- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Spared Nerve Injury Model of Neuropathic Pain in Mice
Published: Vol 8, Iss 6, Mar 20, 2018 DOI: 10.21769/BioProtoc.2777 Views: 18764
Reviewed by: Gary LiuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2670 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views
Abstract
Experimental models of peripheral nerve injury have been developed to study mechanisms of neuropathic pain in living animals. The spared nerve injury (SNI) model in rodents is a partial denervation model, in which the common peroneal and tibial nerves are injured, producing consistent and reproducible tactile hypersensitivity in the skin territory of the spared, intact sural nerve. SNI-operated mice require less force applied to the affected limb to elicit a withdrawal behavior as compared to sham mice. This effect is observed as early as 2 days after surgery and lasts for at least 1 month. We describe detailed surgical procedures to establish the SNI mouse model that has been widely used for investigating mechanisms of neuropathic pain.
Keywords: Mouse pain modelBackground
Partial nerve injury animal models have been developed for the purpose of studying the molecular, cellular, and circuit mechanisms of neuropathic pain (Bennett and Xie, 1988; Seltzer et al., 1990; Kim and Chung, 1992). A partial denervation model enables researchers to investigate structural and functional changes in diverse groups of neuronal and non-neuronal cells. Studies can be performed during the initiation, progression (also known as acute) and maintenance (chronic) phases of neuropathic pain, as well as at different anatomical sites along the pain pathway including distal vs. proximal peripheral nerve fibers, dorsal root ganglion, spinal cord, subcortical and cortical areas. The spared nerve injury (SNI) model involves partial nerve injury where the common peroneal and tibial nerves are injured, producing consistent and reproducible pain hypersensitivity in the territory of the spared sural nerve (Decosterd and Woolf, 2000; Shields et al., 2003). This model has proved to be robust, demonstrating substantial and prolonged changes in behavioral measures of mechanical sensitivity and thermal responsiveness (Bourquin et al., 2006). These features closely mimic the cardinal symptoms of clinically described neuropathic pain disorders.
Materials and Reagents
- Cotton-wool applicator
- Double edge razor blades (Baili, catalog number: BP005 )
- Povidone-Iodine Prep Pad (Dynarex, catalog number: 1108 )
- 6-0 nylon suture (Surgical Specialties, Look, catalog number: 916B )
- 8-0 nylon suture (Fine Science Tools, catalog number: 12051-08 )
- C57BL/6J male mice, 8-12 weeks of age (THE JACKSON LABORATORY, catalog number: 000664 )
- Sterile Lubricant Eye Ointment (Stye)
- Ketamine hydrochloride (Ketathesia, NDC 11695-0702-1)
- Xylazine Sterile Solution (AnaSed, NDC 59399-110-20)
- Sterile saline
- Ketamine and xylazine (KX) mixture (see Recipes)
Equipment
- Stereomicroscope (Olympus, model: SZX10 )
- LED surgical light (Schott ACE light source with EKE lamp, Schott, model: A20500 )
- Dissecting scissors and forceps (Fine Science Tools, catalog numbers: 14094-11 , 14084-09 , 15000-08 , 11150-10 )
- Fine forceps (Fine Science Tools, catalog number: 11253-20 )
- Vannas spring scissors (Fine Science Tools, catalog number: 15000-08 )
- Electronic von Frey Anesthesiometer (IITC Life Science, catalog number: 2392 )
Procedure
Note: All procedures in this study were approved by the New York University School of Medicine Institutional Animal Care and Use Committee (IACUC) as consistent with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals to ensure minimal animal use and discomfort.
- Spared nerve injury surgery
- Anesthetize mice with a mixture of KX (0.1 ml/20 g mouse, intraperitoneal injection).
Note: Assess depth of anesthesia with hindlimb or tail pinch. An animal deeply anesthetized does not react to stimulus. The mouse should be placed on a heating blanket for the maintenance of normothermia while undergoing anesthesia. - Apply ophthalmic ointment to the eyes with a cotton-wool applicator.
- Shave the skin on the lateral surface of the left thigh using a razor blade (Figure 1A) followed by topical application of povidone-iodine prep pad.
- Make a single, small skin incision at the mid-thigh level with fine scissors (#14094-11) using the femur as a landmark (Figure 1B) and make blunt dissection using the dull portion of the dissection scissors (# 14084-09) through the biceps femoris muscle (BFM) (Figure 1C). Expose the sciatic nerve and its three branches (Figure 1D).
Note: Perform minimal retraction when exposing the sciatic nerve and its three branches. If there is accidental bleeding from the operation site, apply proper pressure with a cotton bud until coagulation. If bleeding persists, the mouse should not be used for further experiments. - For the SNI operation, distal to the trifurcation of the sciatic nerve, ligate the common peroneal and tibial nerves using 8-0 nylon suture (Figures 1E and 1F) and axotomize with Vannas spring scissors (#15000-08), removing a 2-4 mm piece of each distal nerve stump (Figure 1G). Keep the sural nerve intact (Figures 1E-1G). Avoid any stretching or contact with the spared sural nerve. In the sham operation, the aforementioned manipulations of the sciatic nerve and its branches are not performed.
- Close incisions with muscle and skin sutures (Figure 1H).
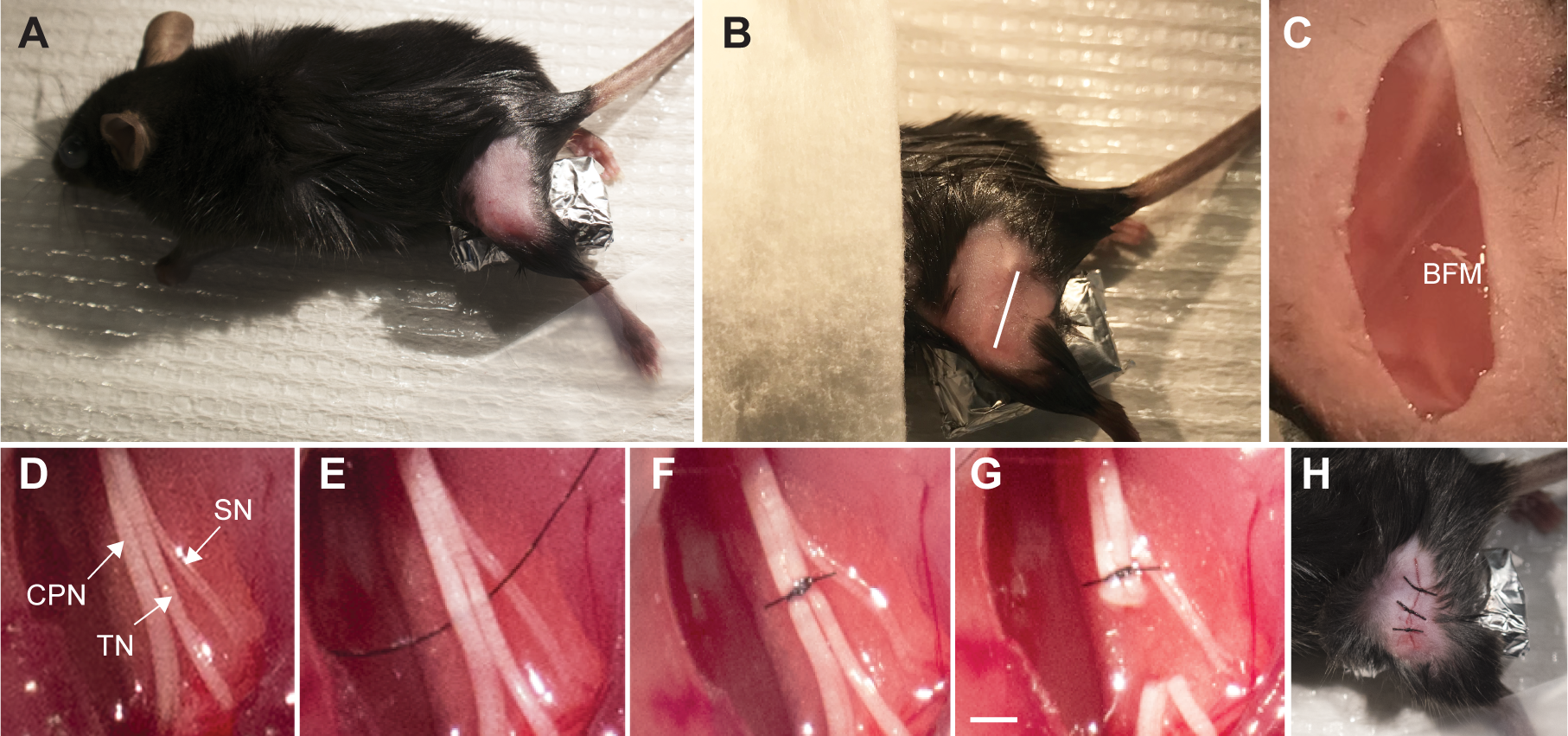
Figure 1. Spared nerve injury surgical procedure to induce neuropathic pain in mice. A. Mouse was anesthetized with KX and positioned prone. Surgical area was then shaved and disinfected. The paw was abducted and elevated from the table. B. White line indicates the incision site on left hindlimb or thigh. C. Following the incision along the white line, the biceps femoris muscle (BFM) was exposed and a careful blunt dissection was made through to expose the trifurcation of the sciatic nerve. D. Exposure of the sciatic nerve and peripheral branches: common peroneal (CPN), tibial (TN) and sural nerves (SN). E. An 8-0 nylon suture was passed under the common peroneal and tibial nerves. F. Ligation of the common peroneal and tibial nerves was performed with a surgical knot. G. The ligated nerves were transected distally and a 2 mm section was removed to prevent nerve regeneration. The surgical steps in panels E-G were not performed in the sham operation. Care was taken to avoid contact with the sural nerve. Scale bar = 2 mm. H. Muscles were reapproximated, followed by overlying skin. The skin was closed with 6-0 nylon suture with at least 3 individual knots along the incision.- The SNI-operated animals should have normal food intake, growth, display regular movements, and grooming.
- Anesthetize mice with a mixture of KX (0.1 ml/20 g mouse, intraperitoneal injection).
- Behavior testing
The von Frey test is used to assess the onset and maintenance of mechanical allodynia over time.- Animals were placed in clear plexiglass cages on an elevated mesh floor and tested after 30 min of habituation (Figure 2A).
Note: During the 30-min habituation before behavior testing, place a small amount of food in testing chambers to help the mice readjust to a new environment, which also lessens their general activity. - In all animal groups, mechanical paw withdrawal threshold was examined using an electronic von Frey anesthesiometer (Figure 2B) with #8 flexible von Frey hair which delivers force up to 11 g (Figure 2C). The anesthesiometer displays the actual force at which paw withdrawal behavior occurs. To perform measurements, first ensure that von Frey hair is securely attached to the anesthesiometer probe. Second, clear the reading on the anesthesiometer before the measurement. Third, direct the von Frey hair through the mesh floor to the lateral plantar aspect (the sural nerve skin territory) of the hind paw (Figure 2D) and record the force displayed.
- Three trials of withdrawal per paw were recorded with intervals of 5 min in between measurements. An average was reported for each day tested (as shown in Figure 2E).
Note: The von Frey test should be performed during the light cycle by the same researcher, who should be blinded to the surgery and treatments. - After SNI, behavioral tests were performed at designated time points (e.g., 2, 7, 14 and 28 days) after surgery (Figure 2E).
Note: The course of SNI-induced neuropathic pain in mice is usually divided into development (1-7 days) and maintenance (8-14 days) phases. Depending on the purpose of each study, behavior tests should be planned accordingly.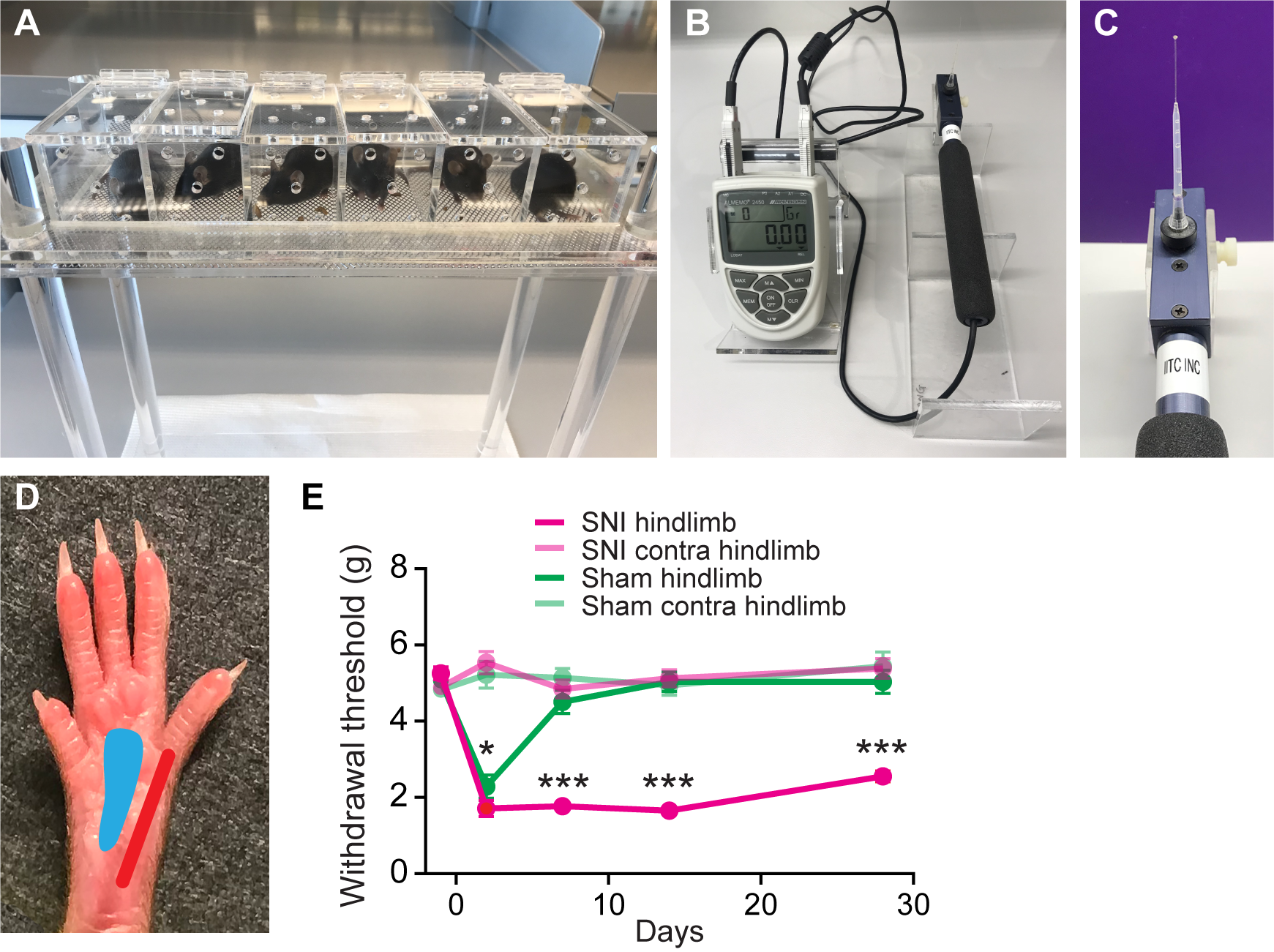
Figure 2. Measuring hindlimb paw withdrawal threshold before and after SNI. A. SNI and sham-operated mice were placed in plexiglass cages on an elevated mesh platform for paw access. B and C. Electronic von Frey anesthesiometer with #8 von Frey hair. D. Plantar view of the left hindlimb paw from a mouse after SNI operation. The red area on the photograph corresponds to the sural nerve skin territory that was tested with the von Frey hair, while the blue area corresponds to the tibial nerve skin territory, which was denervated from SNI surgery and should not be tested during the test. E. Paw withdrawal threshold measured in grams from ipsilateral and contralateral hindlimbs in both SNI and sham-operated mice over 1 month (Two-way ANOVA followed by Tukey’s test; 2 day: P = 0.025; 7 day: P < 0.001; 14 day: P < 0.001; 31 day: P < 0.001. n = 17 in SNI group, and n = 12 in sham group) (Cichon et al., 2017).
- Animals were placed in clear plexiglass cages on an elevated mesh floor and tested after 30 min of habituation (Figure 2A).
Data analysis
A complete description of statistics used for analyzing von Frey behavioral experiments is presented in Cichon et al. (2017).
Notes
- Positive aspects: SNI surgery is a simple procedure to carry out and can be performed by researchers with some surgical experience. Also, following SNI surgery, mice reliably display mechanical hypersensitivity as early as 2 days after injury, and develop long-term hypersensitivity for at least 30 days. Sham-operated mice initially show increased mechanical sensitivity (e.g., 2 days after surgery), which could be related to the surgical inflammation, but should return to baseline levels within days (Figure 2E). Cortical neurons in the awake behaving SNI/sham mice could be imaged with two-photon microscopy (Yang et al., 2013; Cichon et al., 2017). Thus, experiments can be performed to study mechanisms for the initiation, progression and maintenance of neuropathic pain.
- Negative aspects: SNI model induces lesions in the peroneal and tibial nerves, leaving the sural nerve intact. Because the sural nerve innervates the skin on the lateral aspect of the hind paw (Figure 2D), experience and repetitive measurements are required to improve the accuracy and precision of paw withdrawal testing.
Recipes
- Ketamine and xylazine mixture
To make 50 ml of KX:
10 ml ketamine (100 mg/ml)
7.5 ml xylazine (20 mg/ml)
32.5 ml of sterile saline (0.9% NaCl), mix well
Store it away from light exposure and at room temperature
Acknowledgments
This protocol is adapted from the previously published paper (Cichon et al., 2017). This work was supported by National Institutes of Health grants R01GM107469 and R21NS106469 to G.Y. The authors have nothing to disclose.
References
- Bennett, G. J. and Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1): 87-107.
- Bourquin, A. F., Suveges, M., Pertin, M., Gilliard, N., Sardy, S., Davison, A. C., Spahn, D. R. and Decosterd, I. (2006). Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 122(1-2): 14 e11-14.
- Cichon, J., Blanck, T. J. J., Gan, W. B. and Yang, G. (2017). Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat Neurosci 20(8): 1122-1132.
- Decosterd, I. and Woolf, C. J. (2000). Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87(2): 149-158.
- Kim, S. H. and Chung, J. M. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50(3): 355-363.
- Seltzer, Z., Dubner, R. and Shir, Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43(2): 205-218.
- Shields, S. D., Eckert, W. A., 3rd and Basbaum, A. I. (2003). Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain 4: 465-470.
- Yang, G., Pan, F., Chang, P. C., Gooden, F. and Gan, W. B. (2013). Transcranial two-photon imaging of synaptic structures in the cortex of awake head-restrained mice. Methods Mol Biol 1010: 35-43.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cichon, J., Sun, L. and Yang, G. (2018). Spared Nerve Injury Model of Neuropathic Pain in Mice. Bio-protocol 8(6): e2777. DOI: 10.21769/BioProtoc.2777.
Category
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











