- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Primary Cultures of Embryonic Rat Hippocampal and Cerebrocortical Neurons
Published: Vol 7, Iss 18, Sep 20, 2017 DOI: 10.21769/BioProtoc.2551 Views: 18436
Reviewed by: Kae-Jiun ChangEmmanuelle BerretAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1417 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2411 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 211 Views
Abstract
This protocol aims at standardizing the procedure to obtain primary cultures of hippocampal and cerebrocortical neurons for in vitro experiments. Cultures should be prepared from cells isolated during embryonic development when neuronal precursor cells are not yet fully differentiated. This helps increasing the quality and quantity of cells, while offering minimal cell death that often occurs during dissociation of differentiated neurons. Cells plated under the appropriate conditions, either in Petri-dishes or in multi-well plates, will develop and establish synaptic contacts over time since the neuronal culture medium provides the nutrients and trophic factors required for differentiation. In this protocol we describe the methodology for the preparation of both cortical and hippocampal neuronal cultures.
Keywords: Primary culturesBackground
The present protocol describes the preparation of primary cultures of rat hippocampal and cerebrocortical neurons, using Neurobasal medium supplemented with NeuroCultTM SM1 (Chen et al., 2008). The composition of NeuroCultTM SM1 is based on the formulation of the B27 supplement (Brewer et al., 1993), but the former cocktail was found to improve the quality of neuronal cultures, in part by replacement of apo-transferrin with holo-transferrin (Chen et al., 2008). Furthermore, the chemical composition of NeuroCultTM SM1 was described in more detail in the original publication, allowing a better control of the experimental conditions. Neuronal cultures prepared with chemically defined culture media are characterized by the presence of a low percentage of astrocytes. The proliferation of astrocytes in cultures maintained for longer periods of time, in order to allow differentiation of neurons, is prevented by adding the chemical inhibitor of mitosis 5-Fluoro-2’-deoxyuridine.
Materials and Reagents
- Petri dish, 55 mm Polysterene aseptic non-tissue culture treated (Labbox, catalog number: PDIP-06N-500 )
- Petri dish, 150 mm glass soda-lime (DWK Life Sciences, Duran, catalog number: 23 755 52 )
- 15 ml conical tube (Corning, Falcon®, catalog number: 352096 )
- 5 ml glass pipetes (VWR, catalog number: 612-4124 )
- 10 ml glass pipetes (VWR, catalog number: 612-4125 )
- 50 ml conical tube (Corning, Falcon®, catalog number: 352070 )
- Cell strainer 70 μm (Corning, Falcon®, catalog number: 352350 )
- Poly-D-lysine-coated multi-well plate (see step 16 in ‘Procedures’ for instructions)
- Coverslips #1.5 (e.g., Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 1014355110NR15 ; for 10 mm coverslips)
- Mixed cellulose ester filter, ME Range (ME 24), 0.2 μm pore size for filtration unit (GE Healthcare, Whatman, catalog number: 10406970 )
- Acetate cellulose filters of Ø25 mm, 0.20 μm, sterile (FRILABO, catalog number: 1520012 )
- Filtration unit (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: DS0320-5033 )
- Stericup-GV, 0.20 µm, PVDF, 500/1,000 ml, radio-sterilized (Merck, catalog number: SCGVU10RE )
- Pregnant female Wistar rats (E17-E18 days gestation)
- Trypan blue solution, 0.4% (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- Poly-D-lysine hydrobomide (Sigma-Aldrich, catalog number: P7886 )
- Boric acid (Merck, catalog number: 1001651000 )
- Nitric acid (Applichem, catalog number: 133255.1612 )
- Ethanol absolute 99.8+% (Fisher Scientific, catalog number: 10342652 )
- 5-Fluoro-2’-deoxyuridine (5-FDU) (Sigma-Aldrich, catalog number: F0503 )
- Potassium chloride (KCl) (AppliChem, catalog number: 131494.1211 )
- Potassium phosphate dibasic (K2HPO4) (Merck, catalog number: 1051041000 )
- Sodium chloride (NaCl) (Applichem, catalog number: 131659.1211 )
- Sodium bicarbonate (NaHCO3) (Acros Organics, catalog number: 123360010 )
- Sodium phosphate dibasic dihydrate (Na2HPO4·2H2O) (Merck, catalog number: 1065800500 )
- D(+)-Glucose monohydrate (VWR, catalog number: 24371.297 )
- Sodium pyruvate (Sigma-Aldrich, catalog number: P5280 )
- HEPES (Fisher Scientific, catalog number: BP310-1 )
- Phenol red (Sigma-Aldrich, catalog number: P4758 )
- Trypsin (Thermo Fisher Scientific, GibcoTM, catalog number: 27250018 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270106 )
- Minimum essential medium Eagle (MEM) (Sigma-Aldrich, catalog number: M0268 )
- Neurobasal Medium® (Thermo Fisher Scientific, GibcoTM, catalog number: 21103049 )
- NeuroCultTM SM1 Neuronal Supplement (STEMCELL Technologies, catalog number: 05711 )
- L-Glutamine (Sigma-Aldrich, catalog number: G8540 ; Thermo Fisher Scientific, GibcoTM, catalog number: 25030024 )
- Glutamate (Sigma-Aldrich, catalog number: G1626 )
- Horse serum (Thermo Fisher Scientific, GibcoTM, catalog number: 16050122 )
- Sodium hydroxide solution
- Hanks’ balanced salt solution (HBSS) (see Recipes)
- Trypsin solution (2 mg/ml) (see Recipes)
- 10% FBS (see Recipes)
- Neuronal Plating Medium (see Recipes)
- Supplemented neuronal culture medium (see Recipes)
- Boric acid solutions (see Recipes)
Equipment
- Large and small scissors
- Forceps with straight tip (Fine Science Tools, model: Dumont #5 )
- Forceps with curved tip (Fine Science Tools, model: Dumont #5 /45 forceps–Dumont standard tip)
- Pipetboy (Integra Biosciences, model: PIPETBOY acu 2 )
- Magnification glass
- Laminar flow hood
- Water-bath (Medingen Labortechnik, model: T100 )
- Phase contrast inverted microscope equipped with a 10x objective (Nikon Instruments, model: Eclipse TS100 )
- Hemocytometer (Marienfeld-Superior, catalog number: 0640010 )
- Humidified incubator
Procedure
- Remove E18 embryos (to prepare cultures of hippocampal neurons) from a pregnant Wistar rat, anesthetized and sacrificed as previously described, by cesarean-section (Caldeira et al., 2013; Mele et al., 2014; Curcio et al., 2015; Mele et al., 2017).
Note: To culture cerebrocortical neurons, it is recommended to use E17 embryos. The same procedure also applies. - Transfer the embryos to a 150 mm glass Petri dish filled with ice-cold HBSS (150 ml should be enough; see Recipes).
- Sacrifice the embryos by decapitation with scissors and remove the brains (Figure 1). Separate the brain hemispheres and remove the cerebellum with forceps (and other non-essential parts; Figure 2). Carefully remove the meninges under a dissecting microscope using both curved and straight forceps (Video 1).

Figure 1. Brain of an E18 rat embryo observed at different magnification levels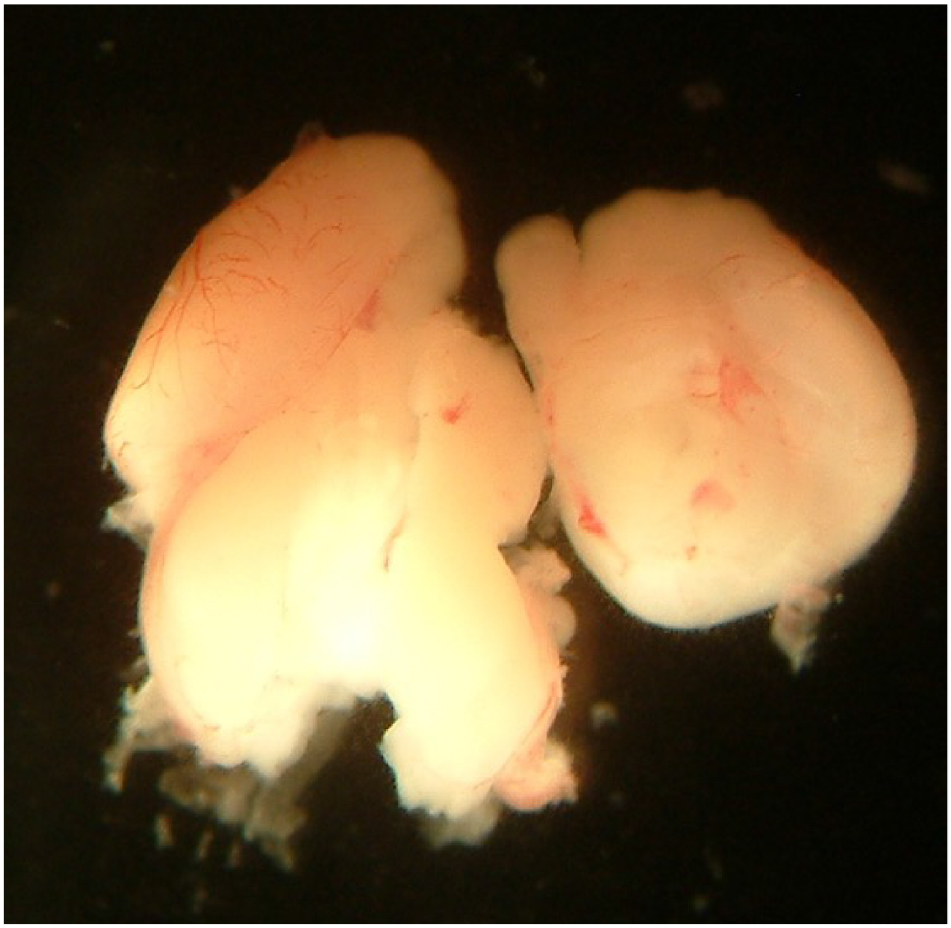
Figure 2. Cerebral cortex from the brain of an E18 rat embryo with the hippocampus still enclosed. The meninges can still be observed at the surface of the cerebral cortex.
Video 1. Initial steps in the dissection of the rat hippocampal - Dissect and isolate the hippocampi and transfer the tissue to a 55 mm plastic Petri dish filled with HBSS (5-7 ml should be enough; see Figure 3 for end result). The use of a dissecting microscope will facilitate this procedure.
Notes:- We recommend to use the hippocampi from all the embryos obtained from the pregnant rat since the cell yield is low.
- To prepare cerebrocortical neurons, dissect the two cortices (see Figure 3 for end result).
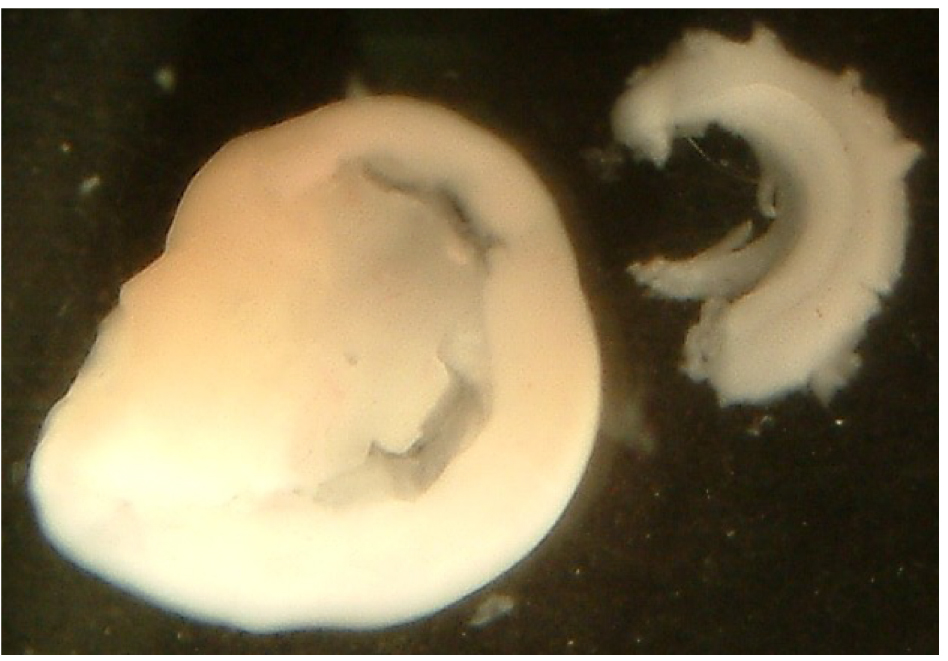
Figure 3. Dissected cerebral cortex (left side) and the isolated hippocampus (right side) - We recommend to use the hippocampi from all the embryos obtained from the pregnant rat since the cell yield is low.
- Take the Petri dish with all the hippocampi obtained from the pregnant rat to a laminar flow hood for sterile conditions.
- Collect all intact hippocampi (or cortices) by pipetting them to a 15 ml conical tube and bring the volume of HBSS to about 6 ml.
Notes:- Use a glass pipette of 10 ml. This type of pipette has a wider tip that avoids damaging the tissue.
- For cerebrocortical neurons, wash the tissue three times with HBSS (5-8 ml is enough) by allowing the tissue to deposit by gravity; the obtained final volume after the second washing step should be as mentioned in step 6.
- Tip: Use four hemispheres for each 15 ml conical tube for preparation of cerebrocortical neuron cultures.
- Use a glass pipette of 10 ml. This type of pipette has a wider tip that avoids damaging the tissue.
- Add 1.5 ml of the trypsin solution (see Recipes) to the medium containing the hippocampi and incubate for 15 min at 37 °C. At the end of the incubation period the tissue should be sedimented.
Notes:- A water-bath system can be used for this incubation.
- For cerebrocortical neurons, this process should last 10 min.
- A water-bath system can be used for this incubation.
- Allow the tissue to deposit, and carefully remove the supernatant in the laminar flow hood.
- Add 8 ml of previously warmed (37 °C) 10% FBS solution (see Recipes) to the sedimented hippocampi and agitate gently.
- Allow the tissue to sediment and wash out the 10% FBS solution by removing the supernatant and rinsing the hippocampi with 10 ml of previously warmed (37 °C) HBSS.
- Remove the supernatant and add 5 ml of previously warmed (37 °C) supplemented neuronal culture medium (see Recipes).
To prepare cultures of cerebrocortical neurons, steps 10-12 must be substituted by washing the tissue five times with ice-cold HBSS (6-9 ml) and by bringing the volume to about 4 ml of HBSS in the last washing step.
Note: This volume was found to be optimal for having a final concentration of cells of about 3-5 x 106/ml of solution. - Use mechanical force provided by the Pipetboy to dissociate the tissue with a sterile 5 ml glass pipette, pipetting up and down the hippocampi suspension.
Notes:- After dissociation of the cells, the suspension becomes cloudy (see Figure 4).
- The mechanical dissociation to prepare cultures of hippocampal neurons is performed in supplemented neuronal culture medium, whereas HBSS should be used in the preparation of cerebrocortical neurons.
- Tip: The tip of the 5 ml glass pipette should be in close proximity with the bottom of the 15 ml conical tube, keeping only a narrow space to allow the passage of the solution. When the suspension is pipetted up and down, the medium will become cloudy as a result of the cell dissociation.
- Since this procedure is rather aggressive to the cells it should not be repeated more than 10 times.

Figure 4. Cell suspension after dissociation of the cerebrocortical tissue (right). The tube on the left contains the non-dissociated cortical tissue. Similar results are obtained after dissociation of hippocampal cells (not shown). - After dissociation of the cells, the suspension becomes cloudy (see Figure 4).
- Collect the cell suspension solution and filter to a sterile 50 ml conical tube through a cell strainer of 70 μm.
Note: This step allows separating the cells in suspension from the small aggregates of non-dissociated tissue that are retained. - To count the number of cells, dilute equal volumes of the following solutions: HBSS, cell suspension, trypan blue (50 μl of each should be enough).
- Add 10 μl of the cell suspension prepared in step 14 to a hemocytometer. Using an inverted phase contrast microscope equipped with a 10x objective, count the cells present in each of the four quadrants located at the corners (area = 0.01 cm2; volume 0.0001 cm3).
Note: The number of cells present in each ml of solution can be calculated using the following formula: average number of cells/square x 3 (dilution factor in step 14) x 104 = number of cells/ml of solution. - Plate the cells in a poly-D-lysine-coated multi-well plate at the appropriate density.
- To prepare the poly-D-Lysine solution, first dilute the 100 mg vial in 100 ml of boric acid (0.1 M, pH 8.2). Aliquots of this solution should be stored at -20 °C until further use. The resulting solution (1 mg/ml) should be further diluted in boric acid (166.3 mM, pH 8.2) and filtered immediately before coating the plates, to obtain a final concentration of 0.1 mg/ml. To coat the plastic dishes, add enough solution to cover the bottom of the wells and incubate for 2 h at 37 °C (overnight incubation in a humidified incubator is recommended). After this coating period, wash each well twice with enough volume of sterile distilled water to cover the bottom of the well, and allow the wells to dry inside the laminar flow hood.
- The protocol for coverslip coating is as follows: 1) wash the coverslips in nitric acid overnight with constant agitation, in a closed glass recipient (we use an 11 x 9 x 7 cm; 0.5 cm thick container); 2) after removing carefully nitric acid, with the help of a plastic pipet, wash abundantly with distilled water (5 washes, 20-30 min each); 3) wash the coverslips for 5 min with absolute ethanol; 4) dry the coverslips at 160 °C for 1 h (in a closed glass container); 5) sterilize the coverslips by UV radiation inside a flow chamber, in a glass Petri-dish for about 30 min. To coat the coverslips, transfer them to the appropriate multi-well plates, and follow the instructions mentioned in step 16a.
Notes:- Tip 1: For a glass container with 11 x 9 x 7 cm we wash a maximum of 100 coverslips. The coverslips may stick onto each other if a higher number is used.
- Tip 2: The poly-D-lysine solution used to coat the wells can be recycled once if used in consecutive days.
- Tip 3: in a 11 x 9 x 7 cm, 0.5 cm thick glass container we use 50 ml of water/absolute ethanol.
- Hippocampal neurons are plated at a density of 90.0 x 103 cells/cm2 whereas cerebrocortical neurons are plated at 92.8 x 103 cells/cm2.
- During plating, hippocampal neurons can be diluted and plated directly in supplemented neuronal culture medium containing 25 μM of glutamate (see Recipes), whereas cerebrocortical neurons must be plated in neuronal plating medium (see Recipes). After 2-3 h of incubation at 37 °C in a humidified incubator with 5% CO2/95% air, replace the neuronal plating medium with fresh supplemented neuronal culture medium without glutamate (only valid for the preparation of cerebrocortical neurons).
- Feed hippocampal neurons once a week, by changing equal parts of volume (1/3 of the initial volume of culture medium), and cerebrocortical neurons twice a week by changing equal parts of volume (¼ of the initial volume of culture medium). At day 3 replace 1/3 of the culture medium with fresh medium (devoid of glutamate) containing 30 μM 5-FDU (final concentration–10 μM; the stock solution is kept at 10 mM) in order to stop the growth of glia cells. In this step always use supplemented neuronal culture medium lacking glutamate. Subsequent neuronal feeding should be done by using supplemented neuronal culture medium devoid of glutamate and 5-FDU. See Figures 5-8 for final results.
- Tip 1: For a glass container with 11 x 9 x 7 cm we wash a maximum of 100 coverslips. The coverslips may stick onto each other if a higher number is used.
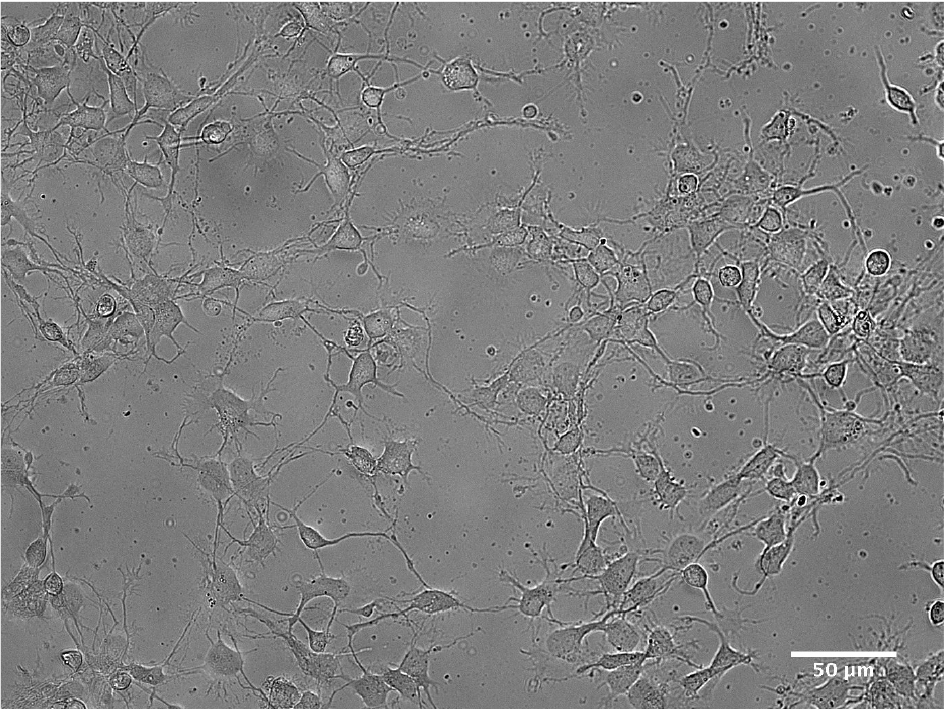
Figure 5. Cultured hippocampal neurons with 1 DIV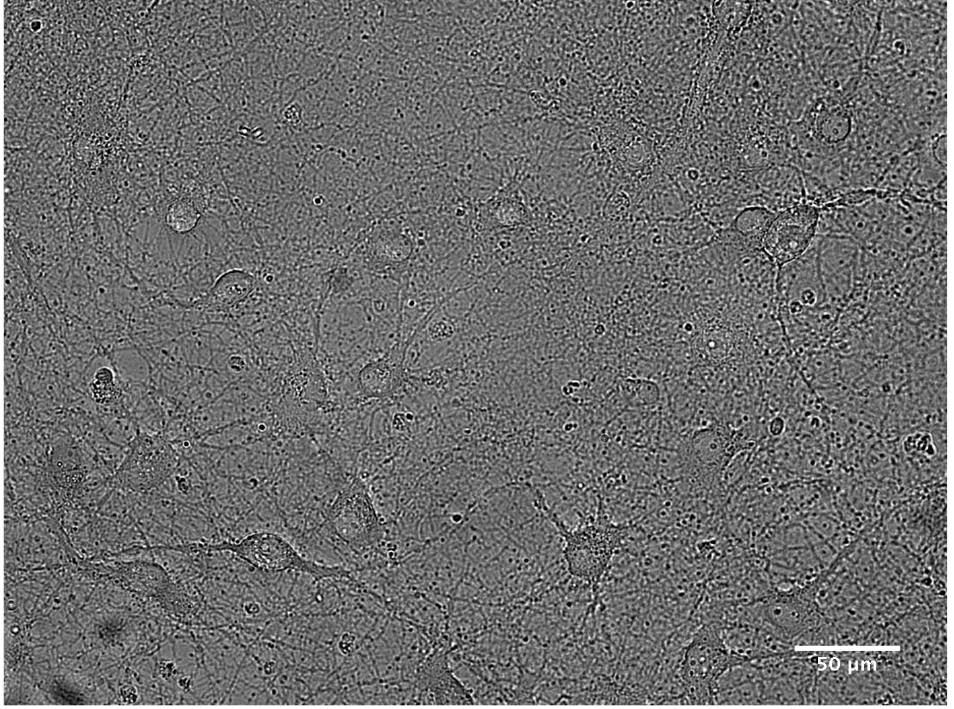
Figure 6. Example of cultured hippocampal neurons with 15 DIV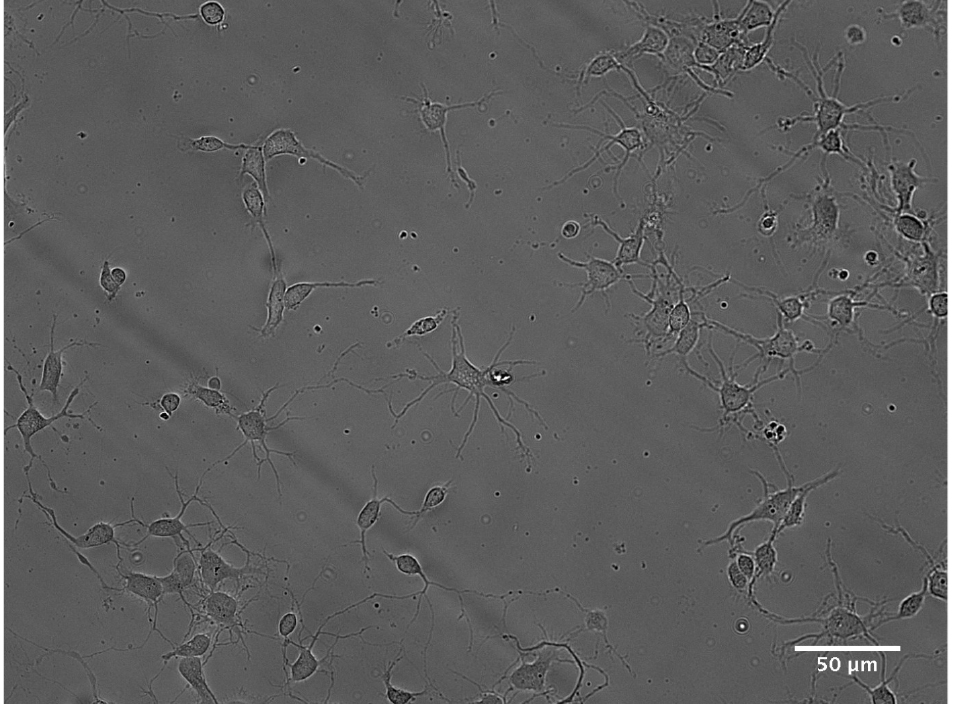
Figure 7. Cultured cerebrocortical neurons with 1 DIV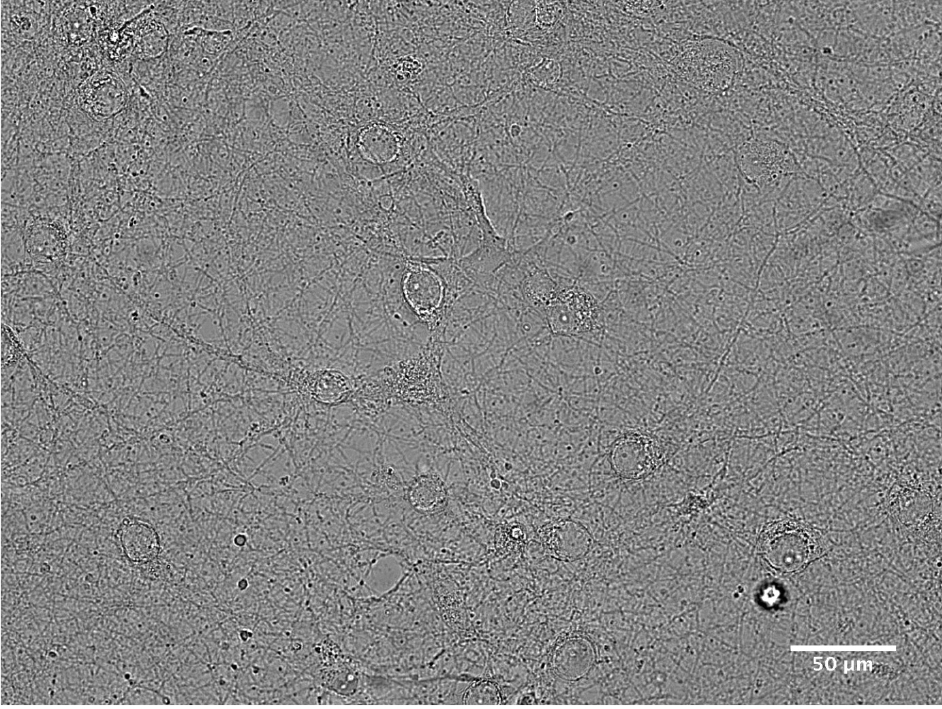
Figure 8. Cultured cerebrocortical neurons with 15 DIV - To prepare the poly-D-Lysine solution, first dilute the 100 mg vial in 100 ml of boric acid (0.1 M, pH 8.2). Aliquots of this solution should be stored at -20 °C until further use. The resulting solution (1 mg/ml) should be further diluted in boric acid (166.3 mM, pH 8.2) and filtered immediately before coating the plates, to obtain a final concentration of 0.1 mg/ml. To coat the plastic dishes, add enough solution to cover the bottom of the wells and incubate for 2 h at 37 °C (overnight incubation in a humidified incubator is recommended). After this coating period, wash each well twice with enough volume of sterile distilled water to cover the bottom of the well, and allow the wells to dry inside the laminar flow hood.
Recipes
- Hanks’ balanced salt solution (HBSS)
5.36 mM KCl
0.44 mM KH2PO4
137 mM NaCl
4.16 mM NaHCO3
0.34 mM Na2HPO4·2H2O
5 mM glucose
1 mM pyruvic acid
10 mM HEPES
0.001% phenol red
Adjust the pH to 7.2 and filter the solution in the laminar flow hood with a filtration unit with a 0.20 μm acetate cellulose filter. Alternatively, use single-use stericups (see Materials and Reagents #10). The solution should be maintained at 4 °C (stable for at least two months) - Trypsin solution (2 mg/ml)
5 ml HBSS
10 mg trypsin
Filter with an acetate cellulose filter of Ø25 mm, 0.20 μm, sterile. Always prepare fresh - 10% FBS
10 ml heat inactivated FBS stock solution
90 ml sterile HBSS
In order to heat inactivate the FBS stock solution, put the original bottle in a water bath pre-warmed at 56 °C for 30 min. After this, FBS can be aliquoted and stored at -20 °C until further usage. 10% FBS solution can be maintained at 4 °C (stable for at least one month) - Neuronal plating medium
9.50 g/L minimum essential medium Eagle
0.6% glucose
1 mM pyruvic acid
3.64 g/L sodium bicarbonate
Adjust the pH to 7.2, filter the solution in the laminar flow hood with a filtration unit with a 0.20 μm acetate cellulose filter. Alternatively, use single-use stericups (see Materials and Reagents #10). The solution should be maintained at 4 °C (stable for at least two months) - Supplemented neuronal culture medium (500 ml)
Neurobasal Medium®
SM1 supplement (1x; 1:50 dilution)
0.5 mM glutamine (1.25 ml of 200 mM stock solution [already sterile])
25 μM glutamate (1.25 ml of 10 mM stock solution [sterile by filtration with an acetate cellulose filter of Ø25 mm, 0.20 μm, sterile])
0.12 mg/ml gentamycin (1.2 ml of 50 mg/ml stock solution)
Solution should be maintained at 4 °C (stable for at least one to two months) - Boric acid solutions
0.1 M boric acid, pH 8.2 (adjust the pH with a sodium hydroxide solution)
166.3 mM boric acid, pH 8.2 (adjust the pH with a sodium hydroxide solution)
After adjusting the pH, filter the solution in the laminar flow hood with a filtration unit with a 0.20 μm acetate cellulose filter. Alternatively, use single-use stericups (see Materials and Reagents #10). Both solutions can be kept at 4 °C for two months
Acknowledgments
Work in the authors laboratory was supported by grants from the Portuguese Science and Technology Foundation (FCT), European Union–European Fund for Economic and Regional Development funding (Operational Competitiveness Program [COMPETE] grants, PEst-C/SAU/LA0001/2013-2014, POCI-01-0145-FEDER-007440, UID/NEU/04539/2013, UID/BIM/4501/2013, SFRH/BPD/84593/2012, SFRH/BPD/115546/2016 and CENTRO-01-0145-FEDER-000008: BrainHealth 2020–Early Detection, Neuromodulation and Advanced Therapies to Brain Disorders).
References
- Brewer, G. J., Torricelli, J. R., Evege, E. K. and Price, P. J. (1993). Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 35: 567-576.
- Caldeira, M. V., Curcio, M., Leal, G., Salazar, I. L., Mele, M., Santos, A. R., Melo, C. V., Pereira, P., Canzoniero, L. M. and Duarte, C. B. (2013). Excitotoxic stimulation downregulates the ubiquitin-proteasome system through activation of NMDA receptors in cultured hippocampal neurons. Biochim Biophys Acta 1832(1): 263-274.
- Chen, Y., Stevens, B., Chang, J., Milbrandt, J., Barres, B. A. and Hell, J. W. (2008). NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods 171: 239-247.
- Curcio, M., Salazar, I. L., Inacio, A. R., Duarte, E. P., Canzoniero, L. M. and Duarte, C. B. (2015). Brain ischemia downregulates the neuroprotective GDNF-Ret signaling by a calpain-dependent mechanism in cultured hippocampal neurons. Cell Death Dis 6: e1645.
- Mele, M., Aspromonte, M. C. and Duarte, C. B. (2017). Downregulation of GABAA receptor recycling mediated by HAP1 contributes to neuronal death in in vitro brain ischemia. Mol Neurobiol 54(1): 45-57.
- Mele, M., Ribeiro, L., Inacio, A. R., Wieloch, T. and Duarte, C. B. (2014). GABA(A) receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol Dis 65: 220-232.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Salazar, I. L., Mele, M., Caldeira, M., Costa, R. O., Correia, B., Frisari, S. and Duarte, C. B. (2017). Preparation of Primary Cultures of Embryonic Rat Hippocampal and Cerebrocortical Neurons. Bio-protocol 7(18): e2551. DOI: 10.21769/BioProtoc.2551.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell growth
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link