- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Photometric Assays for Chloroplast Movement Responses to Blue Light
Published: Vol 7, Iss 11, Jun 5, 2017 DOI: 10.21769/BioProtoc.2310 Views: 8193
Reviewed by: Scott A M McAdamSam-Geun KongAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1688 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 749 Views

A Simple Protocol for Periodic Live Cell Observation of Flagellate Stages in the Lichen Alga Trebouxia
Enrico Boccato [...] Mauro Tretiach
Jan 20, 2026 179 Views
Abstract
Assessment of chloroplast movements by measuring changes in leaf transmittance is discussed, with special reference to the conditions necessary for reliable estimation of blue light–activated chloroplast responses.
Keywords: Arabidopsis thalianaBackground
Following the discovery of phototropins, chloroplast movements activated with blue light absorbed by these photoreceptors began to arouse much interest. Quantitative assessment of chloroplast redistribution in a multilayer leaf relies mainly on measuring transmittance changes of the tissue that are a consequence of this redistribution. Various devices have been used for that purpose, including a recently adapted commercial microplate reader (Wada and Kong, 2011; Johansson and Zeidler, 2016), particularly suited for screening large number of samples. However, samples must be properly pretreated and characterized to make their comparison reliable. This aspect has not been referred to in many papers making use of the transmittance technique. Hence, we found it important to discuss these issues in the current protocol.
Materials and Reagents
- Thin microscope slide with double wells (Ted Pella, catalog number: 260242 )
- Cling film
- Tissue paper
- Optionally, for measurements of water plants or infiltrated leaf pieces
- Parafilm M (Sigma-Aldrich, catalog number: P7793 )
- Silicon grease (Baysilone-Paste, GE Bayer Silicones)
- 2 ml syringe
Equipment
- Custom-made photometer, based on Walczak and Gabryś (1980). This device is a prototype that contains the following commercially available parts (Figure 1):
- Luxeon Royal Blue LXHL-FR5C LED (460 nm) (Luxeon Star, catalog number: LXHL-FR5C )–the source of the blue actinic beam (Figure 1A)
Note: This product has been discontinued. - L-793SRD-B LED (660 nm) (Kingbright, catalog number: L-793SRD-B )–the source of the red measuring light (Figure 1B)
- BPW20RF Planar Silicon PN photodiode (Vishay, catalog number: BPW20RF )–the detector (Figure 1E)
- NI USB-6001 DAQ board (National Instruments, model: USB-6001 )–for signal digitization (Figure 1F)

Figure 1. A double-beam photometer used for measurements of transmittance changes resulting from blue light–activated chloroplast movements. Key components: A. blue LED housing; B. red LED housing; C. measuring chamber; D. 660 nm interference filter; E. receiver photodiode; F. electronic controller.
Software
- Software appropriate for analysis of numerical data, e.g., R, Octave, MATLAB, or Mathematica, equipped with a digital filter appropriate for numerical differentiation (e.g., Savitzky-Golay filter, available in the R software as ‘sgolayfilt’, part of the ‘signal’ library)
Procedure
- Principle of the experiment
The photometric method is based on the measurement of changes in leaf transmittance that result from chloroplast relocations in the cells. In terrestrial angiosperms, chloroplast movements are activated only by blue light and depend on its fluence rate and direction. In weak blue light, chloroplasts redistribute to cell walls perpendicular to the direction of incident light. This response, called accumulation, leads to a decrease in the leaf transmittance. Strong blue light induces chloroplast avoidance. Chloroplasts redistribute to cell walls parallel to the light direction, which results in an increase of the leaf transmittance. Photoreceptors responsible for chloroplast movements are called phototropins. Two phototropins are encoded in the genome of Arabidopsis thaliana: phototropin1 and phototropin2. While the accumulation response is mediated by both phototropins, the avoidance response is primarily controlled by phototropin2 (Jarillo et al., 2001; Kagawa et al., 2001; Sakai et al., 2001). Red light does not induce chloroplast movements in terrestrial angiosperms, thus it is used as measuring light. The measuring red light intensity (0.3 µmol m-2 sec-1) is ca. 40 times less than the light compensation point of photosynthesis (12.8 µmol m-2 sec-1 for red light, Takemiya et al., 2005). Thus, the activity of photosynthesis induced by the measuring light is negligible. - Description of the equipment
- Transmittance measurements are carried out using a custom-made photometer (Figure 1). A detached leaf is mounted on a holder (Figure 2) fitting the measuring chamber (Figure 1C). Two concentric light beams are delivered perpendicular to the dorsal surface of the leaf. These are the actinic blue light of 460 nm from a Luxeon Royal Blue LXHL-FR5C LED (Philips Lumileds Lighting Comp.) (Figure 1A) and the measuring red light of 660 nm, 0.3 µmol m-2 sec-1, from an L-793SRD-B LED (Kingbright) (Figure 1B). Fluence rate of the actinic beam is controlled electronically using voltage-controlled current-source electronics (Figure 1F). An aperture in the sample holder limits the diameter of the transmitted light beam to about ½ of the incident one to ensure uniform illumination of the measured tissue fragment. The aperture diameter of 3.5 mm is used for Arabidopsis thaliana (Figure 2).
Note: Measurements on leaves of other plant species, such as aquatic angiosperms (Banaś and Gabryś, 2007) and mosses (e.g., Lemna trisulca and Funaria hygrometrica, respectively), may require smaller apertures.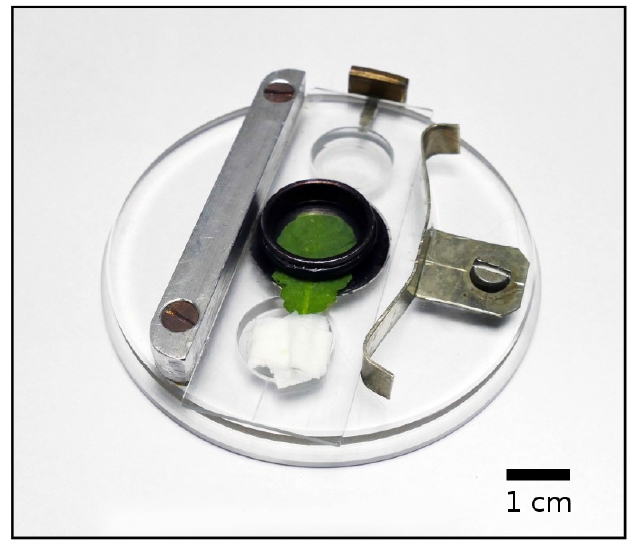
Figure 2. An Arabidopsis leaf on the sample holder
- Transmittance measurements are carried out using a custom-made photometer (Figure 1). A detached leaf is mounted on a holder (Figure 2) fitting the measuring chamber (Figure 1C). Two concentric light beams are delivered perpendicular to the dorsal surface of the leaf. These are the actinic blue light of 460 nm from a Luxeon Royal Blue LXHL-FR5C LED (Philips Lumileds Lighting Comp.) (Figure 1A) and the measuring red light of 660 nm, 0.3 µmol m-2 sec-1, from an L-793SRD-B LED (Kingbright) (Figure 1B). Fluence rate of the actinic beam is controlled electronically using voltage-controlled current-source electronics (Figure 1F). An aperture in the sample holder limits the diameter of the transmitted light beam to about ½ of the incident one to ensure uniform illumination of the measured tissue fragment. The aperture diameter of 3.5 mm is used for Arabidopsis thaliana (Figure 2).
- An opal glass–topped light guide is used to deliver the transmitted light to the signal detector (Figure 1E). This is a planar Silicon PN photodiode, model BPW20RF (Vishay Semiconductor) designed for high precision linear applications. The photodiode is integrated with a 660 nm interference filter and a double lens system (Figure 1D). The measuring beam is square-wave modulated with the frequency of 2 kHz to eliminate contributions from the photodiode dark current and external light sources. This also allows to supplement the actinic blue light with other selected wavelengths (Gabryś, 1985) or to use white light to activate chloroplast movements. The device selectively records changes in the modulated beam due to the application of the lock-in detection technique. The actinic beam intensity can be set to a desired level at any time after the start of the experiment. The device can also generate short pulses of actinic light. Pulse generation is hardware-controlled to ensure repeatability of the pulse duration. The software used to run the photometer is written in LabVIEW (National Instruments)–a software environment for deployment of measurement and control systems.
- Calibration of the equipment
- Before measurements, the instrument must be calibrated. The receiver photodiode signal is measured with the measuring light path blocked by an opaque lid. This value corresponds to 0% transmittance.
- Next, the signal is measured with the sample holder inside the measuring chamber, but without the leaf. This value corresponds to 100% transmittance. The receiver photodiode signal depends linearly on the fluence rate of the transmitted light.
- Before measurements, the instrument must be calibrated. The receiver photodiode signal is measured with the measuring light path blocked by an opaque lid. This value corresponds to 0% transmittance.
- Plant growth conditions
Note: Leaves used for chloroplast movement measurements should be optically thin. Each cell layer absorbs light, so that a fluence rate gradient is formed across the leaf blade. The distal cell layer receives less light. Thus, its chloroplasts respond to weaker light than those in the layer facing the light source. Optically denser layers produce a steeper gradient. To minimize light attenuation through the leaf blade, the transmittance of leaves used for experiments is at least 8%. It is possible to measure thicker leaves after infiltration with water or buffer using a syringe, which increases leaf transmittance. It is also a convenient way of applying inhibitors (Aggarwal et al., 2013) or other chemicals (Anielska-Mazur et al., 2009).- For Arabidopsis thaliana, moderate transmittance levels of leaves are assured by growing at 80-100 µmol m-2 sec-1 of white light in a short day (10 h light/14 h dark).
- Leaves from 4-5-week-old plants are used for photometric measurements.
- For Arabidopsis thaliana, moderate transmittance levels of leaves are assured by growing at 80-100 µmol m-2 sec-1 of white light in a short day (10 h light/14 h dark).
- Sample preparation
- Plants are dark-adapted in a darkroom for at least 7 h to allow chloroplasts to obtain the stationary dark position. Dark adaptation is necessary to ensure that chloroplasts start from the same positions in the cell, which is crucial for quantitative comparison of chloroplast responses in various leaves. Otherwise transmittance changes correspond to different phases of redistribution and cannot be compared. Routinely, leaves are transferred to darkness at the end of the photoperiod light cycle.
- All experimental manipulations are performed under dim green light (< 0.05 µmol m-2 sec-1).
- Following dark adaptation, a single leaf is detached from the plant and placed on a thin microscope slide mounted on the holder (Figure 2).
- The leaf is flattened and positioned in the middle of the slide directly above the measuring aperture. It is important to avoid putting the major vein into the measuring area.
- The leaf is covered with cling film stretched over a metal ring and its petiole is wrapped in a piece of water-soaked tissue paper to avoid drying during the experiment.
Note: Big leaves, e.g., Nicotiana tabaccum, may be cut into small fragments and immersed in a drop of water for the measurement. In that case, the leaf fragment is placed in a chamber formed by a Parafilm ring and the cling film (Anielska-Mazur et al., 2009). To prevent evaporation, the chamber is sealed with a high viscosity silicon grease. - The holder with the leaf is placed inside the measuring chamber and the initial transmittance level is recorded immediately.
Note: Ideally, the dark transmittance values of all leaves used in the same study should be similar. As the photometric method is an indirect method of chloroplast movement assessment, the reliability of the results may be adversely affected by differences in leaf anatomy. This must be taken into account while comparing chloroplast responses in mutant and wild type leaves (Eckstein et al., 2016). Variation in leaf anatomy is often reflected in different dark transmittance. The effect of dark transmittance on the movement parameters calculated from the photometric curves (e.g., amplitudes and velocities, see below) should always be determined. Thus, it is advisable to plot the parameters against the dark transmittance. This effect can also be taken into account at the stage of statistical analysis of results (see the Data analysis section).
- Plants are dark-adapted in a darkroom for at least 7 h to allow chloroplasts to obtain the stationary dark position. Dark adaptation is necessary to ensure that chloroplasts start from the same positions in the cell, which is crucial for quantitative comparison of chloroplast responses in various leaves. Otherwise transmittance changes correspond to different phases of redistribution and cannot be compared. Routinely, leaves are transferred to darkness at the end of the photoperiod light cycle.
- Measurements under continuous light–multiple illumination steps of increasing intensity
Note: Chloroplast relocations in response to increasing light intensities can be measured in one leaf. The outcome is a fluence rate–response curve. For Arabidopsis, typical light intensities used start from 0.1 µmol m-2 sec-1 and increase stepwise up to 120 µmol m-2 sec-1 (Jarillo et al., 2001) (Figure 3A).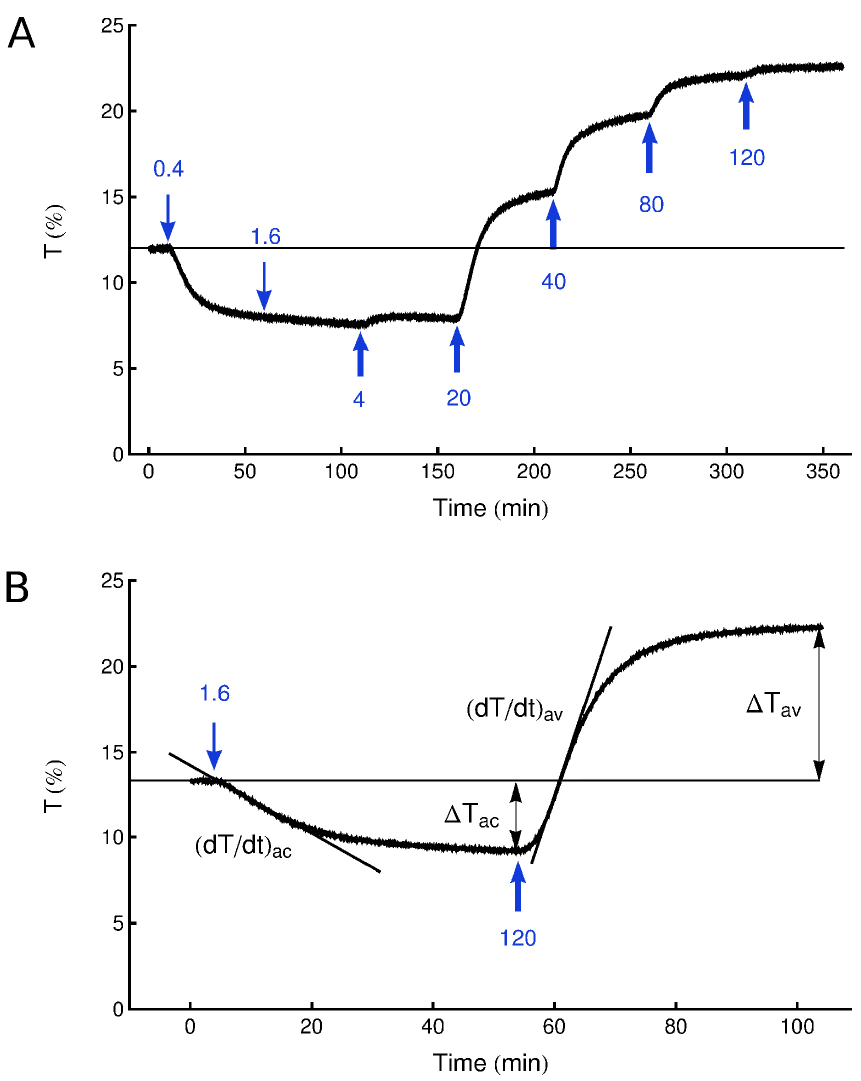
Figure 3. Typical curves obtained using the photometric method. A. A fluence rate–response curve. A dark-adapted leaf of A. thaliana with initial transmittance of 12% was exposed to continuous blue light of fluence rates 0.4-120 µmol m-2 sec-1. Each irradiation step lasted 50 min. B. A typical recording trace, explaining the way in which amplitudes and velocities are calculated. Arrows indicate start of illumination with weak (↓1.6 µmol m-2 sec-1) or strong (↑120 µmol m-2 sec-1) blue light. ΔTac, ΔTav–amplitudes, and (dT/dt)ac, (dT/dt)av–velocities of transmittance changes corresponding to the accumulation and avoidance response, respectively.- The transmittance of a dark-adapted leaf is recorded for several minutes in physiological darkness to make sure that its level is stable.
- Blue light of the desired fluence rate is turned on and the changes in transmittance are recorded for at least 45 min.
Note: This is a compromise time, usually too short to obtain the stationary phase of the response. However, the transmittance drift in the final stage of the response is very small and can be neglected. In the case of slow responses, e.g., at low temperature, the time of light exposure/measurement should be increased (Łabuz et al., 2015). - After the response reaches a plateau, the blue light intensity can be changed.
Note: To measure only the maximal accumulation and avoidance, saturating light for each response can be used. A typical experiment consists of recording the dark transmittance level for 5 min, followed by weak blue light (1.6 µmol m-2 sec-1) for at least 45 min and strong blue light (120 µmol m-2 sec-1) for at least 45 min (Figure 3B).
- The transmittance of a dark-adapted leaf is recorded for several minutes in physiological darkness to make sure that its level is stable.
- Measurements under continuous light–one intensity at a time
Another approach is to measure chloroplast responses only to one light intensity at a time. In this case, we always illuminate a dark-adapted leaf and obtain a family of curves, each corresponding to one intensity (Jarillo et al., 2001). This is a standard approach if we want to compare the kinetics of chloroplast responses in various samples under various fluence-rates, as it ensures that chloroplasts start from the same position in the cell. For response amplitudes, both approaches are equivalent provided that amplitudes do not depend significantly on previous illumination but only on the current fluence-rate. This must be verified experimentally for the studied species. In the case of A. thaliana wild type, amplitudes depend only on the current illumination. However, mutants may behave differently. For example, the phot2 mutant shows practically no response to strong blue light when chloroplasts have already reached the accumulation position, while a biphasic response is observed when strong light is applied directly after dark adaptation (Jarillo et al., 2001). - Measurements of responses to light pulses
Note: Transmittance changes after light pulses are lower in magnitude than responses to continuous light, therefore they require a good signal-to-noise ratio of the recording system.- The dark transmittance level is recorded for 5 min.
- A very short pulse of blue light is applied and chloroplast relocations are recorded in darkness for the following 45 min. For experiments with A. thaliana, we typically use a series of strong blue light (120 µmol m-2 sec-1, saturating the avoidance response) pulses for 0.1, 0.2, 1, 2, 10 and 20 sec (Sztatelman et al., 2016).
Note: The pulse duration and light intensity can be adjusted in broad ranges. For pulses resulting only in transient accumulation, the chloroplast response follows the Bunsen-Roscoe reciprocity law. Thus, it is determined by the total amount of radiant energy delivered (product of fluence rate and the exposure time) (Gabryś et al., 1981). - If the goal of the experiment is to compare effects of different pulse durations on chloroplast movements in the same plant line, it is convenient to record the whole series on the same leaf. After the transmittance curve is recorded, the leaf is transferred back to darkness for recovery and the response in a second leaf is measured. This dark recovery may be shorter than the initial adaptation period because the responses to pulses and the respective transmittance changes are small and usually level off within 1.5 h. Next, the first leaf is taken back and challenged with a light pulse of increased duration.
- The dark transmittance level is recorded for 5 min.
Data analysis
- Calculation of movement parameters
- To quantify the chloroplast movements, amplitudes (ΔT) and maximal velocities (dT/dt) of transmittance change during each illumination phase are calculated. These parameters can be estimated on a curve print-out using a ruler. Alternatively, they can be determined using software appropriate for analysis of numerical data, e.g., R, MATLAB or Mathematica. Amplitudes are calculated relative to the dark transmittance level (Figure 3B). If the noise is substantial, the accuracy of the calculated values of amplitudes can be improved by smoothing the curves with a low-pass digital filter. Savitzky Golay filter (available e.g., in the free software R) is appropriate for curves with points spaced at equal distances, as is usually the case. If the points are irregularly spaced, the curve can be smoothed using local regression (also available in R). Maximal velocity can be determined by drawing a straight line, tangent to the transmittance curve at the point where the response is the fastest. The slope of the line is equal to the magnitude of the velocity. Numerical estimation of the velocity is possible with an appropriate digital filter. Savitzky-Golay filter can also be used for this purpose. It works by fitting a polynomial to points within a sliding window. The point in its center is replaced with a value predicted by the polynomial fit (for smoothing) or with the derivative of the fit (for velocity calculation). The degree of the polynomial should be low. We use quadratic polynomials. The width of the window is also specified by the user. Wider window allows for more efficient noise removal. However, the use of a too wide window may result in underestimation of the maximal velocity of movements. Thus, the width should be adjusted to the noise level and sampling rate of the equipment. For velocity calculation, we use the window width that corresponds to a 2 min interval.
Note: To better characterize the kinetics of chloroplast movements, it is advisable to plot rate (dT/dt) against time as in Łabuz et al., 2015 (Figures 1C and 1E-1F, therein). This facilitates estimation of the maximum velocity and the time after which the maximum velocity is achieved. This also helps to assess whether the new light intensity was applied after reaching the stationary phase of the preceding response. - The chloroplast movements triggered by pulses can be quantified similarly to responses to continuous light. However, the response to a single pulse often consists of two phases–a fast, transient avoidance phase followed by a slower accumulation response (Sztatelman et al., 2006). Thus, two amplitudes and two velocities should be calculated for a single pulse.
Note: Additional parameters may be calculated from photometric curves. The time between the onset of the pulse and the maximum of transient avoidance or accumulation is useful in characterizing the dynamics of chloroplast responses to light pulses (Sztatelman et al., 2006).
- To quantify the chloroplast movements, amplitudes (ΔT) and maximal velocities (dT/dt) of transmittance change during each illumination phase are calculated. These parameters can be estimated on a curve print-out using a ruler. Alternatively, they can be determined using software appropriate for analysis of numerical data, e.g., R, MATLAB or Mathematica. Amplitudes are calculated relative to the dark transmittance level (Figure 3B). If the noise is substantial, the accuracy of the calculated values of amplitudes can be improved by smoothing the curves with a low-pass digital filter. Savitzky Golay filter (available e.g., in the free software R) is appropriate for curves with points spaced at equal distances, as is usually the case. If the points are irregularly spaced, the curve can be smoothed using local regression (also available in R). Maximal velocity can be determined by drawing a straight line, tangent to the transmittance curve at the point where the response is the fastest. The slope of the line is equal to the magnitude of the velocity. Numerical estimation of the velocity is possible with an appropriate digital filter. Savitzky-Golay filter can also be used for this purpose. It works by fitting a polynomial to points within a sliding window. The point in its center is replaced with a value predicted by the polynomial fit (for smoothing) or with the derivative of the fit (for velocity calculation). The degree of the polynomial should be low. We use quadratic polynomials. The width of the window is also specified by the user. Wider window allows for more efficient noise removal. However, the use of a too wide window may result in underestimation of the maximal velocity of movements. Thus, the width should be adjusted to the noise level and sampling rate of the equipment. For velocity calculation, we use the window width that corresponds to a 2 min interval.
- Randomization and experimental design
- Reproducibility of the chloroplast movement assay requires both randomization and proper control over plant growth conditions. The conditions in the growth chamber (availability of water, light intensity, etc.) may vary from place to place. To reduce the risk of systematic errors, plants from different lines should be placed in the chamber in a randomized manner. To reduce variance due to differences in growth, pots may be reshuffled several times during plant growth; this is especially important in growth chambers with side illumination. If the experiment requires use of several growth chambers, plants from all lines (or, in general, all experimental groups) should be grown simultaneously in every chamber. The results obtained from different batches of plants may vary. To ensure the reproducibility of results, the measurements should be repeated at least three times, using batches of plants that were sown and grown independently.
- In experiments that involve leaf pretreatment (e.g., infiltration with an inhibitor), whole plants may be either allocated randomly to each treatment level (e.g., inhibitor concentration), or leaves from the same plant, similar in size and shape, may be allocated to each level. This choice determines the type of the statistical method that can be used for data analysis. These two methods of allocation to treatment levels must not be mixed in a single experiment. Otherwise it would be impossible to analyze the results statistically.
- The number of plants necessary for an experiment depends on the magnitude of the studied effects, the variability of plants and the number of experimental groups. A group is a combination of levels of different factors, e.g., in an experiment on a mutant and the wild type plants, treated with an inhibitor (two concentrations plus control), which was repeated three times on different batches of plants, there are three factors (the plant line, the inhibitor concentration, the plant batch) and 18 groups (2 x 3 x 3). In experiments on A. thaliana, we use 10 or more plants per group when we look for effects of moderate magnitude. Large effects require fewer replicates.
- Reproducibility of the chloroplast movement assay requires both randomization and proper control over plant growth conditions. The conditions in the growth chamber (availability of water, light intensity, etc.) may vary from place to place. To reduce the risk of systematic errors, plants from different lines should be placed in the chamber in a randomized manner. To reduce variance due to differences in growth, pots may be reshuffled several times during plant growth; this is especially important in growth chambers with side illumination. If the experiment requires use of several growth chambers, plants from all lines (or, in general, all experimental groups) should be grown simultaneously in every chamber. The results obtained from different batches of plants may vary. To ensure the reproducibility of results, the measurements should be repeated at least three times, using batches of plants that were sown and grown independently.
- Statistical tests
- The choice of statistical tests depends on the type of variables that may affect the movement parameters. Most often, the variables controlled by the experimenter are fixed categorical variables (factors), which means that their values are levels specified in advance. Examples are plant lines (two levels: wild type vs. mutant plants) or stress treatment (stressed vs. control plants). If the whole experiment was repeated on multiple batches of plants, the batch can be treated as a random variable. Variables may also be continuous, e.g., dark transmittance. The movement parameters can be treated as linearly dependent on the variables. If all variables included in the model are categorical factors, regular Analysis of Variance (ANOVA) is used to test whether the effects of the factors are significant. The differences in means between all pairs of groups can be tested with Tukey test. A selected level, treated as the control can be compared to every other level, with Dunnett’s test. When similar leaves, usually selected from the same plant, are allocated to different treatment levels, a new blocking variable (plant) must be introduced into the ANOVA model. In the special case of just one treatment level and the control, the paired-sample t-test is appropriate.
- The effects of dark transmittance on chloroplast responses may also be included in the model. Usually, the relationship between movement parameters and dark transmittance can be fitted well with a line. In such a case, the effect of dark transmittance can be reduced at the stage of data analysis, by treating dark transmittance as an additional, continuous variable (a covariate), alongside other variables of experimental interest. If the slopes of the best linear fits are similar for all plant lines or treatments, the standard analysis of covariance (ANCOVA), available in the free software R, can be employed.
- The choice of statistical tests depends on the type of variables that may affect the movement parameters. Most often, the variables controlled by the experimenter are fixed categorical variables (factors), which means that their values are levels specified in advance. Examples are plant lines (two levels: wild type vs. mutant plants) or stress treatment (stressed vs. control plants). If the whole experiment was repeated on multiple batches of plants, the batch can be treated as a random variable. Variables may also be continuous, e.g., dark transmittance. The movement parameters can be treated as linearly dependent on the variables. If all variables included in the model are categorical factors, regular Analysis of Variance (ANOVA) is used to test whether the effects of the factors are significant. The differences in means between all pairs of groups can be tested with Tukey test. A selected level, treated as the control can be compared to every other level, with Dunnett’s test. When similar leaves, usually selected from the same plant, are allocated to different treatment levels, a new blocking variable (plant) must be introduced into the ANOVA model. In the special case of just one treatment level and the control, the paired-sample t-test is appropriate.
Acknowledgments
This work was supported from European Union funds within the framework of FP7, Marie Curie ITN CALIPSO, grant No. [GA 2013-ITN-607-607] and from financial resources for science in the years 2013-2017, allocated to the realization of a co-financed international project.
Both the equipment and the protocol have been modified from previous work by Walczak and Gabrys (1980). New type of photometer for measurement of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica 14, 65-72.
References
- Aggarwal, C., Łabuz, J. and Gabrys, H. (2013). Phosphoinositides play differential roles in regulating phototropin1- and phototropin2-mediated chloroplast movements in Arabidopsis. PLoS One 8(2): e55393.
- Anielska-Mazur, A., Bernaś, T. and Gabryś, H. (2009). In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses. BMC Plant Biol 9: 64.
- Banaś A. K and Gabryś, H. (2007). Influence of sugars on blue light-induced chloroplast movements. Plant Signal Behav 4(2): 221-230.
- Eckstein, A., Krzeszowiec, W., Waligórski, P. and Gabryś, H. (2016). Auxin and chloroplast movements. Physiol Plant 156(3): 351-366.
- Gabryś, H. (1985). Chloroplast movement in Mougeotia induced by blue light pulses. Planta 166:134-140.
- Gabryś, H., Walczak, T. and Zurzycki J. (1981). Chloroplast translocations induced by light pulses: Effects of single light pulses. Planta 152: 553-556.
- Jarillo, J. A., Gabrys, H., Capel, J., Alonso, J. M., Ecker, J. R. and Cashmore, A. R. (2001). Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410(6831): 952-954.
- Johansson, H. and Zeidler, M. (2016). Automatic chloroplast movement analysis. Methods Mol Biol 1398: 29-35.
- Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K. and Wada, M. (2001). Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138-2141.
- Łabuz, J., Hermanowicz, P. and Gabryś, H. (2015). The impact of temperature on blue light induced chloroplast movements in Arabidopsis thaliana. Plant Sci 239: 238-249.
- Sakai, T., Kagawa, T., Kasahara, M., Swartz, T. E., Christie, J. M., Briggs, W. R., Wada, M. and Okada, K. (2001). Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. PNAS 98: 6969-6974.
- Sztatelman, O., Łabuz, J., Hermanowicz, P., Banaś, A. K., Bażant, A., Zgłobicki, P., Aggarwal, C., Nadzieja, M., Krzeszowiec, W., Strzałka, W. and Gabryś, H. (2016). Fine tuning chloroplast movements through physical interactions between phototropins. J Exp Bot 67: 4963-4978.
- Takemiya, A., Inoue, S., Doi, M., Kinoshita, T. and Shimazaki, K. (2005). Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17: 1120-1127.
- Wada, M. and Kong, S. G. (2011). Analysis of chloroplast movement and relocation in Arabidopsis. Methods Mol Biol 774: 87-102.
- Walczak, T. and Gabrys, H. (1980). New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica 14: 65-72.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gabryś, H., Banaś, A. K., Hermanowicz, P., Krzeszowiec, W., Leśniewski, S., Łabuz, J. and Sztatelman, O. (2017). Photometric Assays for Chloroplast Movement Responses to Blue Light. Bio-protocol 7(11): e2310. DOI: 10.21769/BioProtoc.2310.
Category
Plant Science > Plant cell biology > Cell imaging
Cell Biology > Cell imaging > Live-cell imaging
Cell Biology > Organelle isolation > Chloroplast
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









