- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Context-driven Salt Seeking Test (Rats)
Published: Vol 8, Iss 7, Apr 5, 2018 DOI: 10.21769/BioProtoc.2456 Views: 6018
Reviewed by: Beatriz CastroXi FengAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Study Spatial Subgoal Learning Using Escape Behavior in Mice
Philip Shamash and Tiago Branco
Jun 20, 2022 2606 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
Changes in reward seeking behavior often occur through incremental learning based on the difference between what is expected and what actually happens. Behavioral flexibility of this sort requires experience with rewards as better or worse than expected. However, there are some instances in which behavior can change through non-incremental learning, which requires no further experience with an outcome. Such an example of non-incremental learning is the salt appetite phenomenon. In this case, animals such as rats will immediately seek out a highly-concentrated salt solution that was previously undesired when they are put in a novel state of sodium deprivation. Importantly, this adaptive salt-seeking behavior occurs despite the fact that the rats never tasted salt in the depleted state, and therefore never tasted it as a highly desirable reward.
The following protocol is a method to investigate the neural circuitry mediating adaptive salt seeking using a conditioned place preference (CPP) procedure. The procedure is designed to provide an opportunity to discover possible dissociations between the neural circuitry mediating salt seeking and salt consumption to replenish the bodily deficit after sodium depletion. Additionally, this procedure is amenable to incorporating a number of neurobiological techniques for studying the brain basis of this behavior.
Background
The salt appetite phenomenon was first discovered by Richter (1936), who found that rats began to immediately consume a 1% salt solution in greater quantities compared to water in a 2-bottle choice test following adrenalectomy and consequently bodily sodium depletion. More recently, Robinson and Berridge (2013) have shown that this immediate increase in salt-seeking behavior occurs to discrete salt-paired cues following sodium depletion in the absence of salt before it has been tasted as a desirable reward. In addition, adaptive salt-seeking has also been observed with contextual cues following sodium depletion (Stouffer and White, 2005).
In the brain, there is clear evidence that the central nucleus of the amygdala (Galaverna et al., 1993; Seeley et al., 1993; Tandon et al., 2012; Hu et al., 2015), lateral hypothalamus (Wolf and Quartermain, 1967; Tandon et al., 2012), and the nucleus accumbens (Roitman et al., 2002; Voorhies and Bernstein, 2006; Loriaux et al., 2011; Tandon et al., 2012) are important for the consumption of salt following sodium depletion in order for animals to replenish the deficit. However, there has been surprisingly little work done on the neural circuitry mediating cue-driven salt seeking following sodium depletion. In other words, it is mostly unclear how the brain enables animals to seek out salt in a novel deprivation state. In a recent study, we showed that the ventral pallidum (VP) plays an important role in this phenomenon (Chang et al., 2017). The VP has previously been shown to track the changes in value of salt-paired cues before and after sodium depletion (Tindell et al., 2009; Robinson and Berridge, 2013). However, it was previously unknown whether the VP is necessary for mediating salt appetite in terms of cue-driven salt seeking or salt consumption. Using a novel CPP procedure, described here, we showed that optogenetic inhibition of the VP impairs context-driven salt seeking but not the consumption of salt itself following sodium depletion (Chang et al., 2017).
The protocol we have used to demonstrate this effect allows for not only optogenetics to be used but also other techniques to manipulate the brain (e.g., DREADDs, intracranial injections, lesions) or to record brain activity (e.g., electrophysiology, calcium imaging). Further study of context-driven salt seeking with this procedure may help elucidate the neural bases of disorders of aberrant motivation that may lead to reduced reward seeking, as in depression, or non-homeostatic reward seeking (e.g., overeating leading to obesity). In addition, this procedure could be easily extended to investigate the neural bases of other nutrient deficit-induced changes in behavior such as calcium appetite (Leshem et al., 1999; Schulkin, 2000).
Materials and Reagents
- Electrical tape (TemflexTM 1700, 3M, catalog number: 1700-1X66FT )
- Disposable weighing boat
- Rat (Long-Evans, 250-300 g; Male; 7 weeks old; Charles River Laboratories)
- Sodium-free food (TestDiet; Order Info: 1816123 (5ANR), TD 90228 1/2)
- Sugar (pure cane granulated, Domino®)
- Salt (iodized, Morton Salt, Inc.)
- Sugar-free Kool-Aid powder (orange and grape, Kool-Aid, Kraft Foods, Inc.)
- Furosemide (Salix®, Merck)
Equipment
- Preference test chamber (29.5 x 12.5 x 21 in., custom designed; Dartmouth Apparatus Shop)
- 16 oz. screw-top bottles (Ancare, catalog number: MST16 )
- Screw-top bend ball pt. tubes (5 in. long with 1 in. bend, Ancare, catalog number: PCST51BTD )
- Digital video camera (Sony)
- 2 portable luminaires (UL, catalog number: E196460 )
- 2 red light bulbs (Sunlite, catalog number: SL24/R )
Procedure
- Preparation of animals
This procedure is designed for rats as the subjects. Rats are food restricted and maintained at 85% of their ad libitum weights throughout the experiment to motivate them to drink the sucrose and salt solutions. - Preparation of apparatus
- Behavioral training is conducted in a custom-designed place preference chamber (29.5 x 12.5 x 21 in.), which we had made by the Dartmouth Apparatus Shop (Figure 1). The outside walls of the chamber are made of transparent acrylic, and there are acrylic inserts that can be placed in the chamber to divide it into three separate contexts. All training and testing is conducted in the dark except for the red light provided by the portable luminaires (see Figure 1) to provide enough illumination for recording purposes.

Figure 1. Setup for investigating context-driven salt seeking and close up views of the place preference chamber - Two contexts (12.5 x 12.5 x 18.5 in.) within the chamber are paired with one of two solutions, while the third middle context (5 x 12.5 x 18.5 in.) connects the other two contexts and is paired with nothing. One of the paired contexts is designated the Grid context. The Grid context has a transparent acrylic floor in a grid-like pattern with electrical tape on the outside walls that form a grid-like pattern. The other paired context is designated the Striped context. The Striped context has the same grid-patterned floor, but the outside walls have electrical tape in a parallel diagonal pattern. Each of these two contexts also has a distinctive object to aid in discrimination (e.g., a metal binder clip or an empty plastic microscope coverslip case). Both contexts have holes on either end to allow for a spout connected to a bottle filled with a solution to be available for the rats to drink from.
- The third, middle context that connects the Grid and Striped contexts is designated the Neutral context that consists only of clear acrylic walls and is the starting point for each training and testing session. Although individual rats can sometimes naturally favor one context, it is important to first establish that there is no general bias towards one context of the other. If there is, adjustments can be made (e.g., to the lighting, chamber orientation, etc.).
- Behavioral training is conducted in a custom-designed place preference chamber (29.5 x 12.5 x 21 in.), which we had made by the Dartmouth Apparatus Shop (Figure 1). The outside walls of the chamber are made of transparent acrylic, and there are acrylic inserts that can be placed in the chamber to divide it into three separate contexts. All training and testing is conducted in the dark except for the red light provided by the portable luminaires (see Figure 1) to provide enough illumination for recording purposes.
- Behavioral training and testing protocol
Step 1: Training- Rats undergo 8 days of initial training in which they learn to associate one context (Grid or Striped) with access to 3% sucrose in distilled water, and the other context with access to 3% salt in distilled water. Each solution is mixed with 2 g of sugar-free Kool-Aid powder (orange or grape) to aid discrimination between the two contexts and solutions. The assignment of context, solution, and Kool-Aid flavor pairings are counterbalanced across rats but kept constant within each rat.
- On each training day (4 days total in each context), rats are placed into the Neutral context and allowed to freely explore one context (Grid or Striped) for 20 min. Access to the other paired context is blocked. During these sessions, rats are allowed to freely drink from a bottle of 100 ml of either sucrose or salt (orange and purple bottles as in Step 1 of Figure 2). The order of context exposure is pseudorandomly assigned between rats.
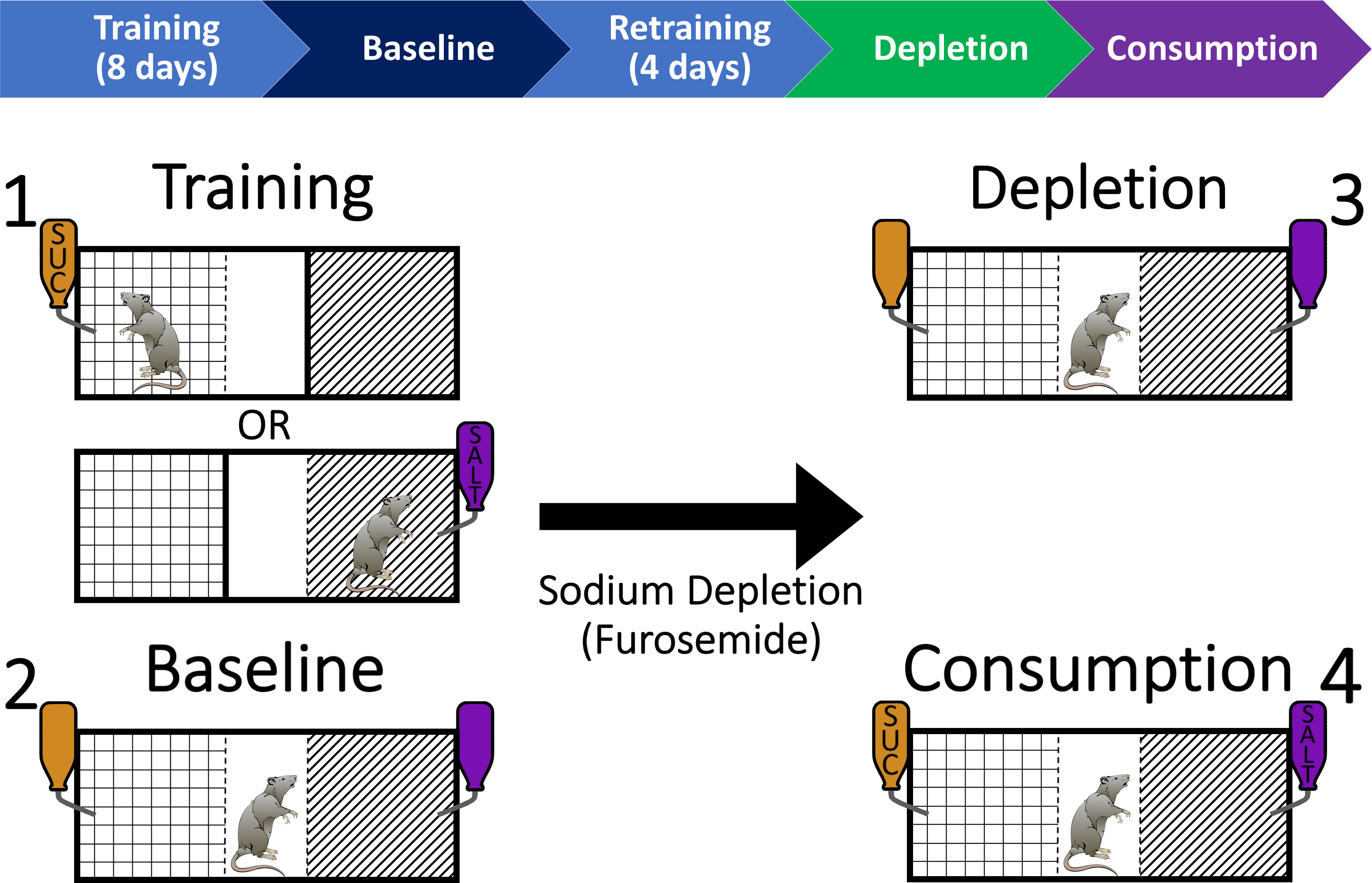
Figure 2. Behavioral training and testing procedure to investigate context-driven salt seeking in rats. Step 1: Rats are trained over 8 days to associate one context with access to sucrose (e.g., Grid) and another context with access to salt (e.g., Striped). Step 2: Rats are then given a baseline test session in which they have access to both contexts without sucrose or salt (but flavored solutions still present). Following 4 days of retraining (2 days in each context), rats are then depleted of bodily sodium through furosemide injections (i.p.). Step 3: After 48 h, rats are placed back into the chamber for a depletion test that is identical to the baseline test session. Step 4: Finally, rats are given a consumption test that is identical to the previous test sessions but sucrose and salt are now available. All training and testing sessions are 20 min in length.
Step 2: Baseline test- Once initial training is complete, rats are then given a 20 min baseline preference test in which they are allowed to explore the entire chamber, including both the Grid and Striped contexts, for the first time (both wall inserts are removed). The Grid and Striped contexts in this test session are identical as in training. One important exception is that sucrose and salt are not present in the flavored solutions. We conduct the baseline preference test in this way to gauge rats’ ability to use the contextual cues of the chamber to guide their behavior. The test serves as an important comparison point for an identical test conducted after sodium depletion.
Retraining and sodium depletion- Following the baseline test session, the rats are given an additional 4 days of training (2 sessions in each context) that is identical to the initial training sessions (Step 1). We give rats an additional 4 days of training to re-strengthen the associations between sucrose and salt with their respective contexts, which may be weakened following the baseline preference test. After the final training session, rats are given systemic injections of the diuretic furosemide (10 mg/ml/kg; i.p.; Merck). In order to maintain sodium deprivation, rats are also maintained on a sodium-free diet (TestDiet) and distilled water immediately following furosemide injections.
Step 3: Depletion test- Forty-eight hours after the furosemide injection, rats are then given another context preference test (depletion test). This test is identical to the baseline preference test (i.e., the full chamber is accessible; flavored solutions are available, but contain no salt or sucrose), except now the rats are sodium-deprived. We perform the depletion test this way in order to directly compare the elevation in time spent in the salt-paired context following sodium depletion compared to the baseline preference test.
Step 4: Consumption test- The next day, rats are given a final context preference test with sucrose and salt now present in the flavored solutions (consumption test). This allows us the opportunity to confirm that rats are indeed sodium-depleted. It also further provides an opportunity to investigate any possible neural dissociations between context-driven salt seeking (depletion test) and the consumption of salt (consumption test) following sodium depletion. Upon completion of this test, rats are placed back on their normal food and water diet.
- Rats undergo 8 days of initial training in which they learn to associate one context (Grid or Striped) with access to 3% sucrose in distilled water, and the other context with access to 3% salt in distilled water. Each solution is mixed with 2 g of sugar-free Kool-Aid powder (orange or grape) to aid discrimination between the two contexts and solutions. The assignment of context, solution, and Kool-Aid flavor pairings are counterbalanced across rats but kept constant within each rat.
Data analysis
- Video of test sessions is recorded by a digital camera (Sony) that is attached to a tripod facing the side of the chamber and positioned so that the entire chamber is visible. The primary measures to analyze are the amount of solutions consumed (in ml) during training and test sessions, the number of entries into each paired context during test sessions, the amount of time spent in each paired context (in sec) during test sessions, and the amount of time spent consuming solutions (in sec) during test sessions. The amount of time spent in each context and the amount of time spent consuming solutions is also broken down into 5-min blocks to give a more detailed record of how rats’ behavior changes over time. Behavior is hand-scored offline by looking at the head placement of each rat and what context it is located in, calculated to the nearest second. Additionally, calculations of elevation scores of each measure can be made by computing the total difference between depletion and baseline tests. Automated video-tracking devices can be used as an alternative to video hand-scoring. Spout lickometers can also be incorporated to assess lick microstructure in addition to volume consumption.
- For analyses with multiple data points from each subject (e.g., training consumption data or 5-min block data), repeated measures ANOVAs are used. For analyses with a single data point from each subject (e.g., elevation scores) based on a priori test plans, generalized linear models can be used. Significant interactions are followed up with Bonferroni-corrected generalized linear models. Otherwise, all analyses have a rejection criterion of P < 0.05.
- Figure 3 presents what one would expect to observe for a control group of rats in our salt appetite procedure (n = 19 male rats; group means ± SEM; adapted from Chang et al., 2017). Rats should drink more sucrose than salt over the course of training (Figure 3A). Following sodium depletion, rats should show an elevation in time spent in the salt paired context during the depletion test compared to the baseline test (Figure 3B). Finally, rats should show in elevation in the consumption of salt and a decrease in the consumption of sucrose compared to the last training day with each solution following sodium depletion (Figure 3C). Neural manipulations during either the depletion and consumption tests may reveal dissociations between context-driven salt seeking and salt consumption following sodium depletion. One salient example is our prior study (Chang et al., 2017), in which we observed a specific deficit in context-driven salt seeking but not salt consumption following sodium depletion with optogenetic inhibition of the VP.
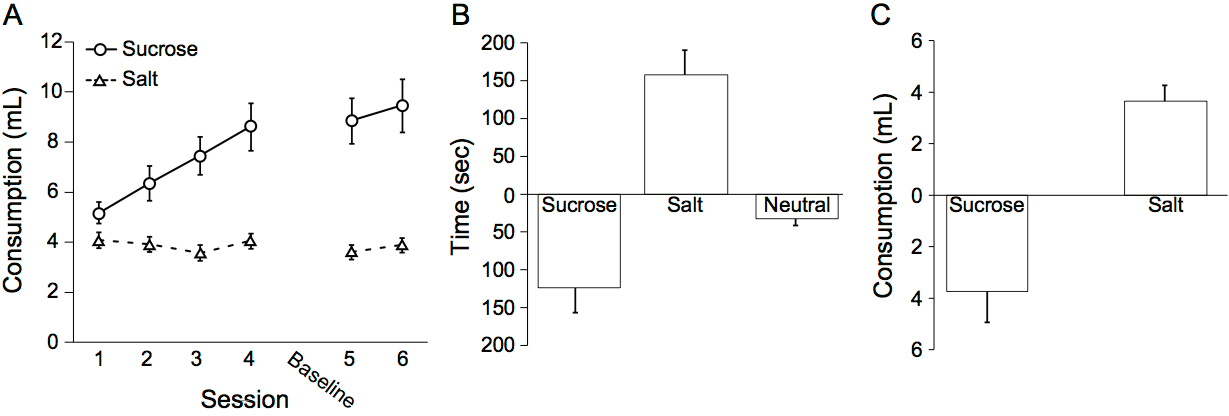
Figure 3. Expected results with our salt appetite procedure. A. Rats should drink more sucrose than salt over the course of training. B. Rats should show an elevation in time spent in the salt-paired context and a decrease in time spent in the sucrose-paired context following sodium depletion during the depletion test compared to the baseline test. C. Rats should show an elevation in the consumption of salt and a decrease in the consumption of sucrose compared to the last training day with each solution following sodium depletion. Figures adapted from Chang et al., 2017.
Notes
- Although we use ball pt. tubes, there may still be some leaking of solutions when placing bottles into position before the start of each session. To minimize the amount of solution lost from leaking, we place a collection dish (e.g., a disposable weighing boat) underneath the spout on the floor of the chamber to collect any solution that may leak before the rat is placed in the chamber. Once the bottle has been in position for approximately 5 min, the collection dish is then removed before the rat is placed into the chamber. The solution in the collecting dish is then added to what is remaining in the bottle following each session to provide a more accurate measurement of how much rats actually consumed during each session.
- Some individual rats may show a bias towards one context of the chamber in terms of time spent in each context regardless of which solution it is paired with during the baseline test session. However, what is most critical is the elevation in time spent in each context during the depletion test that is the primary measure for gauging the salt appetite phenomenon. We have consistently observed a robust elevation in time spent in the salt-paired context following sodium depletion in our control rats using this method of measurement despite any inherent biases rats may have towards one context or the other.
- We have only run this procedure using male rats, but we would not expect to see differences between male and female rats.
Recipes
- For training sessions, we make each solution in 1 L batches. Thus, 30 g of either sugar or salt are weighed out and placed into a 1 L bottle. The bottle is then filled up to 1 L with distilled water. Finally, 2 g of sugar-free Kool-Aid powder (orange or grape) is added. The solution is then mixed until either the sugar or salt (and Kool-Aid powder) is dissolved.
- For test sessions, 1 L batches are filled with distilled water and 2 g of Kool-Aid powder only.
- Furosemide is initially at a 5% concentration, which we dilute it to 1% with distilled water (e.g., 5 ml of furosemide and 20 ml of distilled water) for a concentration of 10 mg/ml.
Acknowledgments
This work was supported by funding from National Institutes of Health Grant F32MH106178 (SEC) and from Whitehall Foundation Research Grant 2014-05-77 (KSS). The authors declare no competing financial interests. This protocol was adapted from Chang et al., 2017.
References
- Chang, S. E., Smedley, E. B., Stansfield, K. J., Stott, J. J. and Smith, K. S. (2017). Optogenetic inhibition of ventral pallidum neurons impairs context-driven salt seeking. J Neurosci 37(23): 5670-5680.
- Galaverna, O. G., Seeley, R. J., Berridge, K. C., Grill, H. J., Epstein, A. N. and Schulkin, J. (1993). Lesions of the central nucleus of the amygdala. I: Effects on taste reactivity, taste aversion learning and sodium appetite. Behav Brain Res 59(1-2): 11-17.
- Hu, B., Qiao, H., Sun, B., Jia, R., Fan, Y., Wang, N., Lu, B. and Yan, J. Q. (2015). AT1 receptor blockade in the central nucleus of the amygdala attenuates the effects of muscimol on sodium and water intake. Neuroscience 307: 302-310.
- Leshem, M., Del Canho, S. and Schulkin, J. (1999). Calcium hunger in the parathyroidectomized rat is specific. Physiol Behav 67(4): 555-559.
- Loriaux, A. L., Roitman, J. D. and Roitman, M. F. (2011). Nucleus accumbens shell, but not core, tracks motivational value of salt. J Neurophysiol 106(3): 1537-1544.
- Richter, C. P. (1936). Increased salt appetite in adrenalectomized rats. Am J Physiol 115: 155-161.
- Robinson, M. J. and Berridge, K. C. (2013). Instant transformation of learned repulsion into motivational "wanting". Curr Biol 23(4): 282-289.
- Roitman, M. F., Na, E., Anderson, G., Jones, T. A. and Bernstein, I. L. (2002). Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci 22(11): RC225.
- Schulkin, J. (2000). Calcium hunger: Behavioral and biological regulation. Cambridge University Press.
- Seeley, R. J., Galaverna, O., Schulkin, J., Epstein, A. N. and Grill, H. J. (1993). Lesions of the central nucleus of the amygdala. II: Effects on intraoral NaCl intake. Behav Brain Res 59(1-2): 19-25.
- Stouffer, E. M. and White, N. M. (2005). A latent cue preference based on sodium depletion in rats. Learn Mem 12(6): 549-552.
- Tandon, S., Simon, S. A. and Nicolelis, M. A. (2012). Appetitive changes during salt deprivation are paralleled by widespread neuronal adaptations in nucleus accumbens, lateral hypothalamus, and central amygdala. J Neurophysiol 108(4): 1089-1105.
- Tindell, A. J., Smith, K. S., Berridge, K. C. and Aldridge, J. W. (2009). Dynamic computation of incentive salience: "wanting" what was never "liked". J Neurosci 29(39): 12220-12228.
- Voorhies, A. C. and Bernstein, I. L. (2006). Induction and expression of salt appetite: effects on Fos expression in nucleus accumbens. Behav Brain Res 172(1): 90-96.
- Wolf, G. and Quartermain, D. (1967). Sodium chloride intake of adrenalectomized rats with lateral hypothalamic lesions. Am J Physiol 212(1): 113-118.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chang, S. E. and Smith, K. S. (2018). Context-driven Salt Seeking Test (Rats). Bio-protocol 8(7): e2456. DOI: 10.21769/BioProtoc.2456.
- Chang, S. E., Smedley, E. B., Stansfield, K. J., Stott, J. J. and Smith, K. S. (2017). Optogenetic inhibition of ventral pallidum neurons impairs context-driven salt seeking. J Neurosci 37(23): 5670-5680.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link