. R., Pernet, V., Lemmon, V. P. and Park, K. K. (2017). Optic nerve regeneration in mammals: Regenerated or spared axons? Exp Neurol 296: 83-88.
- Fischer, D., Pavlidis, M. and Thanos, S. (2000). Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci 41(12): 3943-3954.
- Hanisch, U. K. and Kettenmann, H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11): 1387-1394.
- Heppner, F. L., Greter, M., Marino, D., Falsig, J., Raivich, G., Hovelmeyer, N., Waisman, A., Rulicke, T., Prinz, M., Priller, J., Becher, B. and Aguzzi, A. (2005). Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11(2): 146-152.
- Hilla, A. M., Diekmann, H. and Fischer, D. (2017). Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci 37(25): 6113-6124.
- Howell, G. R., Libby, R. T., Jakobs, T. C., Smith, R. S., Phalan, F. C., Barter, J. W., Barbay, J. M., Marchant, J. K., Mahesh, N., Porciatti, V., Whitmore, A. V., Masland, R. H. and John, S. W. (2007). Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol 179(7): 1523-1537.
- Jiao, X., Peng, Y. and Yang, L. (2014). Minocycline protects retinal ganglion cells after optic nerve crush injury in mice by delaying autophagy and upregulating nuclear factor-κB2. Chin Med J (Engl) 127(9): 1749-1754.
- Kerrison, J. B., Flynn, T. and Green, W. R. (1994). Retinal pathologic changes in multiple sclerosis. Retina 14(5): 445-451.
- Kitayama, M., Ueno, M., Itakura, T. and Yamashita, T. (2011). Activated microglia inhibit axonal growth through RGMa. PLoS One 6(9): e25234.
- Kreutzberg, G. W. (1996). Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8): 312-318.
- Leibinger, M., Andreadaki, A., Gobrecht, P., Levin, E., Diekmann, H. and Fischer, D. (2016). Boosting central nervous system axon regeneration by circumventing limitations of natural cytokine signaling. Mol Ther 24(10): 1712-1725.
- Leibinger, M., Andreadaki, A., Golla, R., Levin, E., Hilla, A. M., Diekmann, H. and Fischer, D. (2017). Boosting CNS axon regeneration by harnessing antagonistic effects of GSK3 activity. Proc Natl Acad Sci U S A 114(27): E5454-E5463.
- Leibinger, M., Muller, A., Andreadaki, A., Hauk, T. G., Kirsch, M. and Fischer, D. (2009). Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci 29(45): 14334-14341.
- Levkovitch-Verbin, H., Kalev-Landoy, M., Habot-Wilner, Z. and Melamed, S. (2006). Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol 124(4): 520-526.
- Magharious, M. M., D'Onofrio, P. M. and Koeberle, P. D. (2011). Optic nerve transection: a model of adult neuron apoptosis in the central nervous system. J Vis Exp(51): 2241.
- Nimmerjahn, A., Kirchhoff, F. and Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726): 1314-1318.
- Prinz, M. and Priller, J. (2014). Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15(5): 300-312.
- Rice, R. A., Spangenberg, E. E., Yamate-Morgan, H., Lee, R. J., Arora, R. P., Hernandez, M. X., Tenner, A. J., West, B. L. and Green, K. N. (2015). Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J Neurosci 35(27): 9977-9989.
- Silver, J. and Miller, J. H. (2004). Regeneration beyond the glial scar. Nat Rev Neurosci 5(2): 146-156.
- Spangenberg, E. E., Lee, R. J., Najafi, A. R., Rice, R. A., Elmore, M. R., Blurton-Jones, M., West, B. L. and Green, K. N. (2016). Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-β pathology. Brain 139(Pt 4): 1265-1281.
- Thanos, S. (1991). The relationship of microglial cells to dying neurons during natural neuronal cell death and axotomy-induced degeneration of the rat retina. Eur J Neurosci 3(12): 1189-1207.
- Thanos, S., Mey, J. and Wild, M. (1993). Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci 13(2): 455-466.
- Waisman, A., Ginhoux, F., Greter, M. and Bruttger, J. (2015). Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol 36(10): 625-636.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hilla, A. M. and Fischer, D. (2018). Studying the Role of Microglia in Neurodegeneration and Axonal Regeneration in the Murine Visual System. Bio-protocol 8(16): e2979. DOI: 10.21769/BioProtoc.2979.
- Hilla, A. M., Diekmann, H. and Fischer, D. (2017). Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci 37(25): 6113-6124.
Category
Neuroscience > Nervous system disorders > Animal model
Neuroscience > Cellular mechanisms > Microglia
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link