- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Microvascular Endothelial Cells
Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2886 Views: 16144
Reviewed by: Ivan ZanoniLokesh KalekarSalma Merchant

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2233 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1460 Views
Abstract
The vascular endothelium is essential to normal vascular homeostasis. Its dysfunction participates in various cardiovascular disorders. Murine endothelial cell culture is an important tool for cardiovascular disease research. This protocol demonstrates a quick, efficient method for the isolation of microvascular endothelial cells from murine tissues without any special equipment. To isolate endothelial cells, the lung or heart were mechanically minced and enzymatically digested with collagenase and trypsin. The single cell suspension obtained was then incubated with an anti-CD31, anti-CD105 antibody and with biotinylated isolectin B-4. The endothelial cells were harvested using magnetic bead separation with rat anti-mouse Ig- and streptavidin-conjugated microbeads. Endothelial cells were expanded and collected for subsequent analyses. The morphological and phenotypic features of these cultures remained stable over 10 passages in culture. There was no overgrowth of contaminating cells of non-endothelial origin at any stage.
Keywords: Primary cultureBackground
Microvascular endothelial cells play a central role in the development of immune responses by regulating leukocyte recirculation and as antigen presenting cells to T lymphocytes. The wellbeing of the endothelium is essential to vascular homeostasis. The dysfunctional endothelium participates in various cardiovascular disorders, including atherosclerosis, vasculitis and ischemia/reperfusion injuries (Cid et al., 2004; Wang et al., 2007). Therefore, in vitro endothelial cell cultures are important tools for studying vascular physiology and disease pathology. However, the isolation of primary murine endothelial cells is considered particularly difficult because most protocols described have involved the perfusion of organs or large vessels with digesting enzymes and time-consuming purification process (Gumkowski et al., 1987).
The purpose of this protocol is to provide a simple method to isolate and expand endothelial cells from the lung/heart without using any special equipment. Using this method, we previously complemented in vivo studies demonstrating the importance of CD31 signaling in endothelial cells cytoprotection (Cheung et al., 2015).
Materials and Reagents
- Materials
- Pipette tips
- Multiwell plate (cell culture grade) (Greiner Bio One International, catalog number: 662160 )
- 50 ml centrifuge tubes (cell culture grade) (Greiner Bio One International, catalog number: 210261 )
- 10 ml disposable pipette (Greiner Bio One International, catalog number: 607160 )
- Cell strainers (100 µm, Corning, catalog number: 352360 ; 70 µm, Corning, catalog number: 352350 )
- Scalpel
- miniMACS separation unit (Miltenyi Biotec, catalog number: 130-042-102 )
Note: Magnetic cell sorting of labeled EC was performed using a miniMACS separation unit (Miltenyi Biotec, Bisley, Surrey, UK) including two magnets. Labeled cells were incubated with MACS magnetic goat anti-rat IgG (H+L) (Miltenyi Biotec) MicroBeads and streptavidin (Miltenyi Biotec) MicroBeads and then separated using a high gradient magnetic separation column (MS+ columns, Miltenyi Biotec) placed on the separation unit, according to the manufacturer’s instructions. - High gradient magnetic separation column (MS+ columns) (Miltenyi Biotec, catalog number: 130-042-201 )
- Pipette tips
- Animals
Mice (Balb/c, age 6 weeks up to 1 year from Charles River, UK or the in-house breeding facility) - Reagents
- Ice
- Isoflurane
- Phosphate buffered saline solution (PBS, Gibco)
- Collagenase type II (Thermo Fisher Scientific, GibcoTM, catalog number: 17101015 )
- EC media
- DNaseI solution
- 0.125% trypsin in 0.2% EDTA (Life Technologies)
- Dako mounting media (Dako)
- MicroBeads and streptavidin (Miltenyi Biotec, catalog number: 130-048-101 )
- Antibodies
- Biotinylated isolectin B4 (purchased from Vector Laboratories, Peterborough, UK)
Note: The anti-CD40 mAb 3/23 (rat IgG2a) (Van Den Berg et al., 1996) was a kind gift from Dr. G. Klaus (National Institute for Medical Research, London, UK). - Rat IgG2a (clone R35-95, BD, PharmingenTM, catalog number: 553927 )
- Hamster Igs (BD, CompBeadTM, catalog number: 552845 )
- Mouse IgG1 (TdT Cocktail Control, Harlan Sera-Lab, Oxon, UK, Thermo Fisher Scientific, catalog number: 31903 )
Note: The above mAbs 16b and 16c were used as isotype-matched control antibodies in staining experiments: rat IgG2a (clone R35-95); hamster Igs. To block Fc receptors, mouse IgG2a and mouse IgG1 (TdT Cocktail Control, Harlan Sera-Lab, Oxon, UK) were used. - MACS magnetic goat anti-rat IgG (H+L) (Miltenyi Biotec, catalog number: 130-048-501 )
- Rat IgG2b anti-mouse CD16/CD32 monoclonal antibody (BD, catalog number: 553141 )
- Secondary antibody conjugated rhodamine red-X (Molecular Probes)
- Biotinylated isolectin B4 (purchased from Vector Laboratories, Peterborough, UK)
- FACS
Note: The following antibodies were purchased from Pharmingen (La Jolla, CA). - Immuno Fluorescence Staining
PECAM-1(MEC 13.3) (Santa Cruz Biotechnology, catalog number: sc-18916 ) - Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific, GibcoTM, catalog number: 41966-052 )
- Glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030 )
- 10,000 U/ml Penicillin-Streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Sodium pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11360039 )
- HEPES (Thermo Fisher Scientific, GibcoTM, catalog number: 15630056 )
- 1% non-essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number: 11140050 )
- 2-mercaptoethanol (Thermo Fisher Scientific, GibcoTM, catalog number: 31350010 )
- Heat-inactivated fetal calf serum (FCS; Globepharm, Esher, UK)
- EC growth supplement (Sigma-Aldrich, catalog number: E0760 )
- 2% gelatin (type B from bovine skin, Sigma-Aldrich, catalog number: G7765 ) coated tissue culture flasks (Nunc, Life Technologies, Paisley, UK)
- Working medium (see Recipes)
- Ice
Equipment
- Pipettes
- Sterile beakers 100-150 ml (sterilize at 180 °C)
- Laminar flow work bench
- Tweezers (sterilize at 180 °C)
- Scissors (sterilize at 180 °C)
- Shaker
- Water bath
- Centrifuge (Hettich Instruments, model: UNIVERSAL 320 R )
- Fixed-angle rotor (Hettich Instruments, catalog number: 1620A )
- EPICS Profile Cytometer (Coulter Electronics, Luton, UK)
- Fluorescence microscopy (Zeiss epi-fluorescent microscope)
Procedure
Note: For the EC purification described, tissue from no more than one to two animals is required (see Figure 1 for diagram).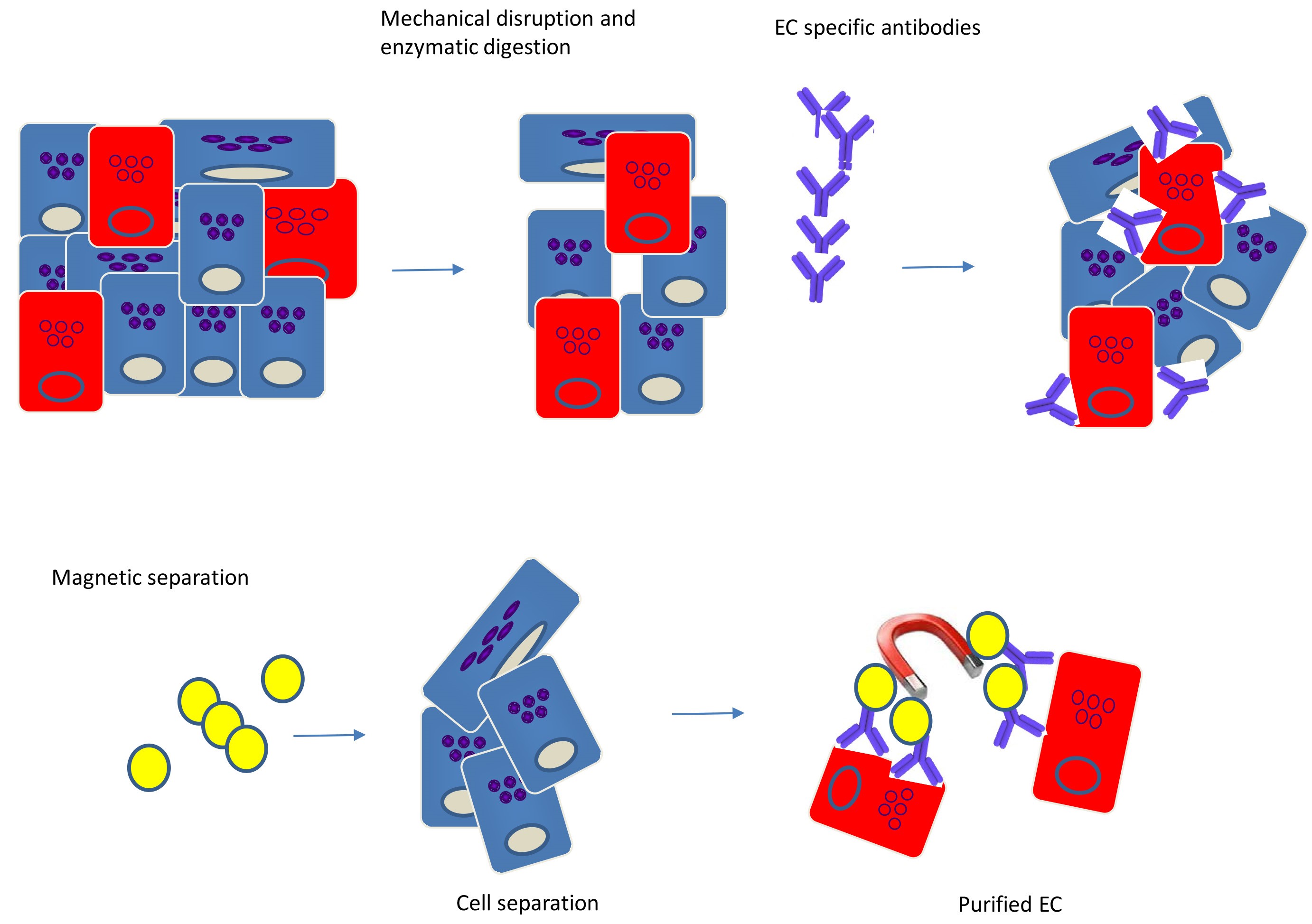
Figure 1. Isolation of endothelial cells (ECs) from tissues by immunomagnetic separation. Tissues consist of diverse cell types and matrix components, and in general, the endothelial content constitutes 1-2% of the total tissue mass. Tissues are mechanically disrupted and enzymatically digested to create a single-cell suspension. Endothelial-specific Abs are added to the single-cell suspension to label the EC. ECs are separated from the other components by using magnetic beads, resulting in highly pure population of ECs.
- Euthanize mice according to your local animal regulations. We use an overdose of isoflurane, which leads to breathing arrest within one minute.
- Working as sterilely as possible remove murine organs such as heart, lung, and liver using autoclaved instruments and rinse in PBS to remove blood.
- In a Petri dish, using sterile crossed scalpels, dissect tissue into 2-mm3 blocks.
- Wash twice in PBS by low-speed centrifugation (210 x g, 1 min).
- Incubate diced tissue in a solution of collagenase (0.5 mg/ml) for 1 h at 37 °C in a humid incubator.
Note: We use Gibco® Collagenase Type II because compared to other collagenase preparations it has a higher clostripain activity and is well-suited for the digestion of heart, bone, thyroid, cartilage, and liver tissues. N.B. it is isolated from Clostridium histolyticum and packaged as a lyophilized, non-sterile powder for research use in cell or tissue dissociation and organ perfusions. Gibco® Collagenase Type II activity is guaranteed to be greater than 125 units/mg. - Subsequently, add 75 µl DNase I solution per 10 ml cell suspension and incubate for another 30 min in a 37 °C water bath with continuous agitation.
- Pass digested tissue through a cell strainer to remove undigested blocks.
- Rinse the cell strainer twice with PBS supplemented with 2.5% FCS to collect any remaining cell.
- Incubate for a further 10 min in 0.25% trypsin (1 ml of trypsin for every 100 mg of tissue) to obtain single cell suspension.
- Wash once in 500 µl PBS supplemented with 2.5% FCS.
- Incubate for 30 min at 4 °C with murine immunoglobulins to block Fc receptors.
Note: Mouse BD Fc Block is a purified rat IgG2b anti-mouse CD16/CD32 monoclonal antibody. - Wash twice in cold PBS supplemented with 2.5% FCS.
- Incubate for 45 min at 4 °C with rat anti-mouse CD31, rat anti-mouse CD105 and biotinylated isolectin B4.
- Wash twice in cold 500 µl PBS supplemented with 0.5% FCS and count cells.
- Resuspend pellet and incubate with PBS 0.5% FCS (200 μl/L, 2.5 x 107 cells), rat anti-mouse Ig (25 μl/L, 2.5 x 107 cells)- and streptavidin-conjugated microbeads (25 μl/L, 2.5 x 107 cells) for 15 min at 4 °C (total volume 250 μl). Meanwhile, load columns onto the separation unit (use one column every 1-2.5 x 107 cells) and wash each column with 500 μl PBS 0.5% FCS as per manufacturer’s instructions.
- Load each column with 250 μl cell suspension. The magnetically labeled cells are retained in the column(s) while non-labeled cells pass through. After the cell suspension has flowed through the column, wash the column twice with 500 μl PBS 0.5% FCS. For detailed procedure, please refer to the video on the manufacturer's website.
- Unload the column(s) from the magnet and elute the magnetically retained cells with PBS 0.5% FCS using the plunger provided.
- Wash the eluted cells and centrifuge at 200 x g for 5 min. Resuspend in EC medium (105 cells/ml) and plate out in dishes/plates (Table 1) pre-coating with gelatin. The phenotype and morphology of these cultures remain stable over 10-15 passages in culture, and no overgrowth of contaminating cells of non-endothelial origin is observed at any stage. You can get about 80% of yield.
Note: Coat P100 dishes with gelatin, let sit in an incubator(37 °C) for at least 30 min, wash in PBS and leave to dry.
Table 1. Passaging Seeding Density and Volume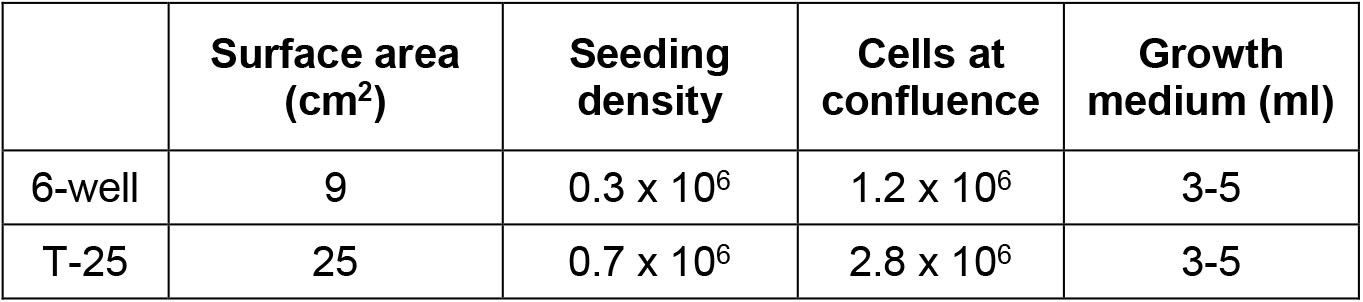
- After overnight culture on 5% CO2, remove non-adherent cells and replace medium with 75 μg/ml EC growth supplement.
- At confluence, detach EC with either trypsin-EDTA or cell dissociation solution and characterize.
Note: To preserve the features of EC physiology and gene expression, the tissue specimen should preferably be processed immediately after resection. It is advisable to have ready-to-use aliquots of the work solutions in storage.
Data analysis
- FACS analysis
ECs were detached from the culture flasks with trypsin/EDTA (Life Technologies), washed and resuspended in phosphate buffered saline solution (PBS, Gibco) containing 1% FCS (Globepharm). Cells (106 cells/ml) were then incubated with the indicated monoclonal antibody for 30 min at 4 °C. Cells were then washed twice in cold PBS with 1% FCS and incubated for a further 30 min at 4 °C with the appropriate FITC conjugated secondary antibody. After two additional washes, the cells were analyzed using an EPICS Profile Cytometer (Coulter Electronics, Luton, UK). - Immunocytochemistry
Primary antibody diluted in 3% bovine serum albumin was applied overnight at 4 °C, followed by incubation with a secondary antibody conjugated rhodamine red-X (Molecular Probes) for 1 h at room temperature. Nuclei were labeled with Hoechst 33258 diluted in 3% bovine serum albumin for 15 min at room temperature. All cells were mounted using Dako mounting media (Dako), and fluorescence images were captured using fluorescence microscopy (Zeiss epi-fluorescent microscope). - Results
- The surface molecule expression pattern of primary cultures EC express CD31, and CD105 was determined by flow cytometry. As demonstrated, isolated ECs show high-level expression of CD31 and CD105 in both heart and lung after isolation (Figure 2). In addition, murine EC grew in several clusters formed monolayers and demonstrated spindle-shaped and cobblestone-like appearances after ten days as demonstrate under bright field miscopy Figures 3A and 3B. We further investigated the availability of these cells using dye exclusion trypan assay and found that the viability remains high, reaches over 80% after the second passage as shown in Figures 3C and 3D. Figure 3E shows statistical analysis of percentage cell survival in day 2 and day 10 EC seeding.
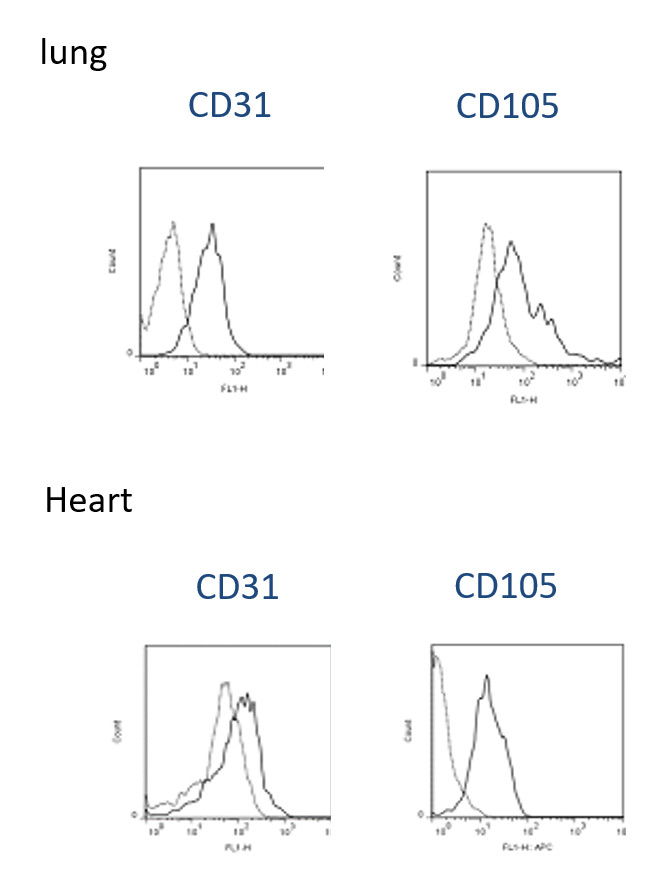
Figure 2. Phenotypic characterization of EC purified from murine lung and heart. EC isolates from murine lung (passage 1) were stained with the antibodies specific for the surface molecules indicated within each graph. Isotype-matched irrelevant antibodies were used as a control.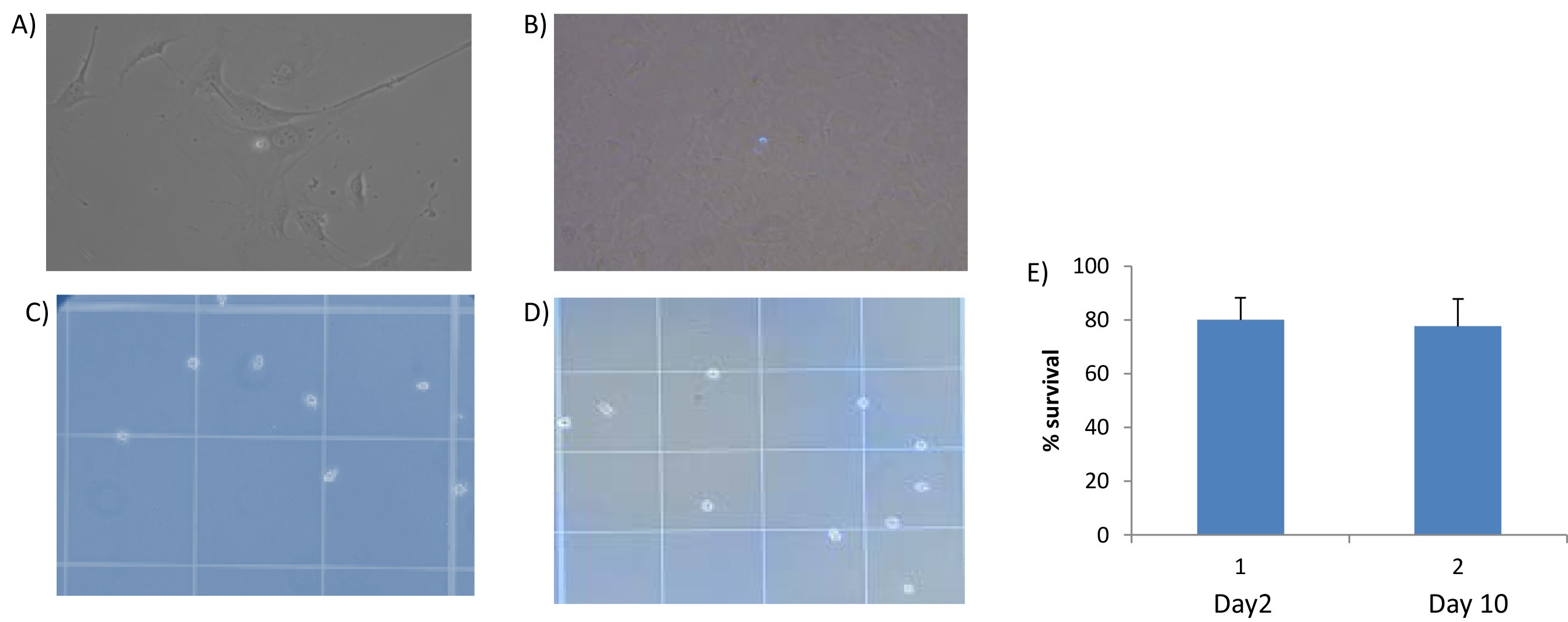
Figure 3. Bright field images of primary murine lung and heart endothelial cell after isolation. A and B. Isolated ECs were subjected to light microscopy analysis for morphology day 2 (A) and day 7 (B). C, D and E. Trypan blue counting indicates 80% of the cells are surviving on day 2 and 10. - In this protocol, the cultured cells retain their morphological and functional key characteristics of in vivo ECs. We were also able to show the immunofluorescent staining positive cells for CD31 (Figure 4).
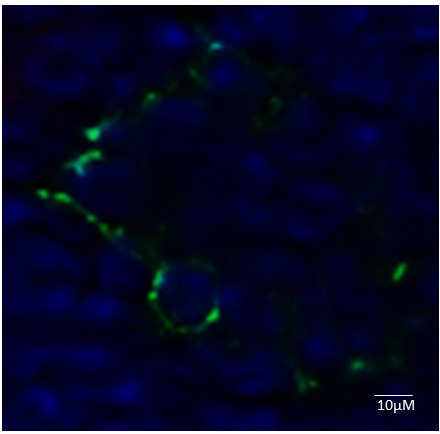
Figure 4. Immunofluorescent characterizations of isolated endothelial cells (ECs). Cells were spun onto microscope slides and subjected to immunofluorescence staining against the indicated antigens. PECAM-1 (MEC 13.3) (green) with 4,6-diamidino-2-phenylindole (DAPI) (blue). - The significance applications of primary cultured mouse endothelial cells
The protocol provides a great opportunity to study the endothelial-specific activities of targeted molecules. The ability to yield high numbers of mouse ECs makes it very useful in cardiovascular research. This method also reduces cost and improves the potential of studying EC-based therapy in murine models through engraftment of endothelial cells.
- The surface molecule expression pattern of primary cultures EC express CD31, and CD105 was determined by flow cytometry. As demonstrated, isolated ECs show high-level expression of CD31 and CD105 in both heart and lung after isolation (Figure 2). In addition, murine EC grew in several clusters formed monolayers and demonstrated spindle-shaped and cobblestone-like appearances after ten days as demonstrate under bright field miscopy Figures 3A and 3B. We further investigated the availability of these cells using dye exclusion trypan assay and found that the viability remains high, reaches over 80% after the second passage as shown in Figures 3C and 3D. Figure 3E shows statistical analysis of percentage cell survival in day 2 and day 10 EC seeding.
- Conclusion
Here we follow this simple and quick method to generate primary EC lines. This can be used for multiple passages for vascular research. - Statistical analysis
Results are expressed as mean ±SD or SEM, as indicated. The Student’s t-test and ANOVA were used. All reported P values are two-sided. A P-value of less than 0.05 was regarded as significant.
Recipes
- EC medium
Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Paisley, Scotland)
2 mM glutamine (Gibco)
100 U/ml penicillin (Gibco)
100 μg/ml streptomycin (Gibco)
1 mM sodium pyruvate (Gibco)
20 mM HEPES (Gibco)
1% non-essential amino acids (Gibco)
50 mM 2-mercaptoethanol (Gibco)
Freshly added 20% heat-inactivated foetal calf serum (FCS; Globepharm, Esher, UK)
75 μg/ml EC growth supplement (Sigma, Poole, UK)
Prepared in 2% gelatin (type B from bovine skin, Sigma) coated tissue culture flasks (Nunc, Life Technologies, Paisley, UK)
Acknowledgments
K.CP. Cheung and F.M.Marelli-Berg are supported by the British Heart Foundation grants CH/15/2/32064 and by the Barts Charity grant MGU0377.
Competing interests
The authors declare no conflict of interest.
References
- Cheung, K., Ma, L., Wang, G., Coe, D., Ferro, R., Falasca, M., Buckley, C. D., Mauro, C. and Marelli-Berg, F. M. (2015). CD31 signals confer immune privilege to the vascular endothelium. Proc Natl Acad Sci U S A 112(43): E5815-5824.
- Cid, M. C., Segarra, M., Garcia-Martinez, A. and Hernandez-Rodriguez, J. (2004). Endothelial cells, antineutrophil cytoplasmic antibodies, and cytokines in the pathogenesis of systemic vasculitis. Curr Rheumatol Rep 6(3): 184-194.
- Gumkowski, F., Kaminska, G., Kaminski, M., Morrissey, L. W. and Auerbach, R. (1987). Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels 24(1-2): 11-23.
- Van Den Berg, T. K., Hasbold, J., Renardel De Lavalette, C., Dopp, E. A., Dijkstra, C. D. and Klaus, G. G. (1996). Properties of mouse CD40: differential expression of CD40 epitopes on dendritic cells and epithelial cells. Immunology 88(2): 294-300.
- Wang, J. M., Huang, Y. J., Wang, Y., Xu, M. G., Wang, L. C., Wang, S. M. and Tao, J. (2007). Increased circulating CD31+/CD42- microparticles are associated with impaired systemic artery elasticity in healthy subjects. Am J Hypertens 20(9): 957-964.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cheung, K. C. and Marelli-Berg, F. M. (2018). Isolation of Microvascular Endothelial Cells. Bio-protocol 8(12): e2886. DOI: 10.21769/BioProtoc.2886.
Category
Cancer Biology > Inflammation > Biochemical assays
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link