- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Enhancement of Mucus Production in Eukaryotic Cells and Quantification of Adherent Mucus by ELISA
Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2879 Views: 11772
Reviewed by: David CisnerosSongjie CaiEmiel P.C. van der Vorst

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detection of Amylin-β-amyloid Hetero-Oligomers by Enzyme-Linked Immunosorbent Assay
Noah S. Leibold [...] Florin Despa
Feb 5, 2025 1415 Views

Western Blotting and Immunoprecipitation of Native Human PIEZO1 Channels
Jinyuan Vero Li [...] Charles D. Cox
Jul 20, 2025 3560 Views

Cluster FLISA—A Method to Compare Protein Expression Efficiency Between Cell Lines and Subunit Clustering of Proteins
Sabrina Brockmöller and Lara Maria Molitor
Nov 5, 2025 1207 Views
Abstract
The mucosal surfaces of the gastrointestinal, respiratory, reproductive, and urinary tracts, and the surface of the eye harbor a resident microflora that lives in symbiosis with their host and forms a complex ecosystem. The protection of the vulnerable epithelium is primarily achieved by mucins that form a gel-like structure adherent to the apical cell surface. This mucus layer constitutes a physical and chemical barrier between the microbial flora and the underlying epithelium. Mucus is critical to the maintenance of a homeostatic relationship between the microbiota and its host. Subtle deviations from this dynamic interaction may result in major implications for health. The protocol in this article describes the procedures to grow low mucus-producing HT29 and high mucus-producing HT29-MTX-E12 cells, maintain cells and use them for mucus quantification by ELISA. Additionally, it is described how to assess the amount of secreted adherent mucus. This system can be used to study the protective effect of mucus, e.g., against bacterial toxins, to test the effect of different culture conditions on mucus production or to analyze diffusion of molecules through the mucus layer. Since the ELISA used in this protocol is available for different species and mucus proteins, also other cell types can be used.
Keywords: MucusBackground
The interface of the body with the external environment is formed by mucosal surfaces. These mucosal epithelial tissues can be found for example in the gastrointestinal, respiratory, reproductive, and urinary tracts, and the surface of the eye. Due to their exposure to the external environment many microorganisms populate these tissues. Therefore, these epithelia have evolved multiple mechanisms of defense in response to their vulnerability to microbial attack. Many defensive compounds are secreted into the mucosal fluid, including mucins, antibodies, defensins, protegrins, collectins, cathelicidins, lysozyme, histatins, and nitric oxide (Kagnoff and Eckmann, 1997; Lu et al., 2002; Raj and Dentino, 2002).
To date, more than 20 genes encoding mucins have been identified in humans (Corfield, 2015). The human mucin (MUC) family comprises membrane-bound (MUC1, MUC3A/B, MUC4, MUC12, MUC13, MUC15 – 17, MUC20 and MUC21) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 – 9, MUC19) (Moran et al., 2011; Tailford et al., 2015).
The mucus layer of the intestinal epithelial surface is mainly composed of the secreted mucin MUC2, but the membrane-bound mucins MUC1, MUC3 and MUC4 are also expressed (Kim and Ho, 2010). In addition, the intestinal mucus layer differs in terms of composition, organization and thickness along the gastrointestinal tract (Tailford et al., 2015). The secreted mucins form a gel-like structure adherent to the apical cell surface that constitutes a physical and chemical barrier between the luminal contents and the underlying epithelium (Allan, 2011). Inflammasome activity controls the secretion of mucus in goblet cells and increased secretion of mucus is observed as the microbiota becomes more diverse (Jakobsson et al., 2015). It is becoming apparent that mucus plays a crucial role in maintaining a homeostatic balance between microbiota and its host. Even small deviations from this dynamic interaction can have significant health effects, among which are colitis, colorectal cancer and susceptibility to infection (McGuckin et al., 2011; Hansson, 2012; Chen and Stappenbeck, 2014).
In this protocol, we describe the culture of in vitro models producing different amounts of mucus depending on their culture condition (static vs. semi-wet with mechanical stimulation (Navabi et al., 2013). These models are based on the little to no mucus-producing HT29 cell line, a human colon adenocarcinoma cell line, and its high mucus-producing derivative HT29-MTX-E12 (E12). Furthermore, it is described how to quantify the mucus produced in the different models by ELISA. In addition, the mucolytic compound N-acetyl-L-cysteine (NAC) is used to remove adherent mucus in order to quantify the amount of secreted adherent mucus. A schematic overview of the workflow described in this protocol is provided in Figure 1.
The method described in this protocol is suitable to study the protective effect of mucus against bacterial toxins (Reuter et al., 2018), to test the effect of different culture conditions on mucus production (Navabi et al., 2013) or to analyze diffusion of molecules through the mucus layer. Since the ELISA used in this protocol is available for different species and mucus proteins, other cell types can also be used.
Materials and Reagents
- 1.5 ml microcentrifuge tubes (SARSTEDT, catalog number: 72.690.001 )
- 15 ml centrifuge tubes (Corning, Falcon®, catalog number: 352196 )
- Absorbent paper
- 175 cm2 flask (Greiner Bio One International, CellStar®, catalog number: 660160 )
- Transwell® Inserts with 0.4 µm porous polyester membranes and 12 mm diameter (Corning, Transwell®, catalog number: 3460 )
- 12-well plate (included in Corning Transwell® package; if additional plates are required: Corning, Costar®, catalog number: 3513 )
- Cell lines HT29 (European Collection of Authenticated Cell Cultures (ECACC), catalog number: 91072201 ) and HT29-MTX-E12 (European Collection of Authenticated Cell Cultures (ECACC), catalog number: 12040401 ) or other cells to be tested for their mucus production
- Deionized water
- 70% ethanol
- Dulbecco’s modified Eagle’s medium (DMEM), high glucose, GlutaMAX (Thermo Fisher Scientific, GibcoTM, catalog number: 31966021 )
- Heat-inactivated fetal calf serum (FCS)
- 100x Penicillin/Streptomycin solution (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- 100x Non-essential Amino Acids (Thermo Fisher Scientific, GibcoTM, catalog number: 11140050 )
- Phosphate buffered saline (PBS), pH 7.0-7.2 (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- 0.05% Trypsin-EDTA solution (Thermo Fisher Scientific, GibcoTM, catalog number: 25300054 )
- ELISA kit for mucin (CLOUD-CLONE, catalog number: SEA705Hu )
Note: In this protocol, the ELISA kit SEA705Hu was used for measurement of human mucin 2 (MUC2). Kits for different species and mucus proteins are available at Cloud-Clone Corp., e.g., SEA413Mu for measurement of mouse mucin 1 (MUC1)). - N-acetyl-L-cysteine (Sigma-Aldrich, catalog number: A9165 )
- Cell culture medium (see Recipes)
- N-acetyl-L-cysteine working solution (see Recipes)
Equipment
- Sterile forceps
- Container for wash solution
- Multichannel Pipette (volume range: 20-200 µl)
- Water bath
- Humidified CO2 incubator (Thermo Fisher Scientific, model: HeracellTM 150i )
- Biological safety cabinet
- Hemacytometer (BRAND, Neubauer Improved, catalog number: 717805 )
- 37 °C incubator (e.g., CO2 incubator at 37 °C with CO2 switched off)
- Orbital shaker for use in CO2 incubators (Infors, model: Celltron )
- Ultrasonicator (Ultrasonic Homogenizer, BioLogics, model: 300VT )
- Microcentrifuge (Eppendorf, model: 5418 )
- Swing Bucket Centrifuge (Thermo Fisher Scientific, model: HeraeusTM MegafugeTM 16R )
- Microplate reader (Tecan Trading, model: Infinite® 200 )
Procedure
See Figure 1 for an overview of the workflow described in this protocol.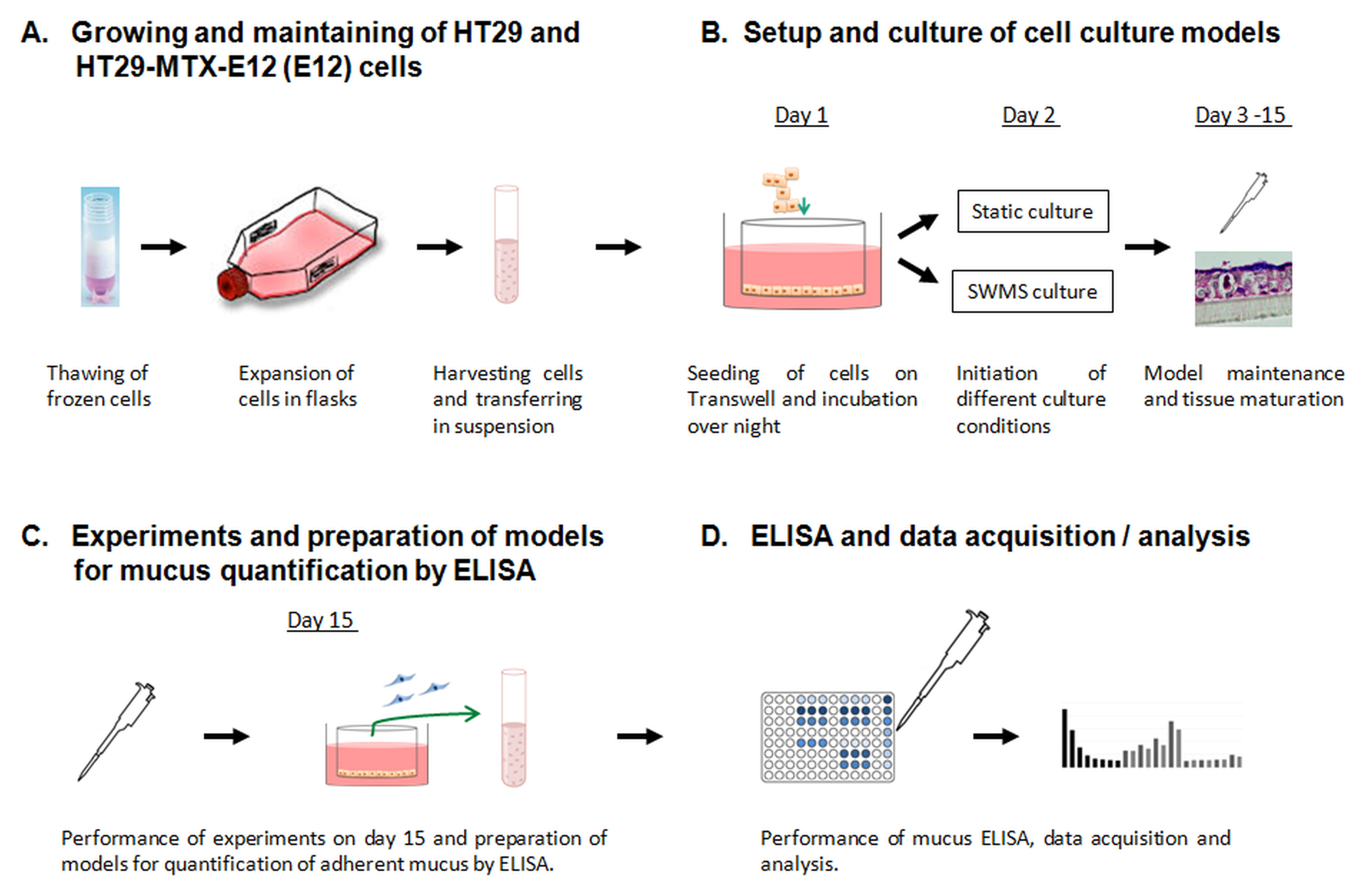
Figure 1. Schematic overview of the workflow described in this protocol. A. The procedure how to thaw, expand, and maintain HT29 and HT29-MTX-E12 (E12) cells in culture is described in Procedure A of this protocol. B. The generation and maintenance of models based on Transwell inserts, and how to initiate the different culture conditions (static vs. semi-wet interface with mechanical stimulation (SWMS)) is specified in Procedure B. C. In Procedure C the removal of adherent mucus and the preparation of models for adherent mucus quantification is described. D. The procedure of the mucus ELISA with subsequent data acquisition and analysis is explained in Procedure D and section “Data analysis” of this protocol.
- Growing and maintaining of HT29 and HT29-MTX-E12 (E12) cells
Note: Before you start, heat the cell culture medium to 37 °C.- Rapidly thaw a vial of frozen HT29 and HT29-MTX-E12 (E12) cells containing 1 x 106 cells by gentle agitation in a 37 °C water bath. To reduce the possibility of contamination, keep the cap of the tube out of the water. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate carefully with 70% ethanol. From this point on all further steps should be carried out under strict aseptic conditions.
- Transfer the vial contents to a 15 ml centrifuge tube containing 9 ml warm cell culture medium and centrifuge at 300 x g for 5 min at room temperature with maximum acceleration/deceleration settings. Discard supernatant. Resuspend cells in 1 ml pre-warmed medium and transfer the cell suspension to a 175 cm2 flask containing 25 ml pre-warmed medium. Place the flask in a humidified CO2 forced-air incubator at 37 °C.
- Twenty four hours after seeding, check the cells under a microscope for attachment, if attached to flask surface, slowly aspirate media to remove dead cells and add 25 ml fresh pre-warmed medium. Change the medium every 2-3 days.
- When the cells have reached 80% confluency, they are ready for passaging. Cells need to be passaged for a minimum of 3 cycles before using them for the experiment.
Note: This is necessary to give the cells time to recover from thawing and may vary from cell type to cell type. The HT29 and E12 cells applied in this protocol were used until passage 20. - For passaging, remove media and wash monolayer with 10 ml PBS. Add 5 ml of pre-warmed (37 °C) trypsin-EDTA solution and incubate at 37 °C for 3-10 min. Check the detachment of cells regularly under the microscope. When cells start to detach, add 5 ml warm media to inactivate trypsin and thoroughly pipette several times to get a uniform cell suspension (mucus-producing cells tend to form cellular lumps).
Note: The incubation time with trypsin-EDTA solution and subsequent detachment of cells varies from cell type to cell type and detachment of cells should be controlled under the microscope regularly to avoid excessively long incubation time. The detachment process can be quickened by gently knocking with the hand against the cell culture flask. - Transfer the cell suspension into a 15 ml centrifuge tube and centrifuge at 300 x g for 5 min at room temperature. Discard supernatant and resuspend cells in 5 ml pre-warmed medium. Count cells using a hemacytometer and transfer 7 x 105 cells into a new 175 cm2 flask with 25 ml fresh pre-warmed medium. The remaining cells can be used for subsequent experiments.
- Rapidly thaw a vial of frozen HT29 and HT29-MTX-E12 (E12) cells containing 1 x 106 cells by gentle agitation in a 37 °C water bath. To reduce the possibility of contamination, keep the cap of the tube out of the water. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate carefully with 70% ethanol. From this point on all further steps should be carried out under strict aseptic conditions.
- Setup and culture of cell culture models
- Unpack the Transwell® inserts and place them in the cavities of a 12-well plate. Subsequently, harvest and count HT29 and E12 cells as described in Steps A5 and A6. Adjust the cell concentration of the obtained cell suspension to a working concentration of 2.73 x 105 cells per ml.
- To set up the models, add 275 µl of the cell suspension containing 7.5 x 104 HT29 or E12 cells to the apical side of the membrane and 1 ml medium to the basal compartment. Incubate the plates in the cell culture incubator overnight.
- On the next day, the medium is changed and the culture condition semi-wet interface with mechanical stimulation (SWMS) is initiated to enhance mucus production.
- To generate the static culture condition, replace the medium in the apical compartment with 275 µl and the basal compartment with 1 ml pre-warmed (37 °C) medium as described in Step B4. Incubate the cells in a cell culture incubator for 15 days and maintain the media volumes during the medium change.
- To generate culture condition semi-wet interface with mechanical stimulation (SWMS), replace the medium in the apical compartment with 75 µl and the basal compartment with 850 µl pre-warmed (37 °C) medium as described in Step B4. Incubate the cells on an orbital shaker with an agitation of 65 rpm in a cell culture incubator for 15 days and maintain the media volumes during the medium change.
- To generate the static culture condition, replace the medium in the apical compartment with 275 µl and the basal compartment with 1 ml pre-warmed (37 °C) medium as described in Step B4. Incubate the cells in a cell culture incubator for 15 days and maintain the media volumes during the medium change.
- A medium exchange should be done every 1-2 days as secreted mucus will acidify the medium (the medium turns yellow). To change the medium, aspirate the medium in the basal compartment first. Then, use sterile forceps to lift out the Transwell® inserts and very carefully aspirate the apical medium without disturbing the cells or the adherent mucus layer. Subsequently, fill very carefully 275 µl or 75 µl of fresh pre-warmed medium into the apical compartment and put the inserts back into their cavities. Finally, fill 1 ml or 850 µl into the lower compartment and hold the plate at a slight angle to avoid air bubbles underneath the membrane. The medium in the inner and outer compartments should have the same level.
- Unpack the Transwell® inserts and place them in the cavities of a 12-well plate. Subsequently, harvest and count HT29 and E12 cells as described in Steps A5 and A6. Adjust the cell concentration of the obtained cell suspension to a working concentration of 2.73 x 105 cells per ml.
- Preparation of models for mucus quantification by ELISA
- After 15 days, the models are ready and can be used for experiments. If quantification of secreted adherent mucus is required a subset of models should be treated as described in Step C2, otherwise continue with Step C3.
Notes:- Perceivable ideas for experiments could be to test the colonization efficacy of bacteria in presence and absence of an adherent mucus layer, or to test the diffusion of particles through the cell layer in presence and absence of an adherent mucus layer.
- If other cell types are used, the optimal culture time for maximal mucus production can be determined by performing the mucus ELISA at different time points. In addition, histological sections can be prepared and stained with PAS/Alcian blue to stain mucus and to obtain a qualitative overview of the mucus localization in the cell layer.
- Perceivable ideas for experiments could be to test the colonization efficacy of bacteria in presence and absence of an adherent mucus layer, or to test the diffusion of particles through the cell layer in presence and absence of an adherent mucus layer.
- The mucolytic agent N-acetyl-L-cysteine (NAC) is used to chemically remove adherent mucus. NAC has a low order of toxicity and reduces the viscosity of mucus by irreversible reduction of disulfide bonds in mucoproteins.
- For this purpose, remove the medium as described in Step B4.
- Add 500 µl pre-warmed (37 °C) NAC working solution to the apical compartment and 1.5 ml pre-warmed (37 °C) medium to the basal compartment in order to balance the medium level in both compartments. Subsequently, place the prepared models on an orbital shaker for 1 h and 65 rpm in the cell culture incubator.
- Wash both compartments once with PBS and continue with Step C3.
- For this purpose, remove the medium as described in Step B4.
- To detach cells, carefully aspirate the medium as described in Step B4 and carefully wash both compartments once with PBS by adding 500 µl to the apical and 1.5 ml to the basal compartment.
- Remove PBS as described in Step B4 and add pre-warmed (37 °C) trypsin-EDTA solution (500 µl apical, 1.5 ml basal) to each model. Incubate at 37 °C for 10-15 min in the cell culture incubator.
- Detach cells by thoroughly pipetting several times to get a uniform cell suspension and transfer the solution into a 1.5 ml tube containing 500 µl medium. Subsequently, count cells in a hemacytometer.
Note: When the cells begin to detach, the fluid becomes cloudy and small “holes” become visible on the membrane. - An equal number of cells should be analyzed by ELISA. Therefore, transfer 4 x 106 cells per approach into a new 1.5 ml tube and centrifuge at 2,000 x g for 5 min at 4 °C. Resuspend pellet in 500 µl ice-cold PBS. Subsequently, subject the cell suspension to ultrasonication for 4 times 10 sec at 20 kHz on ice (avoid foaming).
Note: The number of cells to be analyzed can be varied and should be roughly estimated in advance on the basis of the mucus production of the cells to achieve optimal results in the ELISA. - Centrifuge at 1,500 x g for 10 min at 4 °C to remove cellular debris. The supernatant is required for ELISA. Keep samples on ice until use.
- After 15 days, the models are ready and can be used for experiments. If quantification of secreted adherent mucus is required a subset of models should be treated as described in Step C2, otherwise continue with Step C3.
- ELISA and data acquisition
- In the following step, the assay is performed according to the manufacturer's instructions. Reconstitute the standard included in the kit and prepare a dilution series with 7 points. Reconstitute all other components included in the kit according to the manufacturer's instructions.
- Perform the assay in duplicate readings for each standard, blank (Standard Diluent, included in the kit), and samples. Load the pre-coated ELISA-plate with 100 µl of standard, blank, and samples and incubate for 2 h at 37 °C. Remove the liquid of each well, don’t wash. Add primary antibody and incubate for 1 h at 37 °C. Wash each well three times with wash solution and incubate with HRP-conjugated secondary antibody for 30 min at 37 °C. Wash each well again five times and subsequently incubate with 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate solution for 15-25 min at 37 °C in the dark. Add sulphuric acid to stop the reaction and measure absorption immediately at 450 nm with a Microplate Reader.
Note: The liquid will turn blue by the addition of TMB substrate. The incubation time with TMB substrate is critical and may vary. It is therefore strongly recommended to check the course of the reaction regularly. The strongest blue coloration should be observed at the highest standard. In this protocol, approximately 20 min is sufficient. The liquid will turn yellow by the addition of Stop solution. Mix the liquid until uniform by tapping the side of the plate. In some cases, dilution is required before measurement to obtain optimal measurement results.
- In the following step, the assay is performed according to the manufacturer's instructions. Reconstitute the standard included in the kit and prepare a dilution series with 7 points. Reconstitute all other components included in the kit according to the manufacturer's instructions.
Data analysis
- A typical measurement is shown in Figure 2A. First, average the duplicate readings for each standard, blank, and samples and subtract the average blank optical density. Next, draw a standard curve by plotting the mean optical density (OD) for each standard on the linear y-axis and mucus concentration on the linear x-axis. Create a linear regression line and use the formula of the regression line to calculate the mucus concentrations from the averaged measured OD values of the samples (Figures 2B and 2C). If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.
- To calculate the amount of secreted mucus, the mucus concentration of the NAC-treated samples must be subtracted from the mucus concentration of the untreated samples. The difference represents the amount of secreted mucus (Figure 2C).
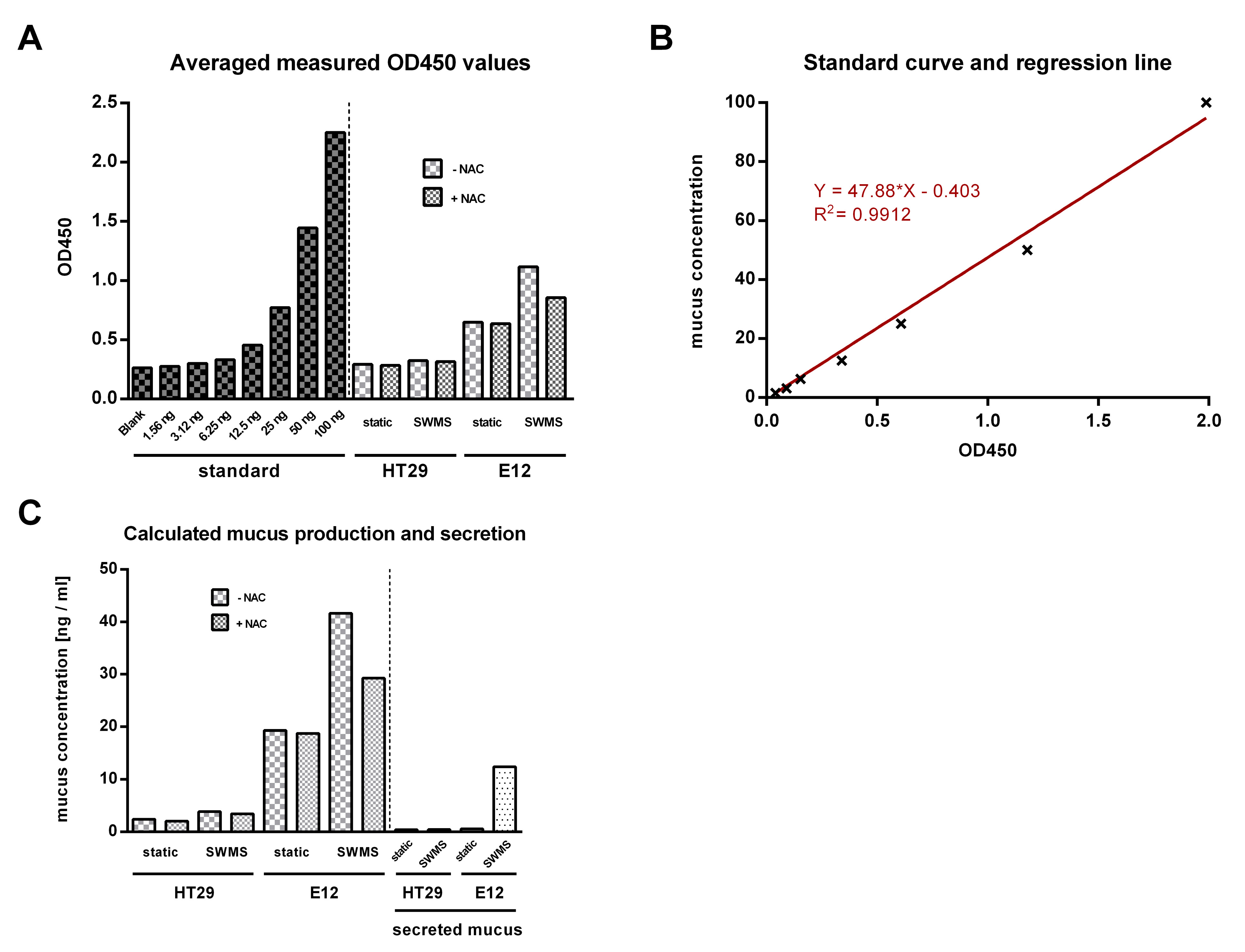
Figure 2. Exemplary data analysis of measurement with MUC2 ELISA in HT29 and HT29-MTX-E12 (E12) cells under different culture conditions. A. A typical absorption measurement with a Microplate Reader at 450 nm is shown after averaging the duplicate reads of blank, standard, and samples. B. The incubation time with TMB substrate was 20 min. Using the measured OD450 values for the standard, a standard curve can be created after subtraction of the averaged blank value. By inserting a linear regression line, the mucus concentration of the samples can be calculated using the regression line equation. C. The calculated mucus concentration for the samples is shown in and after subtraction of the NAC-treated samples the amount of secreted mucus can be determined.
Recipes
- Cell culture medium (500 ml)
440 ml DMEM, high glucose, GlutaMAX
50 ml FCS
5 ml Penicillin/Streptomycin
5 ml non-essential amino acids - N-acetyl-L-cysteine working solution (60 mM)
10 ml DMEM, high glucose, GlutaMAX
97.9 mg N-acetyl-L-cysteine
Acknowledgments
This protocol is adapted from Reuter et al. (2018). The work was partly supported by Ardeypharm GmbH.
Competing interests
The authors do not have any potential conflicts of interest to declare.
References
- Allan, A. (2011). Gastrointestinal mucus. Compr Physiol 359-382.
- Chen, G.Y. and Stappenbeck, T.S. (2014). Mucus, it is not just a static barrier. Sci Signal 7, pe11.
- Corfield, A. P. (2015). Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta 1850(1): 236-252.
- Hansson, G. C. (2012). Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 15(1): 57-62.
- Jakobsson, H. E., Rodriguez-Pineiro, A. M., Schutte, A., Ermund, A., Boysen, P., Bemark, M., Sommer, F., Backhed, F., Hansson, G. C. and Johansson, M. E. (2015). The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16(2): 164-177.
- Kagnoff, M.F. and Eckmann, L. (1997). Epithelial cells as sensors for microbial infection. J Clin Invest 100: 6-10.
- Kim, Y. S. and Ho, S. B. (2010). Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12(5): 319-330.
- Lu, J., Teh, C., Kishore, U. and Reid, K. B. (2002). Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim Biophys Acta 1572(2-3): 387-400.
- McGuckin, M. A., Linden, S. K., Sutton, P. and Florin, T. H. (2011). Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9(4): 265-278.
- Moran, A. P., Gupta, A. and Joshi, L. (2011). Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 60(10): 1412-1425.
- Navabi, N., McGuckin, M.A. and Linden, S.K. (2013). Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS One 8: e68761.
- Raj, P. A. and Dentino, A. R. (2002). Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett 206(1): 9-18.
- Reuter, C., Alzheimer, M., Walles, H. and Oelschlaeger, T.A. (2018). An adherent mucus layer attenuates the genotoxic effect of colibactin. Cell Microbiol 20.
- Tailford, L. E., Crost, E. H., Kavanaugh, D. and Juge, N. (2015). Mucin glycan foraging in the human gut microbiome. Front Genet 6: 81.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Reuter, C. and Oelschlaeger, T. A. (2018). Enhancement of Mucus Production in Eukaryotic Cells and Quantification of Adherent Mucus by ELISA. Bio-protocol 8(12): e2879. DOI: 10.21769/BioProtoc.2879.
Category
Biochemistry > Protein > Immunodetection
Cell Biology > Cell-based analysis > Protein secretion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link