- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Root Aliphatic Suberin Analysis Using Non-extraction or Solvent-extraction Methods
Published: Vol 7, Iss 12, Jun 20, 2017 DOI: 10.21769/BioProtoc.2331 Views: 10611
Reviewed by: Arsalan DaudiSibongile MafuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sorghum bicolor Extracellular Vesicle Isolation, Labeling, and Correlative Light and Electron Microscopy
Deji Adekanye [...] Jeffrey L. Caplan
Oct 5, 2024 2107 Views

Closed Systems to Study Plant–Filamentous Fungi Associations: Emphasis on Microscopic Analyses
Vasiliki Skiada and Kalliope K. Papadopoulou
Feb 20, 2025 2854 Views

PhosphoLIMBO: An Easy and Efficient Protocol to Separate and Analyze Phospholipids by HPTLC From Plant Material
Louise Fougère [...] Yohann Boutté
Sep 5, 2025 1334 Views
Abstract
Here we describe both non-extraction and solvent-extraction methods for root aliphatic suberin analysis. The non-extraction method is fast as roots are directly depolymerized using acidic transmethylation. However, suberin aliphatic components are isolated together with all the other acyl chains making up the lipids (e.g., membranes) present in roots. For the solvent-extraction method, roots are first delipidated before transmethylation. This method is longer but allows separation of soluble and polymerized root lipids. This protocol is optimized for tissue culture- or soil-grown Arabidopsis thaliana plants, but can be used with roots of other plants.
Keywords: SuberinBackground
Suberin is an extracellular plant lipid polymer deposited in the cell walls of various tissues such as endodermis, exodermis and periderm of roots. Suberin acts as a barrier controlling water and solute fluxes and restricting pathogen infections (Ranathunge et al., 2011; Andersen et al., 2015; Vishwanath et al., 2015; Barberon et al., 2016). Suberin is a complex heteropolymer made up of aliphatics, phenolics, and glycerol, which is associated with solvent-extractable waxes (Bernards, 2002). In the model plant Arabidopsis thaliana, the suberin polymer is primarily made of ω-hydroxy acids and α,ω-dicarboxylic acids, but it also contains unsubstituted fatty acids and primary fatty alcohols (Domergue et al., 2010; Vishwanath et al., 2013), whereas the associated waxes are in the form of alkyl hydroxycinnamates (AHCs; Kosma et al., 2012; Delude et al., 2016). The non-extraction method described here allows for high-throughput and rapid analysis of suberin composition, which is particularly advantageous when screening large numbers of plant lines (e.g., mutants, overexpressing transgenic lines, or natural variants). A more traditional and accurate solvent extraction method applicable when soluble and polymerized lipids (i.e., suberin polyester) need to be analyzed separately is included for comparison.
Materials and Reagents
- Petri dish (90 mm diameter) (Fisher Scientific)
- Peat moss, vermiculite and perlite (3:1:1, v/v/v; Medan S.A.)
- Paper towels
- 8 ml glass tubes (Dutscher, catalog number: 065307B ) with polytetrafluoroethylene (PTFE)-lined caps (Dutscher, catalog number: 001031 )
- Pots for Arabidopsis (Polystyrene, 9 x 9 x 9.5 cm) (SOPARCO, catalog number: 4686 )
- 2 ml GC vials with caps (Agilent Technologies, catalog number: 5182-0557 ) and 400 μl flat bottom glass inserts (Agilent Technologies, catalog number: 5181-3377 )
- Arabidopsis seeds
- 95% ethanol
- Sodium hypochlorite (Bleach)
- Distilled water
- Isopropanol (Fisher Scientific, catalog number: 10315720 )
- Chloroform (Sigma-Aldrich, catalog number: 32211 )
- Methanol (Sigma-Aldrich, catalog number: 34885 )
- Nitrogen (H2O < 3 ppm; CnHm < 0.5 ppm; O2 < 2 ppm)
- Sulfuric acid (H2SO4) (CARLO ERBA Reagents, catalog number: E410391 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886 )
- Methyl tert-butyl ether (MTBE) (Sigma-Aldrich, catalog number: 20256 )
- Tri(hydroxymethyl)aminomethane (Tris base) (Sigma-Aldrich, catalog number: T6066 )
- N,O-Bis(trimethylsilyl)-trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) (Sigma-Aldrich, catalog number: T6381 )
- Heptane (Sigma-Aldrich, catalog number: 32287 )
- Toluene (Sigma-Aldrich, catalog number: 32249 )
- Murashige and Skoog medium (Duchefa Biochemie, catalog number: M0222.0050 )
- Vitamins
- Plant agar (Duchefa Biochemie, catalog number: P1001.1000 )
- 2-(N-Morpholino)-ethane sulfonic acid (MES) (Euromedex, catalog number: EU0033-B )
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: P5958 )
- Internal standards:
Heptadecanoic acid (C17:0) (Sigma-Aldrich, catalog number: H3500 )
Pentadecanol (C15:0-OH) (Sigma-Aldrich, catalog number: 412228 )
ω-pentadecalactone (yielding ω-OH-C15:0) (Sigma-Aldrich, catalog number: W284009 ) - Murashige and Skoog (MS) medium (see Recipes)
- Stock solutions (5 mg/ml) of internal standards (see Recipes)
Equipment
- Growth chamber (Fitotron, Weiss Technik)
- Pair of scissors (Holtex) and tweezers (Hammacher)
- Dry heating block (Fisher Scientific, catalog number: FB15103 )
- Polytron (IKA, model: T 25 digital )
- Tube rotator (Cole-Parmer, Stuart, model: SB3 )
- Fume hood (Delagrave)
- Dessicator (Nalgene)
- Centrifuge (Hettich Lab Technology, model: ROTORFIX 32 A , 6000RPM max)
- Glass Pasteur pipets (e.g., VWR)
- Weighing scales (Sartorius, model: SECURA124-1S )
- Temperature-controlled evaporator connected to nitrogen tank (Meyer N-Evap Organomation)
- Agilent 6850 (Agilent Technologies, model: Agilent 6850 ) gas chromatograph equipped with an HP-5MS column (length 30 m, id 0.25 mm, film thickness 0.25 μm) and an Agilent 5975 mass spectrometric detector (70 eV, mass-to-charge ratio 50-750) (or equivalent GC-MS)
- pH meter (Hanna Instruments, model: Hi4222 )
- Autoclave
Procedure
- Plant growth and root harvest
- Sow sterilized Arabidopsis seeds (1 min in 95% ethanol, 15 min in Bleach [6-14% sodium hypochlorite], and 6 washes with sterilized water) on Petri dish with Murashige and Skoog (MS) medium supplemented with 0.7% agar and 2.5 mM MES, pH 5.7 (see Recipes).
- Stratify seeds in the dark for 3 to 4 days at 4 °C, and transfer plates to a controlled-environment growth chamber under long-day conditions (16 h of light and 8 h of darkness) at a temperature of 22 °C. Grow seedlings for up to 4 weeks.
- For soil-grown plants, transfer 2 week-old seedlings grown on MS medium plates to pots containing pre-wetted peat moss-vermiculite-perlite (3:1:1, v/v/v) growing medium (about 700 cm3 per pot). Five plants per pots. Place the pots at 22 °C in a controlled-environment growth chamber with ambient humidity under long-day conditions.
- For collecting roots from tissue culture-grown seedlings, use a pair of scissors to eliminate the aerial portions of the seedlings, remove the entire agar medium disc from the Petri dish and place it upside down in the dish cover. With tweezers, collect the roots from the agar. It is not necessary to wash the roots after collecting them from the agar medium.
- For collecting roots from soil-grown plants, carefully eliminate the soil growing medium by soaking in water, use a pair of scissors to separate roots from other parts of the plant, and rinse roots thoroughly with distilled water to remove as much as possible the remaining growth medium.
- Sow sterilized Arabidopsis seeds (1 min in 95% ethanol, 15 min in Bleach [6-14% sodium hypochlorite], and 6 washes with sterilized water) on Petri dish with Murashige and Skoog (MS) medium supplemented with 0.7% agar and 2.5 mM MES, pH 5.7 (see Recipes).
- Non-extraction method
- Pool roots to form bundles of roots. Make 4 to 5 replicates.
- Dry roots as much as possible by pressing the material several times between paper towels, (i.e., until paper towels stay dry).
- Measure the fresh weight (FW). For Arabidopsis, 5 to 25 mg of FW roots is necessary per replicate.
- Pool roots to form bundles of roots. Make 4 to 5 replicates.
- Solvent-extraction method
- Pool roots to form bundles of roots.
- Dry roots with a paper towel and measure the fresh weight (FW). For Arabidopsis, 70 to 100 mg of FW roots is necessary per replicate.
- Place freshly collected roots in glass extraction tube containing 4 ml hot (85 °C) isopropanol and incubate for 30 min at 85 °C in dry heating block. After cooling to room temperature, reduce to a fine powder with a Polytron when large root or lots of material is analyzed to allow efficient delipidation.
- After sedimentation of the residue, transfer isopropanol phase to a new glass tube and delipidate further the roots by extracting soluble lipids successively with:
- 4 ml chloroform:methanol (2:1, v/v)
- 4 ml chloroform:methanol (1:1, v/v)
- 4 ml chloroform:methanol (1:2, v/v)
- 4 ml 100% methanol
- Perform all delipidation steps at room temperature for 24 h on a tube rotator with the wheel rotating at 40 rpm. Following each delipidation step, collect the solvent fraction in the same 9 ml glass tube, with the newly collected solvent being reduced each time under a gentle stream of nitrogen gas such that no solvent splashes out of the tube (but not completely dry so as to minimize oxidation of lipids). At the end, fully dry the resulting pooled extract, which corresponds to the soluble lipid fraction, resuspend it in 1 ml of chloroform/methanol (1:1; [v:v]), and store it at 4 °C until further analysis as described in the fatty acyl-chain analysis section.
- Following final delipidation step with methanol, dry the resulting solvent-extracted root material in a fume hood at room temperature for 2-3 days. This delipidated dry residue will contain the suberin polymer. Place the tubes containing the delipidated roots (without caps) in a dessicator for another 2 days before measuring the dried residues (DR) weight (usually representing 10 to 15% of FW).
- Pool roots to form bundles of roots.
- Fatty acyl-chain analysis by GC-MS
- Add 1 ml of 5% (v/v) sulfuric acid in methanol containing 5 µg each of heptadecanoic acid (C17:0), pentadecanol (C15:0-OH) and C15-hydroxypentadecanoic acid (ω-OH-C15:0) as internal standards to the freshly collected roots (non-extraction method), or the solvent extracted dried residues, or to one fifth (200 µl) of the soluble lipid fraction (solvent extraction method).
- Close tubes tightly and incubate for 3 h at 85 °C in a dry heating block without agitation. This transmethylation step depolymerizes the suberin polymer as well as releases the acyl-chains making them all soluble lipids.
- After cooling at room temperature, add to each tube 1 ml of NaCl (2.5%, w/v) and 2.2 ml of methyl tert-butyl ether (MTBE) and mix by shaking vigorously.
- Centrifuge at 800 x g for 5 min at room temperature to allow phase separation, and transfer the upper MTBE phase to a clean glass tube using a glass Pasteur pipet.
- Add 1 ml of 100 mM Tris base pH 8.0 containing 0.09% (w/v) NaCl to the MTBE phase, mix by shaking vigorously, and centrifuge at 800 x g for 5 min at room temperature to allow phase separation.
- Collect the upper MTBE phase in a clean glass tube using a glass Pasteur pipet, avoiding any aqueous (lower) phase, and evaporate MTBE under a gentle stream of nitrogen. It is better to leave behind some of the upper MTBE phase than to contaminate with any aqueous (lower) phase.
- Add 100 μl of 99% BSTFA (N,O-Bis(trimethylsilyl)-trifluoroacetamide) with 1% TMCS, close tube tightly, and incubate at 110 °C for 15 min in a dry heating block without agitation. This step trimethylsilylates the free hydroxyl groups making compounds containing these groups more amenable to separation by gas chromatography.
- After cooling at room temperature, evaporate the solvent under a gentle stream of nitrogen and dissolve the products in 250 μl heptane:toluene (1:1, v/v).
- Transfer each sample into a GC vial containing a glass insert.
- For GC-MS analysis, a 1 µl aliquot of the sample dissolved in heptane:toluene (1:1) is injected in splitless mode. The temperature of the injector is held at 250 °C. The column oven temperature is held at 50 °C for 1 min and then increased from 50 °C to 200 °C at a rate of 25 °C per minute, followed by a 1 min hold, and then is ramped up again at a rate of 10 °C per minute to a final temperature of 320 °C, which is held for 8 min. The total run time is 28 min. High purity helium is used as the carrier gas at a flow rate of 1.5 ml per min.
- Add 1 ml of 5% (v/v) sulfuric acid in methanol containing 5 µg each of heptadecanoic acid (C17:0), pentadecanol (C15:0-OH) and C15-hydroxypentadecanoic acid (ω-OH-C15:0) as internal standards to the freshly collected roots (non-extraction method), or the solvent extracted dried residues, or to one fifth (200 µl) of the soluble lipid fraction (solvent extraction method).
Data analysis
- Typical chromatograms of total (non-extraction method), soluble and polymerized (solvent extraction method) acyl chains of roots from 4-week old wild-type seedlings grown in tissue culture are shown in Figure 1.
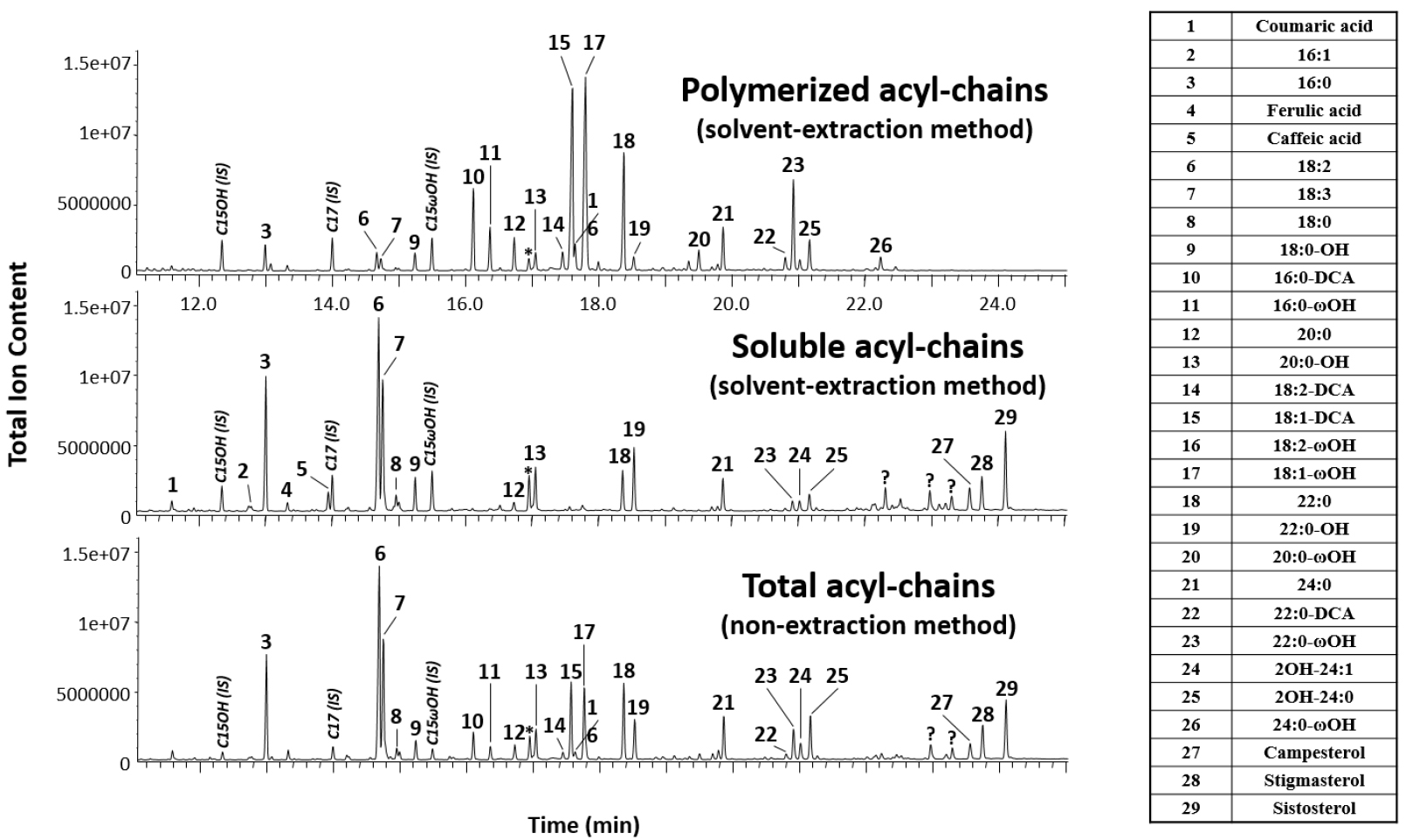
Figure 1. Representative chromatograms of total, soluble and polymerized acyl chains of roots from 4-weeks old wild-type seedlings grown in tissue culture. The identity of all the major monomers (representing more than 1% of the total) is indicated on the left side table in the order of elution. X:Y stands for fatty acids with X carbon atoms and Y unsaturations, X:Y-OH stands for fatty alcohols, X:Y-ωOH stands for ω-hydroxy fatty acids, X:Y-DCA stands for dicarboxylic fatty acids and 2OH-X:Y stands for 2-hydroxy fatty acids. IS stands for internal standards:pentadecanol (C15OH), heptadecanoic acid (C17) and 15-hydroxy-pentadecanoic acid (C15ωOH). Question marks and star indicate unidentified compounds and a contaminant, respectively. - Quantification of monomers is based on peak areas in the GC-MS chromatograms, identified using their retention time, and using the peak area of the respective internal standards (C17:0 for fatty acids and dicarboxylic acids, ω-OH-C15:0 for ω-hydroxyacids, and C15:0-OH for fatty alcohols). Constituent amounts (in μg) are calculated by multiplying the internal standard amount (5 μg) with the peak area of the constituent of interest and dividing by the peak area of the internal standard. Divide the constituent amounts by the fresh weight (FW) or dry residue (DR) of roots to obtain values in µg/mg FW or DR for non-extraction and solvent-extraction method, respectively.
- The global acyl-chain composition of suberin (including polymerized as well as soluble acyl-chains, principally fatty alcohols, in the form of suberin associated waxes) is then calculated as follows:
- From the non-extraction method, suberin comprises all dicarboxylic acids, ω-hydroxyacids, and fatty alcohols, but only about 3% of the C16 and C18 fatty acids (which are mainly coming from the lipids making membranes), 50% of 20:0 and 22:0 fatty acids, and 25% of very-long chain fatty acids above C22 (since suberin is enriched in very-long chain fatty acids, especially in 20:0 and 22:0) as previously described (Delude et al., 2016).
- From the solvent-extraction method, suberin comprises all dicarboxylic acids, ω-hydroxyacids, and fatty alcohols present in both polymerized and soluble fraction, but only the polymerized fatty acids.
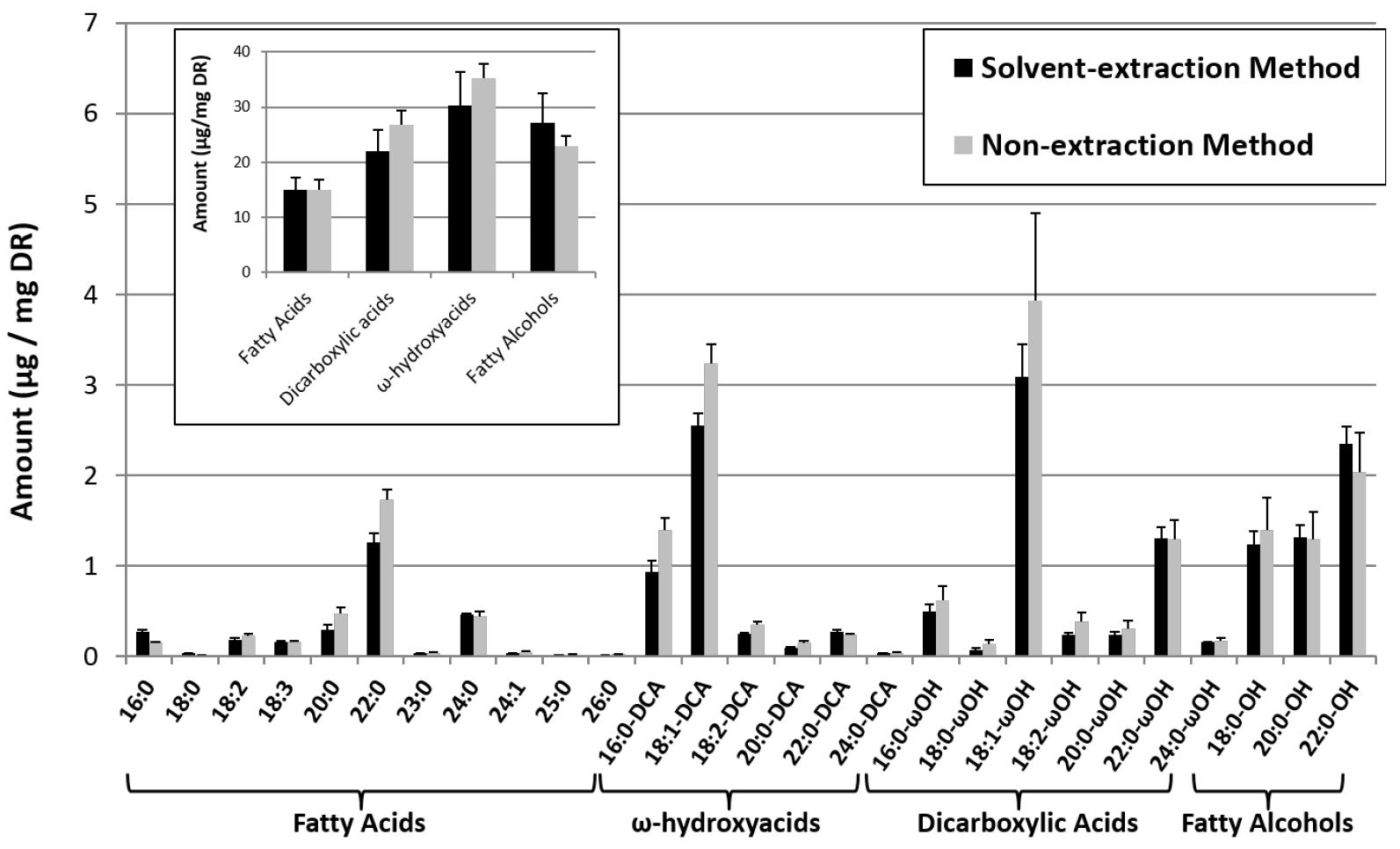 Figure 2. Global acyl-chain composition of the suberin from roots of 4 week-old seedlings were harvested and the suberin aliphatic composition was analyzed according to the solvent- and non-extraction methods. X:Y stands for fatty acids with X carbon atoms and Y unsaturations. Roots from 4 week-old seedlings were harvested and the suberin aliphatic composition was analyzed according to the solvent- and non-extraction methods. In the solvent-extraction method, root fresh weight (FW) and dry residue weight (DR) were measured, indicating that DR was about 14% of FW. This correlation was used to calculate the suberin composition in μg/mg DR in the non-extraction method.
Figure 2. Global acyl-chain composition of the suberin from roots of 4 week-old seedlings were harvested and the suberin aliphatic composition was analyzed according to the solvent- and non-extraction methods. X:Y stands for fatty acids with X carbon atoms and Y unsaturations. Roots from 4 week-old seedlings were harvested and the suberin aliphatic composition was analyzed according to the solvent- and non-extraction methods. In the solvent-extraction method, root fresh weight (FW) and dry residue weight (DR) were measured, indicating that DR was about 14% of FW. This correlation was used to calculate the suberin composition in μg/mg DR in the non-extraction method. - From the non-extraction method, suberin comprises all dicarboxylic acids, ω-hydroxyacids, and fatty alcohols, but only about 3% of the C16 and C18 fatty acids (which are mainly coming from the lipids making membranes), 50% of 20:0 and 22:0 fatty acids, and 25% of very-long chain fatty acids above C22 (since suberin is enriched in very-long chain fatty acids, especially in 20:0 and 22:0) as previously described (Delude et al., 2016).
Notes
- Plasticware should be not be used at any point in the protocol or else plastic contamination may obscure peaks in the GC traces. Pre-rinse all glassware and Hamilton syringes three times with chloroform. New glass Pasteur pipettes do not need to be solvent rinsed.
Recipes
- Murashige and Skoog (MS) medium (400 ml)
Mix 1.8 g MS with Vitamins, 0.2 g MES, 2.8 g agar in 400 ml distilled water
Adjust pH to 5.7 with 1 N KOH (5.61 g in 100 ml water)
Autoclave for 20 min at 120 °C, cool, and then pour into the Petri dishes (20 to 25 ml per dish) - Stock solutions (5 mg/ml) of internal standards
Stock solutions (5 mg/ml) of internal standards (heptadecanoic acid, C17:0; pentadecanol, C15:0-OH; C15-hydroxypentadecanoic acid, ω-OH-C15:0) should be prepared in methanol and stored in glass tube with polytetrafluoroethylene (PTFE)-lined caps at -20 °C until use
Acknowledgments
The solvent extraction method described here is adapted from Delude et al., 2016. This work was supported by the French Ministère de l’Enseignement Supérieur et de la Recherche (doctoral fellowship to C.D.), by the Natural Sciences and Engineering Research Council of Canada (grant to O.R.), and by grant no. MetaboHUB–ANR–11–INBS–0010 to the Functional Genomic Center of the Bordeaux-Metabolome/Lipidome platform.
References
- Andersen, T. G., Barberon, M. and Geldner, N. (2015). Suberization - the second life of an endodermal cell. Curr Opin Plant Biol 28: 9-15.
- Barberon, M., Vermeer, J. E., De Bellis, D., Wang, P., Naseer, S., Andersen, T. G., Humbel, B. M., Nawrath, C., Takano, J., Salt, D. E. and Geldner, N. (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164(3): 447-459.
- Bernards, M. A. (2002). Demystifying suberin. Can J Bot 80: 227-240.
- Delude, C., Fouillen, L., Bhar, P., Cardinal, M. J., Pascal, S., Santos, P., Kosma, D. K., Joubès, J., Rowland, O. and Domergue, F. (2016). Primary fatty alcohols are major components of suberized root tissues of Arabidopsis in the form of alkyl hydroxycinnamates. Plant Physiol 171(3): 1934-1950.
- Domergue, F., Vishwanath, S. J., Joubes, J., Ono, J., Lee, J. A., Bourdon, M., Alhattab, R., Lowe, C., Pascal, S., Lessire, R. and Rowland, O. (2010). Three Arabidopsis fatty acyl-coenzyme A reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol 153(4): 1539-1554.
- Kosma, D. K., Molina, I., Ohlrogge, J. B. and Pollard, M. (2012). Identification of an Arabidopsis fatty alcohol:caffeoyl-Coenzyme A acyltransferase required for the synthesis of alkyl hydroxycinnamates in root waxes. Plant Physiol 160(1): 237-248.
- Ranathunge, K., Schreiber, L. and Franke, R. (2011). Suberin research in the genomics era--new interest for an old polymer. Plant Sci 180(3): 399-413.
- Vishwanath, S. J., Delude, C., Domergue, F. and Rowland, O. (2015). Suberin: biosynthesis, regulation, and polymer assembly of a protective extracellular barrier. Plant Cell Rep 34(4): 573-586.
- Vishwanath, S. J., Kosma, D. K., Pulsifer, I. P., Scandola, S., Pascal, S., Joubes, J., Dittrich-Domergue, F., Lessire, R., Rowland, O. and Domergue, F. (2013). Suberin-associated fatty alcohols in Arabidopsis: distributions in roots and contributions to seed coat barrier properties. Plant Physiol 163(3): 1118-1132.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Delude, C., Vishwanath, S. J., Rowland, O. and Domergue, F. (2017). Root Aliphatic Suberin Analysis Using Non-extraction or Solvent-extraction Methods. Bio-protocol 7(12): e2331. DOI: 10.21769/BioProtoc.2331.
Category
Plant Science > Plant biochemistry > Lipid
Plant Science > Plant cell biology > Tissue analysis
Biochemistry > Lipid > Extracellular lipids
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












