- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Infectious Subviral Particle-induced Hemolysis Assay for Mammalian Orthoreovirus
Published: Vol 8, Iss 2, Jan 20, 2018 DOI: 10.21769/BioProtoc.2701 Views: 6185
Reviewed by: Yannick DebingKristin ShinglerSmita Nair

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

General Maintenance and Reactivation of iSLK Cell Lines
Ariana C. Calderón-Zavala [...] Ekaterina E. Heldwein
Jun 5, 2025 1875 Views

Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2457 Views

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3033 Views
Abstract
Mammalian orthoreovirus (reovirus) utilizes pore forming peptides to penetrate host cell membranes. This step is essential for delivering its genome containing core particle during viral entry. This protocol describes an in vitro assay for measuring reovirus-induced pore formation.
Keywords: VirologyBackground
Reoviruses are nonenveloped, double-stranded RNA viruses that are composed of two concentric protein shells: the inner capsid (core) and the outer capsid (Dryden et al., 1993; Zhang et al., 2005; Dermody et al., 2013). Following attachment, virions are endocytosed (Borsa et al., 1979; Ehrlich et al., 2004; Maginnis et al., 2006; Maginnis et al., 2008) and host cathepsin proteases degrade the σ3 outer capsid protein (Chang and Zweerink, 1971; Silverstein et al., 1972; Borsa et al., 1981; Sturzenbecker et al., 1987; Dermody et al., 1993; Baer and Dermody, 1997; Ebert et al., 2002). This process generates a metastable intermediate, called infectious subviral particle (ISVP), in which the cell penetration protein, µ1, is exposed (Dryden et al., 1993). Reovirus ISVPs undergo a second conformational change to deposit the genome- containing core into the host cell cytoplasm. The altered particle is called ISVP* (Chandran et al., 2002). ISVP-to-ISVP* conversion culminates in the release of µ1-derived pore forming peptides (Nibert et al., 1991; Zhang et al., 2005; Chandran et al., 2002; Odegard et al., 2004; Nibert et al., 2005; Agosto et al., 2006; Ivanovic et al., 2008). The released peptides form pores within endosomal membranes, which are thought to mediate core delivery (Agosto et al., 2006; Ivanovic et al., 2008; Zhang et al., 2009).
Many of the conformational changes that define reovirus entry can be recapitulated in vitro: (i) ISVPs are produced by digesting purified virions with chymotrypsin (Joklik, 1972; Borsa et al., 1973a), and (ii) ISVP* formation can be induced using heat (Middleton et al., 2002), large monovalent cations (Borsa et al., 1973b), µ1-derived peptides (Agosto et al., 2008), red blood cells (Chandran et al., 2002; Sarkar and Danthi, 2010), or lipids (Snyder and Danthi, 2015 and 2016). Thus, questions related to reovirus entry are studied using biochemical and cell-based approaches. In this protocol, we describe an in vitro assay that recapitulates ISVP-to-ISVP* conversion and subsequent pore formation.
Materials and Reagents
- Pipette tips
- PCR 8-well tube strips (VWR, catalog number: 20170-004 )
- 50 ml centrifuge tube (VWR, catalog number: 89039-660 )
- 1.7 ml microcentrifuge tubes (MIDSCI, catalog number: AVSS1700 )
- Vacuum driven and disposable bottle top 0.22 µm filter (Merck, catalog number: SCGPT05RE )
- Flat bottom, 96-well plate (Greiner Bio One International, catalog number: 655180 )
- Purified reovirus stocks (see Berard and Coombs, 2009; Kobayashi et al., 2010 for propagation and purification procedures)
- Crushed ice
- Standard SDS-PAGE materials and reagents (e.g., 10% SDS-polyacrylamide mini gels)
- Coomassie Brilliant Blue stain and destain solutions (Bio-Rad Laboratories, catalog number: 1610435 )
- Citrated bovine calf blood (Colorado Serum Company, catalog number: 31023 )
- Bleach (Biz4USA, Janitorial Supplies, catalog number: CLO30966CT )
- 2-Amino-2-(hydroxymethyl)-1,3-propanediol (Tris) (MP Biomedicals, catalog number: 02103133 )
- Sodium chloride (NaCl) (Merck, catalog number: SX0420-3 )
- 0.1 N hydrochloric acid (Sigma-Aldrich, catalog number: 2104 )
- 0.1 N sodium hydroxide (Sigma-Aldrich, catalog number: 2105 )
- Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK)-treated chymotrypsin (Worthington Biochemical, catalog number: LS001432 )
- Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626 )
- Isopropyl alcohol (Avantor Performance Materials, Macron, catalog number: 3032-02 )
- Dulbecco’s phosphate buffered saline (Thermo Fisher Scientific, GibcoTM, catalog number: 21600044 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M9272 )
- Ultrapure DNase/RNase-free distilled H2O (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10977015 )
- Triton X-100 (TX-100) (Sigma-Aldrich, catalog number: X100 )
- 50% bleach (see Recipes)
- Virus storage buffer (VB) (see Recipes)
- 2 mg/ml TLCK-treated chymotrypsin (see Recipes)
- 100 mM phenylmethylsulfonyl fluoride (PMSF) (see Recipes)
- Phosphate buffered saline supplemented with 2 mM MgCl2 (PBSMg) (see Recipes)
- 10% Triton X-100 (TX-100) (see Recipes)
Equipment
- Personal protective equipment (PPE)
- Laboratory coat
- Gloves
- Eye protection
- Laboratory coat
- Biosafety level 2 (BSL-2) laboratory facility
- BSL-2 certified tissue culture hood
- Solid and liquid waste containers
- Autoclave
- Vacuum pump and aspirator
- Ice bucket
- -20 °C freezer
- Micropipettes
- 0.1-2.5 µl capacity (Eppendorf, catalog number: 3123000012 )
- 2-20 µl capacity (Eppendorf, catalog number: 3123000039 )
- 20-200 µl capacity (Eppendorf, catalog number: 3123000055 )
- 100-1,000 µl capacity (Eppendorf, catalog number: 3123000063 )
- 0.1-2.5 µl capacity (Eppendorf, catalog number: 3123000012 )
- Digital pH meter (VWR, model: SB70P )
- Digital laboratory balance (Mettler Toledo, model: PB1502-S )
- NanoDrop spectrophotometer (Thermo Fisher Scientific, Thermo ScientificTM, model: ND-1000 )
- Hot plate stirrer (VWR, catalog number: 12365-382 )
- Magnetic stir bar (VWR, catalog number: 58948-273 )
- Microcentrifuge (Eppendorf, model: 5424 )
- Thermal cycler (Bio-Rad Laboratories, model: S1000TM )
- Temperature controlled water bath (VWR, catalog number: 89501-466 )
- Gel imaging system (LI-COR, model: Odyssey® Classic )
- Microplate reader (Molecular Devices, model: FilterMax F5 Multi-Mode )
- 250 ml glass beaker (VWR, catalog number: 89000-204 )
- 1,000 ml glass beaker (VWR, catalog number: 89000-212 )
- 100 ml graduated cylinder (VWR, catalog number: 65000-006 )
- 1,000 ml graduated cylinder (VWR, catalog number: 65000-012 )
- 100 ml storage bottle (VWR, catalog number: 89000-234 )
- 1,000 ml storage bottle (VWR, catalog number: 89000-240 )
Note: This product has been discontinued.
Software
- Image Studio Lite (LI-COR)
- SoftMax Pro (Molecular Devices)
Procedure
- Generation of infectious subviral particles (ISVPs)
- Propagate and purify reovirus virions as previously described (Berard and Coombs, 2009; Kobayashi et al., 2010). Using a NanoDrop spectrophotometer, determine particle concentration by measuring the optical density of the purified virus stocks at 260 nm (OD260; 1 unit at OD260 = 2.1 x 1012 particles/ml) (Smith et al., 1969).
- In 1 tube of an 8-well tube strip, dilute 2 x 1011 virions into 90 µl of ice cold VB (see Recipes).
- Add 10 µl of ice cold 2 mg/ml TLCK-treated chymotrypsin (see Recipes) to the diluted virus. Mix by pipetting up and down 3-4 times.
Note: For an undigested control, substitute 10 µl of ice cold VB for 10 µl of TLCK-treated chymotrypsin. - Incubate the reaction for 20 min at 32 °C in a thermal cycler.
Note: Under these conditions, σ3 is degraded (Joklik, 1972; Borsa et al., 1973a) and µ1 is cleaved (Nibert and Fields, 1992; Chandran et al., 1999). - Following digestion, quench chymotrypsin activity by the addition of 1 µl of 100 mM PMSF (see Recipes). Mix by pipetting up and down 3-4 times.
- Incubate the reaction for 20 min on ice.
- To confirm that ISVPs are generated, run 2 x 1010 particles per lane on a 10% SDS-polyacrylamide mini gel. Run the gel for 40-45 min at 200 V constant.
- Visualize the protein bands by Coomassie Brilliant Blue staining (see Data analysis, Figure 1).
- Store ISVPs on ice, and use within 2-3 h for hemolysis experiments.
- Propagate and purify reovirus virions as previously described (Berard and Coombs, 2009; Kobayashi et al., 2010). Using a NanoDrop spectrophotometer, determine particle concentration by measuring the optical density of the purified virus stocks at 260 nm (OD260; 1 unit at OD260 = 2.1 x 1012 particles/ml) (Smith et al., 1969).
- Preparation of bovine red blood cells (RBCs)
- Perform all steps on ice or at 4 °C.
- Transfer 1 ml of citrated bovine calf blood to a microcentrifuge tube.
Note: Citrated bovine calf blood should be used within 2 weeks of the draw date. - Pellet the RBCs by centrifugation at 500 x g for 5 min.
Note: RBCs are the source of membranes for hemolysis experiments. - Aspirate and discard the supernatant.
- Resuspend the RBCs in 1 ml of ice cold PBSMg (see Recipes). Mix by gently pipetting up and down.
- Repeat Steps B3-B5 until the supernatant remains clear after pelleting.
- Resuspend the washed RBCs in ice cold PBSMg at a 30% (vol/vol) concentration. Mix by gently flicking the side of the tube.
Note: Estimate the RBC pellet volume by using the volume markers on the microcentrifuge tube. - Store RBCs on ice, and use immediately for hemolysis experiments
- Perform all steps on ice or at 4 °C.
- ISVP-induced hemolysis assay
- In separate microcentrifuge tubes, assemble the following reactions on ice:
- 33.3 µl VB + 3.7 µl 30% RBCs (0% hemolysis control)
- 30.3 µl VB + 3.7 µl 30% RBCs + 3 µl 10% TX-100 (100% hemolysis control, see Recipes)
- 3.3 µl VB + 3.7 µl 30% RBCs + 30 µl ISVPs
- 33.3 µl VB + 3.7 µl 30% RBCs (0% hemolysis control)
- Mix the reactions by gently flicking the side of the tubes.
- Incubate the reactions for 1 h (T3D reovirus) or for 2 h (T1L reovirus) at 37 °C in a water bath.
Note: Under these conditions, ISVP-to-ISVP* conversion is induced (Chandran et al., 2002; Sarkar and Danthi, 2010) and the µ1-derived pore forming peptides are released (Nibert et al., 1991; Chandran et al., 2002; Odegard et al., 2004; Nibert et al., 2005; Zhang et al., 2005; Agosto et al., 2006; Ivanovic et al., 2008). - Place the reactions on ice for 20 min.
- Pellet intact RBCs by centrifugation at 500 x g for 5 min.
Note: This step should be performed at 4 °C. - Transfer 20 µl of each supernatant to individual wells of a 96-well plate.
- Dilute each transferred supernatant with 80 µl of VB. Mix by pipetting up and down 3-4 times.
- To quantify the amount of hemoglobin released (i.e., RBC lysis), measure the absorbance (A) of the diluted supernatants at 405 nm using a microplate reader. A values are recorded on SoftMax Pro software.
- Calculate the percent hemolysis (see Data analysis).
- In separate microcentrifuge tubes, assemble the following reactions on ice:
Data analysis
- Generation of infectious subviral particles (ISVPs)
- Record and analyze the results using a gel imaging system and Image Studio Lite software (Figure 1).
- Virions contain λ1,2,3, µ1C, σ2, and σ3.
- ISVPs contain λ1,2,3, µ1C, δ, and σ2.
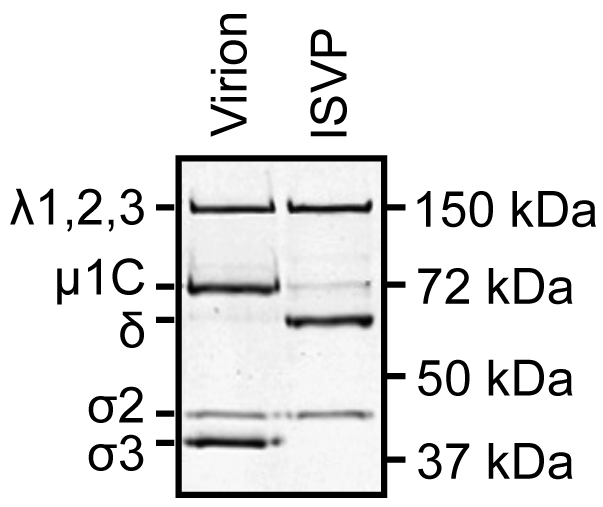
Figure 1. SDS-PAGE gel of reovirus virions and ISVPs - Virions contain λ1,2,3, µ1C, σ2, and σ3.
- Record and analyze the results using a gel imaging system and Image Studio Lite software (Figure 1).
- ISVP-induced hemolysis assay
- All hemolysis experiments should be repeated for at least three independent replicates.
- Calculate the percent hemolysis using the following formula:
[(Asample - Abuffer)/(ATX-100 - Abuffer)] x 100- Abuffer represents the supernatant derived from VB and RBCs.
- ATX-100 represents the supernatant derived from VB, RBCs, and TX-100.
- Asample represents the supernatant derived from VB, RBCs, and ISVPs.
- Abuffer represents the supernatant derived from VB and RBCs.
- When comparing the hemolytic capacity of different reovirus strains, calculate P values using Student’s t-test.
- Use graphing software to plot percent hemolysis.
Note: For T3D reovirus, 40-60% hemolysis is typically observed after 1 h incubation at 37 °C.
- All hemolysis experiments should be repeated for at least three independent replicates.
Notes
- When possible, all procedures are performed in a BSL-2 certified tissue culture hood.
- Laboratory personnel should use appropriate PPE.
- All solid waste is autoclaved prior to disposal.
- All liquid waste is inactivated with 50% bleach prior to disposal.
Recipes
- 50% bleach
In a storage bottle, dilute 50 ml of 100% bleach into 50 ml of ultrapure H2O - Virus storage buffer (VB) (10 mM Tris, pH 7.4, 15 mM MgCl2, and 150 mM NaCl)
- In a glass beaker, dissolve the following into 900 ml of ultrapure H2O:
1.21 g Tris
3.05 g MgCl2·6H2O
8.77 g NaCl - Mix at room temperature using a magnetic stir bar on a stir plate
- Adjust to pH 7.4 with 0.1 N hydrochloric acid
- In a graduated cylinder, bring the final volume up to 1,000 ml with ultrapure water
- Transfer the solution to a storage bottle
- Sterilize by autoclaving
- Store at room temperature3.
- In a glass beaker, dissolve the following into 900 ml of ultrapure H2O:
- 2 mg/ml Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK)-treated chymotrypsin
- In a centrifuge tube, dissolve 100 mg of TLCK-treated chymotrypsin into 50 ml of ultrapure H2O
- Mix at room temperature by gently inverting the tube until the solution becomes clear
- Transfer 1 ml aliquots to microcentrifuge tubes
- Store at -20 °C
- 100 mM phenylmethylsulfonyl fluoride (PMSF)
- In a microcentrifuge tube, dissolve 17.4 mg of PMSF into 1 ml of isopropyl alcohol
- Mix at room temperature by gently inverting the tube until the solution becomes clear
- Store at -20 °C
- Phosphate buffered saline supplemented with 2 mM MgCl2 (PBSMg)
- In a glass beaker, dissolve the following into 900 ml of ultrapure H2O:
9.55 g Dulbecco’s phosphate buffered saline
0.41 g MgCl2·6H2O - Mix at room temperature using a magnetic stir bar on a stir plate
- Adjust to pH 7.4
- In a graduated cylinder, bring the final volume up to 1,000 ml with ultrapure water
- Sterilize by filtering through a 0.22 µm bottle top filter
- Store at room temperature
- In a glass beaker, dissolve the following into 900 ml of ultrapure H2O:
- 10% Triton X-100 (TX-100)
- In a glass beaker, dissolve the following into 80 ml of ultrapure H2O:
0.12 g Tris
0.31 g MgCl2·6H2O
0.88 g NaCl
10 ml of 100% TX-100 - Mix at room temperature using a magnetic stir bar on a stir plate
- Adjust to pH 7.4
- In a graduated cylinder, bring the final volume up to 100 ml with ultrapure water
- Transfer the solution to a storage bottle
- Sterilize by autoclaving
- Store at room temperature
- In a glass beaker, dissolve the following into 80 ml of ultrapure H2O:
Acknowledgments
This protocol was adapted from previously published studies (Chandran et al., 2002; Sarkar and Danthi, 2010). Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers 1R01AI110637 (to P.D.) and F32AI126643 (to A.J.S.) and by Indiana University Bloomington. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The authors declare no conflict of interest.
References
- Agosto,M. A., Ivanovic, T. and Nibert, M. L.(2006). Mammalian reovirus, anonfusogenic nonenveloped virus, forms size-selective pores in a modelmembrane. Proc Natl Acad Sci U S A 103(44): 16496-16501.
- Agosto,M. A., Myers, K. S., Ivanovic, T. and Nibert, M. L. (2008). Apositive-feedback mechanism promotes reovirus particle conversion to theintermediate associated with membrane penetration. ProcNatl Acad Sci U S A 105(30): 10571-10576.
- Baer,G. S. and Dermody, T. S. (1997). Mutationsin reovirus outer-capsid protein sigma3 selected during persistent infectionsof L cells confer resistance to protease inhibitor E64. JVirol 71(7): 4921-4928.
- Berard,A. and Coombs, K. M. (2009). Mammalian reoviruses:propagation, quantification, and storage. CurrProtoc Microbiol Chapter 15: Unit15C 11.
- Borsa,J., Copps, T. P., Sargent, M. D., Long, D. G. and Chapman, J. D. (1973a). Newintermediate subviral particles in the invitro uncoating of reovirus virions by chymotrypsin. JVirol 11(4): 552-564.
- Borsa,J., Morash, B. D., Sargent, M. D., Copps, T. P., Lievaart, P. A. and Szekely,J. G. (1979). Two modes of entry of reovirusparticles into L cells. J Gen Virol 45(1):161-170.
- Borsa,J., Sargent, M. D., Lievaart, P. A. and Copps, T. P. (1981). Reovirus:evidence for a second step in the intracellular uncoating and transcriptaseactivation process. Virology 111(1):191-200.
- Borsa,J., Sargent, M. D., Long, D. G. and Chapman, J. D. (1973b). Extraordinaryeffects of specific monovalent cations on activation of reovirus transcriptaseby chymotrypsin in vitro. JVirol 11(2): 207-217.
- Chandran,K., Farsetta, D. L. and Nibert, M. L. (2002). Strategy for nonenvelopedvirus entry: a hydrophobic conformer of the reovirus membrane penetrationprotein micro 1 mediates membrane disruption. JVirol 76(19): 9920-9933.
- Chandran,K., Walker, S. B., Chen, Y., Contreras, C. M., Schiff, L. A., Baker, T. S. andNibert, M. L. (1999). Invitro recoating of reovirus cores with baculovirus-expressed outer-capsidproteins mu1 and sigma3. J Virol 73(5):3941-3950.
- Chang,C. T. and Zweerink, H. J. (1971). Fate of parental reovirus in infected cell. Virology 46(3): 544-555.
- Dermody,T. S., Nibert, M. L., Wetzel, J. D., Tong, X. and Fields, B. N. (1993). Cellsand viruses with mutations affecting viral entry are selected during persistentinfections of L cells with mammalian reoviruses. JVirol 67(4): 2055-2063.
- Dermody,T. S., Parker, J. S. L. and Sherry, B. (2013). Orthoreoviruses. In: Knipe, D. M.and Howley, P. M. (Eds.). Fields Virology (6th edition). Lippincott Williams & Wilkins: 1304-1346.
- Dryden,K. A., Wang, G., Yeager, M., Nibert, M. L., Coombs, K. M., Furlong, D. B.,Fields, B. N. and Baker, T. S. (1993). Early steps in reovirusinfection are associated with dramatic changes in supramolecular structure andprotein conformation: analysis of virions and subviral particles bycryoelectron microscopy and image reconstruction. JCell Biol 122(5): 1023-1041.
- Ebert,D. H., Deussing, J., Peters, C. and Dermody, T. S. (2002). CathepsinL and cathepsin B mediate reovirus disassembly in murine fibroblast cells. JBiol Chem 277(27): 24609-24617.
- Ehrlich,M., Boll, W., Van Oijen, A., Hariharan, R., Chandran, K., Nibert, M. L. and Kirchhausen,T. (2004). Endocytosis byrandom initiation and stabilization of clathrin-coated pits. Cell 118(5): 591-605.
- Ivanovic,T., Agosto, M. A., Zhang, L., Chandran, K., Harrison, S. C. and Nibert, M. L.(2008). Peptides released fromreovirus outer capsid form membrane pores that recruit virus particles. EMBOJ 27(8): 1289-1298.
- Joklik,W. K. (1972). Studies on the effect ofchymotrypsin on reovirions. Virology 49(3):700-715.
- Kobayashi,T., Ooms, L. S., Ikizler, M., Chappell, J. D. and Dermody, T. S. (2010). Animproved reverse genetics system for mammalian orthoreoviruses. Virology 398(2): 194-200.
- Maginnis,M. S., Forrest, J. C., Kopecky-Bromberg, S. A., Dickeson, S. K., Santoro, S.A., Zutter, M. M., Nemerow, G. R., Bergelson, J. M. and Dermody, T. S. (2006). β1integrin mediates internalization of mammalian reovirus. JVirol 80(6): 2760-2770.
- Maginnis,M. S., Mainou, B. A., Derdowski, A., Johnson, E. M., Zent, R. and Dermody, T.S. (2008). NPXY motifs in the β1 integrincytoplasmic tail are required forfunctional reovirus entry. J Virol 82(7):3181-3191.
- Middleton,J. K., Severson, T. F., Chandran, K., Gillian, A. L., Yin, J. and Nibert, M. L.(2002). Thermostability of reovirusdisassembly intermediates (ISVPs) correlates with genetic, biochemical, andthermodynamic properties of major surface protein mu1. JVirol 76(3): 1051-1061.
- Nibert,M. L. and Fields, B. N. (1992). A carboxy-terminal fragment ofprotein mu 1/mu 1C is present in infectious subvirion particles of mammalianreoviruses and is proposed to have a role in penetration. JVirol 66(11): 6408-6418.
- Nibert,M. L., Odegard, A. L., Agosto, M. A., Chandran, K. and Schiff, L. A. (2005). Putative autocleavage of reovirus mu1 protein inconcert with outer-capsid disassembly and activation for membrane permeabilization. JMol Biol 345(3): 461-474.
- Nibert,M. L., Schiff, L. A. and Fields, B. N. (1991). Mammalian reoviruses contain amyristoylated structural protein. JVirol 65(4): 1960-1967.
- Odegard,A. L., Chandran, K., Zhang, X., Parker, J. S., Baker, T. S. and Nibert, M. L. (2004). Putativeautocleavage of outer capsid protein micro1, allowing release of myristoylated peptide micro1N during particle uncoating,is critical for cell entry by reovirus. JVirol 78(16): 8732-8745.
- Sarkar,P. and Danthi, P. (2010). Determinants ofstrain-specific differences in efficiency of reovirus entry. JVirol 84(24): 12723-12732.
- Silverstein,S. C., Astell, C., Levin, D. H., Schonberg, M. and Acs, G. (1972). Themechanisms of reovirus uncoating and gene activation in vivo. Virology 47(3):797-806.
- Smith,R. E., Zweerink, H. J. and Joklik, W. K. (1969). Polypeptide components ofvirions, top component and cores ofreovirus type 3. Virology 39(4):791-810.
- Snyder,A. J. and Danthi, P. (2015). Lipid membranes facilitateconformational changes required for reovirus cell entry. JVirol 90(5): 2628-2638.
- Snyder,A. J. and Danthi, P. (2016). Lipids cooperate with thereovirus membrane penetration peptide to facilitate particle uncoating. JBiol Chem 291(52): 26773-26785.
- Sturzenbecker,L. J., Nibert, M., Furlong, D. andFields, B. N. (1987). Intracellular digestion ofreovirus particles requires a low pH and is an essential step in the viralinfectious cycle. J Virol 61(8):2351-2361.
- Zhang,L., Agosto, M. A., Ivanovic, T., King, D. S., Nibert, M. L. and Harrison, S. C.(2009). Requirementsfor the formation of membrane pores by the reovirus myristoylated micro1Npeptide. J Virol 83(14): 7004-7014.
- Zhang,X., Ji, Y., Zhang, L., Harrison, S. C., Marinescu, D. C., Nibert, M. L. andBaker, T. S. (2005). Features of reovirus outercapsid protein mu1 revealed by electron cryomicroscopy and image reconstructionof the virion at 7.0 Angstrom resolution. Structure 13(10): 1545-1557.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Snyder, A. J. and Danthi, P. (2018). Infectious Subviral Particle-induced Hemolysis Assay for Mammalian Orthoreovirus. Bio-protocol 8(2): e2701. DOI: 10.21769/BioProtoc.2701.
Category
Microbiology > Microbe-host interactions > Virus
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









