- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mechanical Allodynia Assessment in a Murine Neuropathic Pain Model
Published: Vol 8, Iss 2, Jan 20, 2018 DOI: 10.21769/BioProtoc.2671 Views: 11524
Reviewed by: Khyati Hitesh ShahAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for Measuring Free (Low-stress) Exploration in Rats
Wojciech Pisula and Klaudia Modlinska
Jan 20, 2020 4457 Views

Operant Vapor Self-administration in Mice
Renata C. N. Marchette [...] Khaled Moussawi
May 20, 2021 4562 Views

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1770 Views
Abstract
Experimental animal models are unique tools (i) to study pain transmission and pathophysiology of neuropathic pain, (ii) to identify novel molecular targets and (iii) to test the potential analgesic effect of specific molecules. The chronic constriction injury (CCI) model of neuropathic pain is the first model of post-traumatic painful peripheral neuropathy, originally developed by Bennett and Xie in the late 1980s. The chronic constriction is performed in the sciatic nerve and induces a partial denervation involving myelinated afferent axons and unmyelinated axons. Damage to unmyelinated axons is much more severe than myelinated afferents. As the model induces a partial denervation, it is very useful for the analysis of pain behaviours. Stimulation of the hind paw, a target of the sciatic nerve, induces pain which can be quantitated. Thus, mechanical allodynia is usually assessed 7, 14 and 21 days after CCI of the sciatic nerve by measuring the hind paw withdrawal response to von Frey filament stimulation. Here, we describe in detail the protocol allowing a reliable and reproducible CCI model in mice. Overall, researchers most commonly use this surgical model to discover more efficacious drugs for the pharmacological control of chronic pain states.
Keywords: PainBackground
The chronic constriction injury (CCI) model of neuropathic pain was first developed by Bennett and Xie (1988). The chronic constriction is applied to the sciatic nerve mimicking a post-traumatic painful peripheral neuropathy. This model induces a partial denervation and, therefore, is very useful for a quantitative analysis of pain behaviours and for the evaluation of analgesic effect of novel drugs. The CCI of the sciatic nerve is carried out under isoflurane anesthesia (5% for induction and 2% for maintenance). The biceps femoris and the gluteus superficialis are separated by dissection to expose the sciatic nerve. The CCI is induced by loosely tying one ligature around the sciatic nerve, to preserve epineural circulation.
Analysis of pain behaviour is assessed by measuring mechanical allodynia 7, 14 and 21 days after surgery. Interestingly, one of the advantages of the CCI model is the objective score of pain behaviour in response to von Frey stimulation. Mechanical allodynia is quantitated by measuring the hind paw withdrawal response to von Frey filament stimulation. Mice are placed in a dark box with a wire grid bottom through which the von Frey filaments are applied by using the up-down paradigm previously described (Chaplan et al., 1994). Lack of response to a filament indicates the next filament with a higher bending force in the following stimulation, whereas a positive response indicated the next filament with a lower bending force. Each filament is applied and pressed perpendicularly to the plantar surface of the hind paw until it bends. The filament that evokes 3 paw withdrawals is assigned as the pain threshold in grams. Mice are treated with vehicle, novel potential analgesic drugs, or a classical analgesic drug, as positive analgesic control, and the mechanical thresholds are quantified. This protocol has been recently implemented by our research team (Font et al., 2017).
Overall, CCI model and measurements of mechanical allodynia could be considered as an experimental approach to assess the analgesic activity of any potential drug. Thus, we provide here a complete description of the CCI models and assessment of mechanical allodynia aiming at facilitating its implementation by other scientists.
Materials and Reagents
- Sterile gauze
- Syringes. BD Micro-FineTM Demi, U-100 Insulin, 30 G x ½’’–0.33 x 8 mm (BD, catalog number: 324826 )
- Eppendorf tubes (Eppendorf, catalog number: 0030120086 )
- Scalpel blades (Sigma-Aldrich, catalog number: S2646 )
- 6-0 silk suture (Ethicon, catalog number: MCP492G )
- 5-0 Dermalon suture (Covidien, catalog number: 1756-21 )
- Animals
Adult C57BL/6J male mice (Charles River, Calco, Italy) weighing 20-25 g are used. Six-week-old male mice are allowed to habituate in the animal room for 2 weeks
Note: All animals are housed in groups of five in standard cages with access to food and water ad libitum, and maintained under 12 h dark/light cycle (starting at 7:30 AM), 22 °C temperature, and 66% humidity. All manipulations are carried out between 9:00 and 16:00 h. Procedures were performed in accordance with relevant guidance from the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), the Guide for the Care and Use of Laboratory Animals (Clark et al., 1997) and European Union directives (2010/63/EU). The ethics committees of the IRCCS Neuromed Institute and the Italian Ministry of Health approved the protocol. All efforts were made to minimize suffering and reduce the number of animals used in the experiments. To reach statistical significance, at least 10 animals per group are highly recommended. - Isoflurane (Sigma-Aldrich, catalog number: Y0000858 )
- Potential analgesic drug to be tested
Note: In the example provided here we used raseglurant, a negative allosteric modulator of the metabotropic glutamate type 5 (mGlu5) receptor (Font et al., 2017). - Vehicle
Sterile physiological saline (0.9% NaCl) solution (B. Braun Melsungen, catalog number: 3570380 ) - Classical analgesic drugs for comparative purposes
- Sterile cleaning solution
Note: A 70% ethanol in H2O was used.
Equipment
- Surgical instruments, surgery bench, inhalation anaesthesia apparatus
- Ugo Basile Gas Anaesthesia (Ugo Basile, catalog number: 22100 ), Ugo Basile Induction Box 7900 (Ugo Basile, catalog number: 7900/10 ) (Figure 1)

Figure 1. Apparatus for inhalation anaesthesia in mice and rats with an induction chamber (25 x 13 x 13 cm) - Surgical Microscope Leica Wild M650 (Leica, model: Leica Wild M650) (Figure 2)

Figure 2. Stereomicroscope with an anaesthetized mouse ready to undergo CCI - Fine watchmaker forceps (Sigma-Aldrich, catalog number: T4537-1EA )
- Curved blunt-tipped forceps (Sigma-Aldrich, catalog number: T4787-1EA )
- Micro-dissecting scissor (Sigma-Aldrich, catalog number: S3146-1EA )
- Mathieu needle holder (Fine Science Tools, catalog number: 12510-14 )
- Balances (Sartorius, models: CP124S and BL1500S )
- von Frey filaments (North Coast Medical, Inc., San Jose, CA, USA) (Figure 3)
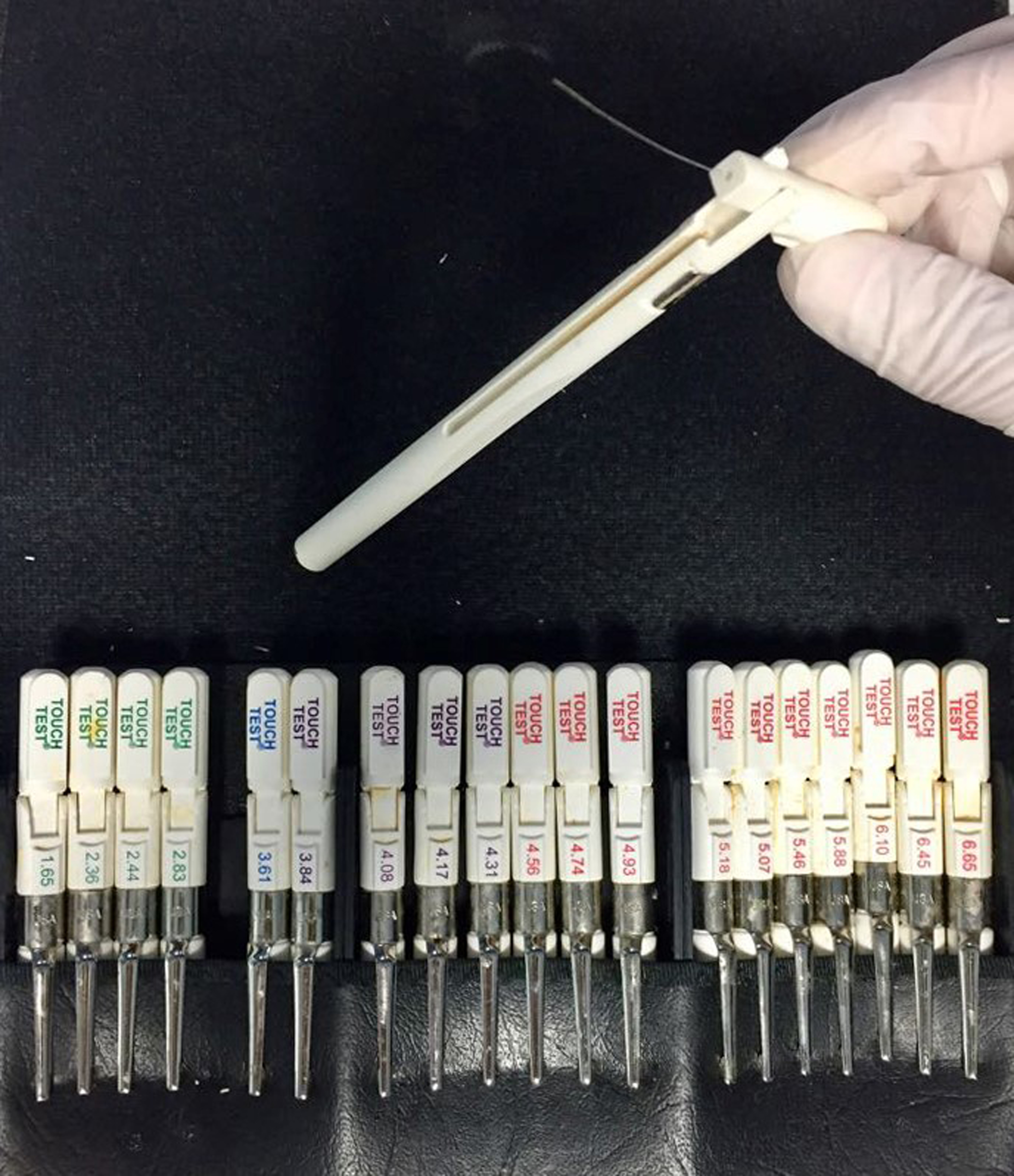
Figure 3. von Frey filaments used to assess mechanical allodynia
Procedure
- Surgical procedure
- Anaesthetize mice in an induction chamber by inhalation of 2.5% isoflurane in N2O/O2 (70:30), maintained at 2% by a facemask throughout surgery.
- Place mouse under a stereomicroscope, remove hair in the right or left hind leg and sterilize the area by the use of a sterile gauze with 70% alcohol.
- Place the mouse in horizontal position with the femur rotated by 90° with the aid of masking tape placed on the foot (Figure 4).
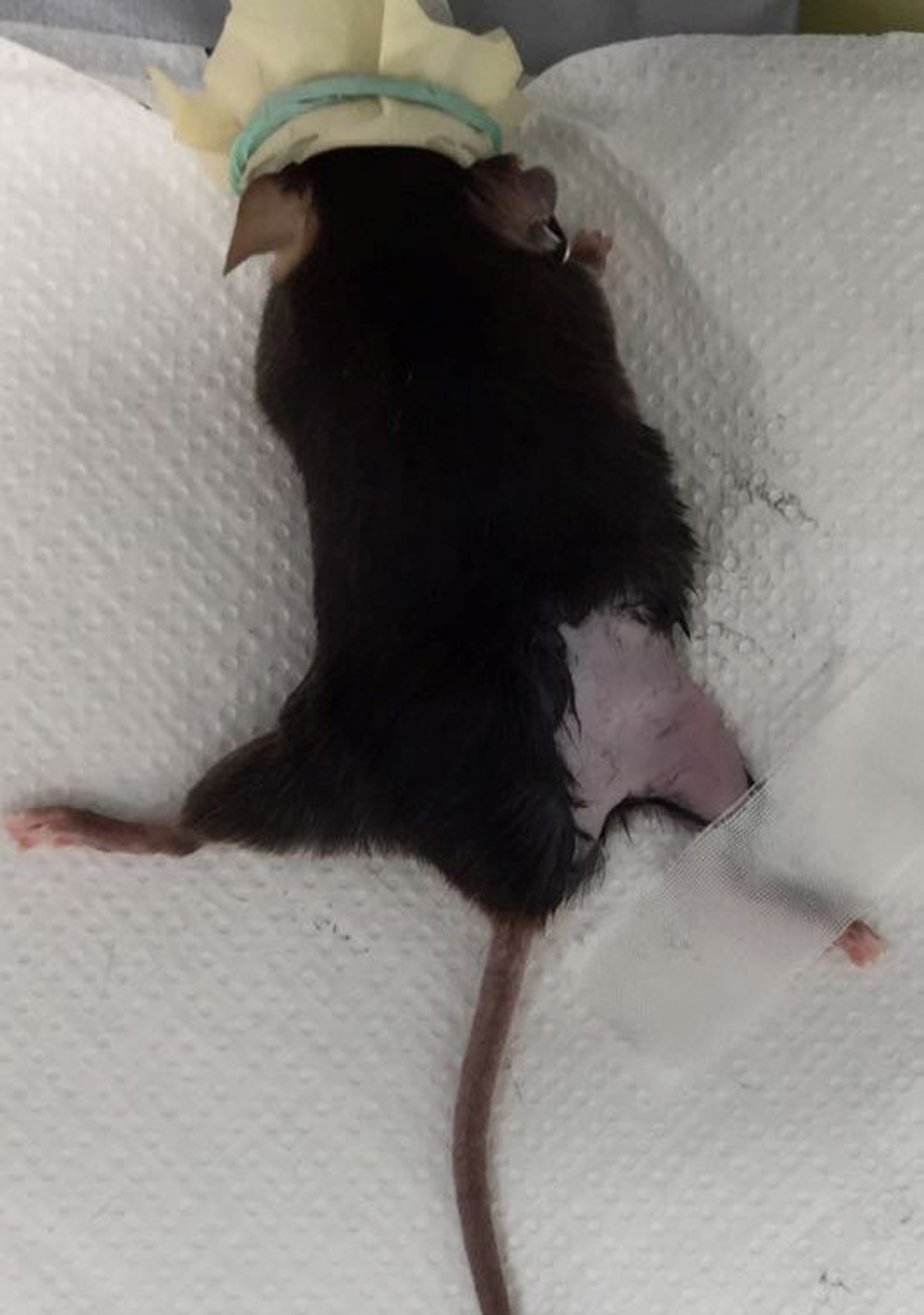
Figure 4. Mouse placed in horizontal position with the right leg rotated at 90° with the aid of masking tape placed on the foot - Make an incision in the skin and separate the muscles between the gluteus superficialis and the biceps femoris by cutting the connective tissue (Figure 5).
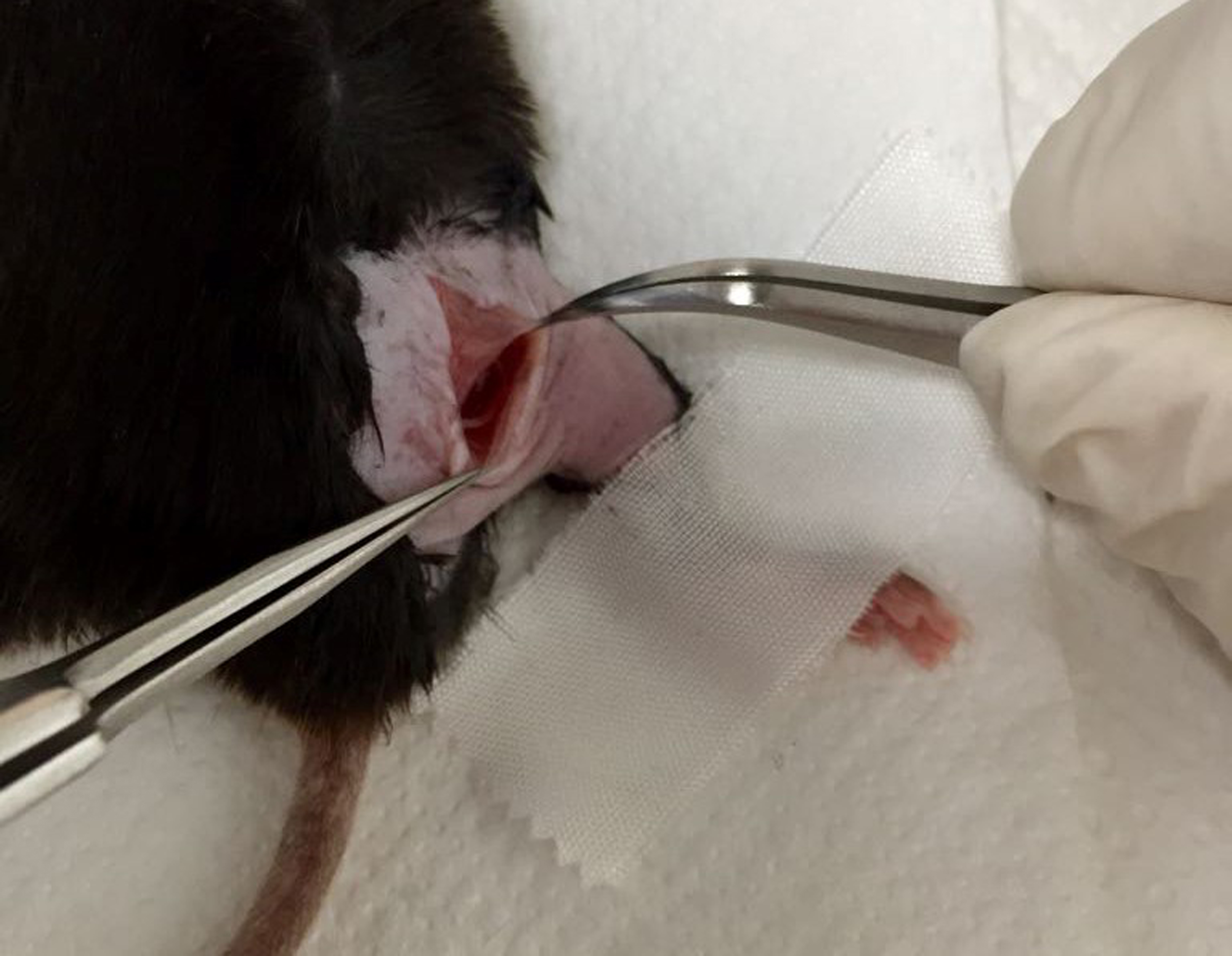
Figure 5. Separation of muscles gluteus superficialis and biceps femoris by cutting the connective tissue - Move the two muscles to uncover the sciatic nerve.
- Using curved blunt-tipped forceps and micro-scissors, softly free the sciatic nerve (proximal to the sciatic trifurcation) from the surrounding connective tissue.
- Under a dissecting microscope, make one ligature, proximal to the nerve trifurcation, while taking care to preserve epineural circulation, around the sciatic nerve with 6-0 silk (Figure 6). The ligature has to be tied loosely around the nerve, until it elicits a brief twitch in the respective hind limb, which prevents over-tightening of the ligation.
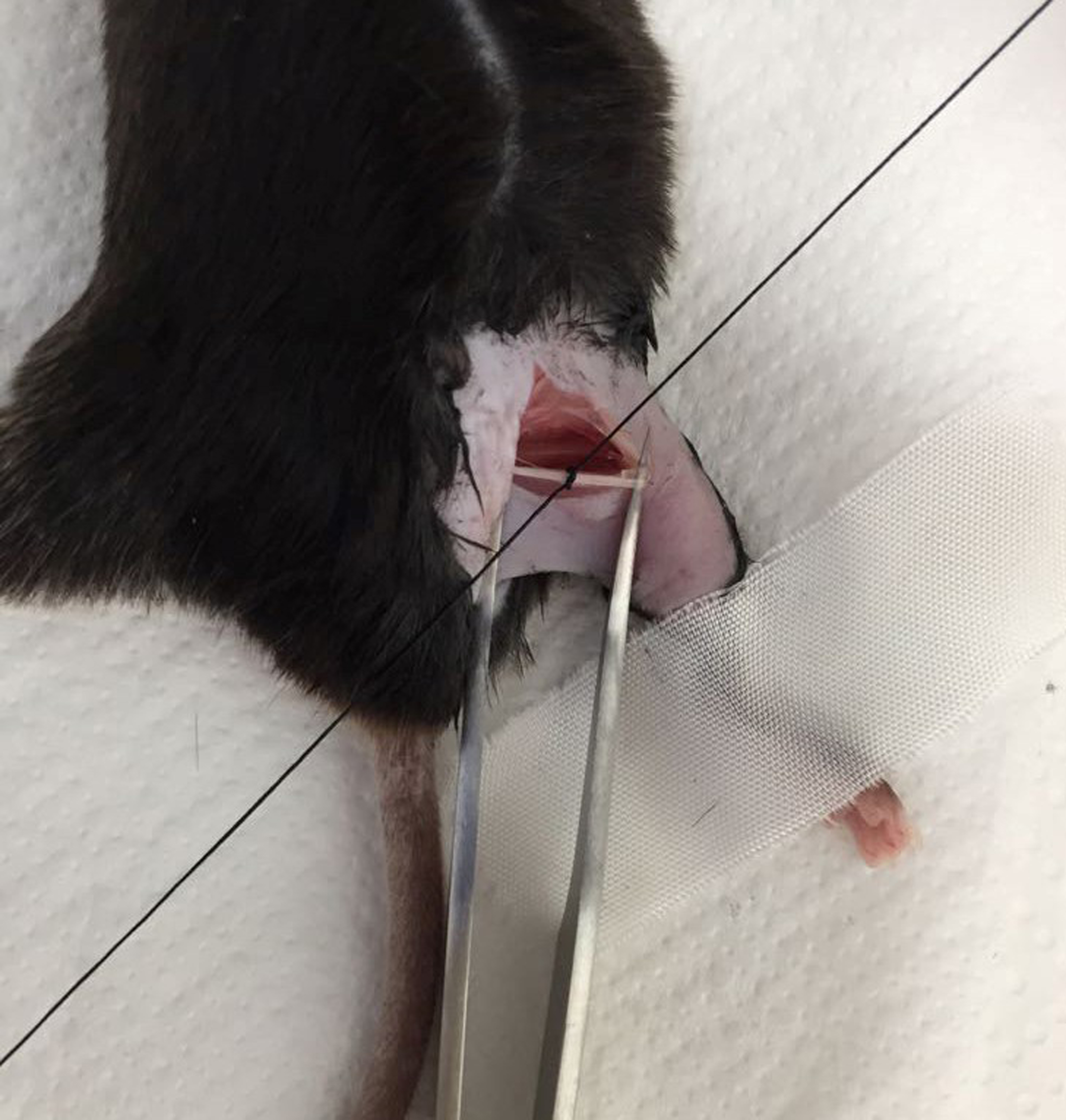
Figure 6. Under the dissecting microscope, free the sciatic nerve by curved blunt-tipped forceps and make one ligature with 6-0 silk - Stitch the muscle layer.
- Clean the incision and close the skin with 2-3 ligatures of 5-0 dermalon. Apply an iodine solution (Rodine, 10% solution) to disinfect the wound.
- Return the mouse to its home cage and check routinely for 72 h.
- Clean all the material used.
- Anaesthetize mice in an induction chamber by inhalation of 2.5% isoflurane in N2O/O2 (70:30), maintained at 2% by a facemask throughout surgery.
- Mechanical allodynia measurement
- Handle mice daily in order to reduce stress.
- Prior to behavioural testing, mice need to be habituated to the testing procedure and the restricted area. The testing environment should be kept quiet and well controlled, with constant temperature and humidity levels. Testing sessions should be carried out at the same time (9:00-14:00).
- Reagents preparation (weight the analgesic drug and dissolve in vehicle).
- Remove the mouse from the house cage and measure its body weight using a balance.
- Administration of the vehicle or analgesic drug.
Administer (i.e., intraperitoneally, i.p.) the analgesic drug to be tested (i.e., raseglurant) or the same volume of vehicle (physiological saline solution) using an insulin syringe.
Note: The amount (400-500 μl) of drug to be administered should be adjusted according to the dose to be tested (i.e., 10 mg/kg of raseglurant) and the mouse body weight (20-25 g). For example, for a mouse of 20 g of weight we will administer (i.p.) 400 μl of a 500 mg/L raseglurant solution in saline. In addition, all animal experimentation should be carried out by a researcher blind to drug treatments. - Place the mouse in a plastic cylinder placed on a wire mesh table. Habituate for 15 min in cylinders prior to testing to allowing mouse to stay calm and still.
- After 20 min, assess mechanical pain thresholds by means of von Frey filaments (Figure 3). Thus, apply filament, bending force range from 0.008 to 3.5 g, to the mid-plantar surface of the hind paw (Figure 7).

Figure 7. Quantification of mechanical thresholds by the von Frey filaments - Apply each filament, starting from the one with a bending force of 0.008 g, and press it perpendicularly to the plantar surface of the hind paw until it bent for five times over a total period of 30 sec (approximately 2 sec per stimulus) and measure the mouse leg withdrawal after each application (Chaplan et al., 1994). Repeat this procedure 5 times with a 3-min interval. Response in 3 out of 5 stimuli is considered as a positive reaction and the filament is assigned as the pain threshold in grams. Lack of response to a filament indicated the next filament with a higher bending force in the following stimulation, whereas a positive response indicated the next filament with a lower bending force.
- Return the mouse to its home cage.
- Clean all the material used.
- Handle mice daily in order to reduce stress.
Data analysis
Representative example of data illustrating the type of results obtained is provided below.
- Administration of the analgesic drug (i.e., raseglurant) significantly increased pain thresholds in CCI mice (Figure 8).
- The results are analyzed either by Student’s t-test or by one-way analysis of variance (ANOVA) followed by a post hoc test, according to the experimental paradigm. P values < 0.05 are considered significant.
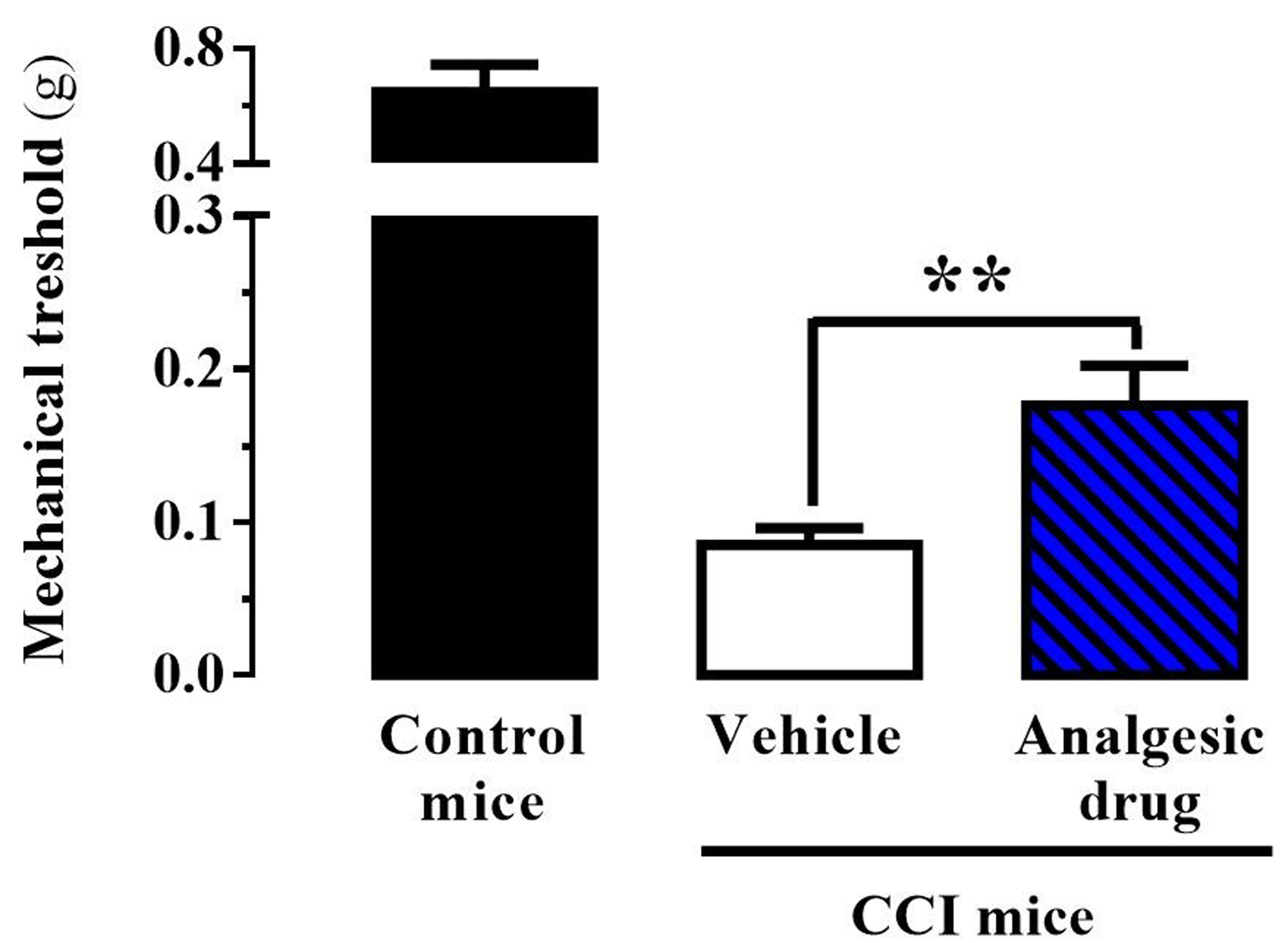
Figure 8. Representative results. Antinociceptive effect of raseglurant (10 mg/kg) as analgesic drug using the CCI of the sciatic nerve animal model. Mechanical allodynia was measured in 21 days post-surgery CCI mice. Thus, animals were intraperitoneally injected with vehicle (Veh, saline) or raseglurant (Ras, 10 mg/kg) and 20 min later the mechanical thresholds were assessed using the von Frey filaments. Values are means ± SEM of 7-9 mice per group. **P < 0.001 Student’s t-test when compared to the vehicle (physiological saline solution) treated animals. Extracted from Font et al., 2017.
Acknowledgments
This work was supported by ERANET Neuron project ‘LIGHTPAIN’. This protocol was adapted from previous work: Font et al., 2017. The authors declare not conflict of interest.
References
- Bennett, G. J. and Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1): 87-107.
- Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. and Yaksh, T. L. (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1): 55-63.
- Clark, J. D., Gebhart, G. F., Gonder, J. C., Keeling, M. E. and Kohn, D. F. (1997). Special report: The 1996 guide for the care and use of laboratory animals. ILAR J 38(1): 41-48.
- Font, J., Lopez-Cano, M., Notartomaso, S., Scarselli, P., Di Pietro, P., Bresoli-Obach, R., Battaglia, G., Malhaire, F., Rovira, X., Catena, J., Giraldo, J., Pin, J. P., Fernandez-Duenas, V., Goudet, C., Nonell, S., Nicoletti, F., Llebaria, A. and Ciruela, F. (2017). Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. Elife 6.
Article Information
Copyright
Notartomaso et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Notartomaso, S., Scarselli, P., Di Pietro, P., Battaglia, G., Llebaria, A., Ciruela, F. and Nicoletti, F. (2018). Mechanical Allodynia Assessment in a Murine Neuropathic Pain Model. Bio-protocol 8(2): e2671. DOI: 10.21769/BioProtoc.2671.
- Font, J., Lopez-Cano, M., Notartomaso, S., Scarselli, P., Di Pietro, P., Bresoli-Obach, R., Battaglia, G., Malhaire, F., Rovira, X., Catena, J., Giraldo, J., Pin, J. P., Fernandez-Duenas, V., Goudet, C., Nonell, S., Nicoletti, F., Llebaria, A. and Ciruela, F. (2017). Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. Elife 6.
Category
Neuroscience > Behavioral neuroscience > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








