- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunostaining of Formaldehyde-fixed Metaphase Chromosome from Untreated and Aphidicolin-treated DT40 Cells
Published: Vol 7, Iss 9, May 5, 2017 DOI: 10.21769/BioProtoc.2259 Views: 10695
Reviewed by: Antoine de MorreeTatsuki KunohXiaoyi Zheng

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Microscopic Detection of DNA Synthesis in Early Mitosis at Repetitive lacO Sequences in Human Cells
Kazumasa Yoshida [...] Masatoshi Fujita
Sep 5, 2022 2658 Views

Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
Mi Rim Lee [...] Yun-Hee Kim
Oct 5, 2025 1703 Views

Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
Nov 20, 2025 1565 Views
Abstract
During mitosis chromosomes are condensed into dense X-shaped structures that allow for microscopic determination of karyotype as well as inspection of chromosome morphology.
This protocol describes a method to perform immunostaining of formaldehyde-fixed metaphase chromosomes from the avian cell line DT40. It was developed to characterize the localization of YFP-tagged TopBP1 on mitotic chromosomes and specifically determine the percentage of TopBP1 foci that formed on breaks/gaps as well as ends of individual metaphase macrochromosomes (Pedersen et al., 2015). For this purpose immunostaining of YFP was applied. However, the protocol may be optimized for other cell lines or epitopes.
Background
Microscopic analysis of stained metaphase chromosomes is a classical cytogenetic technique that is extensively used for both research and diagnostics. The basic principle involves induction of cell cycle arrest in metaphase by a spindle destabilizing reagent such as colcemid, which will trigger the spindle assembly checkpoint and therefore arrests cells in metaphase. This serves to enrich for cells with condensed chromosomes. Subsequently cells are subjected to swelling in hypotonic solution followed by spreading of mitotic cells on a microscope slide. The final result is microscopically detectable chromosomes from single cells convenient for karyotype analysis as well as investigations of individual chromosomes. Traditionally, swollen cells are fixed with methanol and acetic acid (3:1) before spreading on slides (Hungerford, 1965; Ronne et al., 1979).
The method described here uses formaldehyde rather than methanol for fixation. This can be useful for subsequent staining with antibodies that are not compatible with methanol fixation. The protocol is optimized for metaphase spreads from chicken DT40 cells, and immunostaining of YFP-tagged TopBP1 on metaphase macrochromosomes (Pedersen et al., 2015). TopBP1 foci on mitotic chromosomes mark DNA insults that are transmitted to G1 daughter cells (Pedersen et al., 2015; Gallina et al., 2016; Oestergaard and Lisby, 2016). The fluorescent signal of YFP is lost during the preparation of metaphase spreads, therefore this protocol includes immunostaining of the YFP epitope. However, it should be possible to apply the protocol for other cell lines and epitopes by optimizing incubation time in hypotonic buffer and antibody concentrations, respectively.
Aphidicolin is a replication inhibitor, which at low concentration induces formation of gaps and breaks on metaphase chromosomes preferentially at common fragile sites (Durkin and Glover, 2007). As stated by this protocol, DT40 cells may be subjected to 0.5 μM aphidicolin to induce breaks and gaps on metaphase chromosomes in DT40 (Pedersen et al., 2015).
The avian karyotype comprises macrochromosomes as well as mini and microchromosomes. The latter two groups are too small to reliably determine features such as breaks/gaps or ends. They are therefore not included in this analysis.
Materials and Reagents
- 15 ml tubes (such as Sigma-Aldrich, catalog number: T1943 )
- Pipette tips
- Round cover slips (Ø12 mm) (Thermo Fisher Scientific)
- Cytospin slides (Thermo Fisher Scientific, catalog number: 5991059 )
- A DT40 cell line (Baba and Humphries, 1985) carrying endogenous YFP-tagging at the TopBP1 gene (Germann et al., 2014; Pedersen et al., 2015)
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D4540 )
- Aphidicolin (Sigma-Aldrich, catalog number: A0781 )
- Colcemid (Thermo Fisher Scientific, GibcoTM, catalog number: 15212012 )
- Cytofunnels (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: A78710020 )
- Nail polish
- RPMI 1640 medium GlutaMAX (Thermo Fisher Scientific, GibcoTM, catalog number: 61870044 )
- Chicken serum (such as Sigma-Aldrich, catalog number: C5405 or Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 16110082 )
- Fetal bovine serum (FBS) (Heat inactivated) (Thermo Fisher Scientific, GibcoTM, catalog number: 10500 )
- β-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Penicillin/streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Potassium chloride (KCl) (TH GEYER, Chemsolute®, catalog number: 1632 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: 158127 )
- Sodium hydroxide (NaOH)
- Hydrochloric acid (HCl)
- Sodium chloride (NaCl) (Avantor® Performance Materials, J.T. Baker, catalog number: 0278.1000 )
- 2-Amino-2-(hydroxymethyl)-1,3-propanediol (Tris, Trizma base) (Sigma-Aldrich, catalog number: T1503 )
- (Ethylenedinitrilo)tetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: 27285 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100 )
- Tween-20 (Sigma-Aldrich, catalog number: P9416 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A4503 )
- Alexa Fluor 488-conjugated anti-mouse IgG (Thermo Fisher Scientific, Invitrogen, catalog number: A21121 )
- Mouse anti-GFP antibody (Roche Diagnostics, catalog number: 11814460001 )
- Glycerol (AppliChem, catalog number: 142329.1211 )
- n-propyl-gallate (Sigma-Aldrich, catalog number: 02370 )
- 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride, 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich, catalog number: D9542 )
- DT40 cells medium (see Recipes)
- Aphidicolin solution (see Recipes)
- Hypotonic buffer (see Recipes)
- 3% paraformaldehyde in PBS (see Recipes)
- KCM buffer (see Recipes)
- Phosphate buffered saline (PBS) (see Recipes)
- PBS-T (see Recipes)
- Blocking solution (see Recipes)
- Alexa 488-secondary antibody working solution (see Recipes)
- Anti-GFP antibody working solution (see Recipes)
- DAPI-mounting buffer (see Recipes)
Equipment
- Cytocentrifuge, Cytospin4 (Thermo Fisher Scientific, Thermo ScientificTM, model: CytospinTM 4 Cytocentrifuge )
- Pipettes (P1000, P200, P20)
- CO2 incubator (such as Nuaire, model: 4750E , series 6)
- Flasks such as 25 cm2 flasks (TPP, catalog number: 90025 )
- Vortex
- Fume hood
- Coplin jar such as ‘jar staining acc to Coplin W/cover’ (VWR, catalog number: 631-9331 )
- Fluorescence microscope (such as GE Healthcare, DeltaVision Elite; Applied Precision) equipped with a 100x objective lens (NA 1.4; Olympus, model: U-PLAN S-APO ), a cooled EM CCD camera (Photometrics, model: Evolve 512 ), and a solid-state illumination source (Insight; Applied Precision) or similar fluorescence microscope. The microscope should be equipped with appropriate filters to image Alexa488 and DAPI
Software
- SoftWoRx (Applied Precision) software or similar
- Volocity software (PerkinElmer) or ImageJ
Procedure
- Day 1
- Prepare the following two cell cultures
- 5 ml DT40 (TopBP1YFP/+/+) cell culture with a cell density of 0.7 million per ml DT40 cells medium (RPMI 1640 medium GlutaMAX supplemented with 2% chicken serum, 8% FBS, 55 µM β-mercaptoethanol, 50 U/ml penicillin and 50 µg/ml streptomycin) + DMSO (solvent control, diluted similar to aphidicolin).
- 5 ml DT40 (TopBP1YFP/+/+) cell culture with a cell density of 0.7 million per ml DT40 cells medium + 0.5 µM aphidicolin (aphidicolin from 4 mM stock is diluted in culture medium).
- Incubate for 16 h at 39 °C in a CO2 incubator with 5% CO2.
- Day 2
- Add colcemid to 0.1 µg/ml final concentration to the 5 ml cell culture in each of the flasks and incubate for 150 min in the CO2 incubator.
- Harvest the culture with 5 ml of colcemid-treated cells by centrifugation for 5 min at 201 x g in 15 ml tubes.
- Discard the supernatants and gently vortex the cell pellet to resuspend it in the remaining liquid.
- Vortex the tube at very low intensity and add drop by drop 1 ml ice cold hypotonic buffer.
- Add drop by drop another 1 ml ice cold hypotonic buffer (no need to vortex).
- Incubate for 15 min on ice.
- Meanwhile label cytospin slides and carefully place them in the cytofunnel according to manufacturers’ protocol. See Figure 1 for image of the cytofunnel.
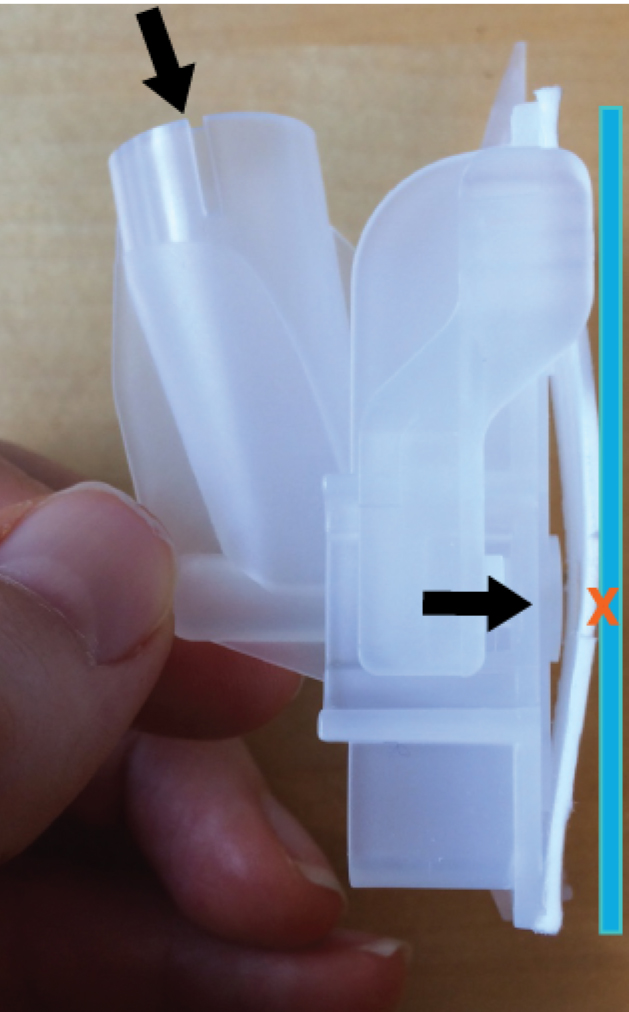
Figure 1. Image showing the cytofunnel. Top arrow indicates the site of loading into the funnel. Bottom arrow indicates the flow direction of the cell suspension toward the slide, which is indicated as a blue line. This takes place during centrifugation with the result that cells are spun onto the slide at the site of the red X. - After the 15 min incubation, transfer 400 μl of the cell suspension from step B5 to the cytofunnel and spin it in a cytospin centrifuge for 5 min at 1,000 rpm corresponding to 113 x g.
- Take the slide out of the cytofunnel.
- To fix the sample on the slide, carefully add 100 μl paraformaldehyde solution to the marked area on the slide and leave for 10 min in the fume hood (the paraformaldehyde should stay as a droplet on the slide).
- Immerse slides in a Coplin jar with 35 ml KCM buffer for 10 min.
- Pour out the KCM buffer (hold the slides in place) and then add 35 ml of PBS to the Coplin jar with the slides.
- Pour out the PBS (hold the slides in place) and then add 35 ml of PBS to the Coplin jar with the slides once again.
Note: At this stage you may store the Coplin jar with slides in PBS in the fridge until next day. - After at least 5 min, take out the slides and drain them well.
Note: Slides should be dry, except from the area within the circle. - Add 30 μl blocking solution to the area within the circle and cover with coverslip.
- Leave for 30 min at room temperature and then very carefully remove the coverslip. (Most of the blocking solution is removed along with the coverslip.)
- Add 20 μl primary anti-GFP antibody solution to the area within the circle, and cover with a coverslip.
- Incubate in a humidified chamber at 37 °C for 25 min.
- Remove the coverslip carefully and wash 2 times, for 3 min each time in PBS-T in a Coplin jar.
- Drain (slides should be dry, except from the area within the circle) and add 20 μl Alexa-488 secondary antibody working solution to the area within the circle. Then cover the area within the circle with a coverslip.
- Incubate in a humidified chamber at 37 °C for 30 min (protected from light).
- Remove coverslip carefully and wash 2 times for 3 min each time in PBS-T (35 ml) in a Coplin jar.
- Drain (slides should be dry, except from the area within the circle) and mount round coverslip onto glass slide with 6 μl DAPI-mounting buffer.
- Seal with a thin layer of nail polish at the edge of the coverslip. See Figure 2 for image of the slide.

Figure 2. Image of the cytospin slide. The sample is within the white circle. A round coverslip covers the area within the circle and nail polish is used to seal the coverslip along the edges/the white circle. - When the nail polish is dry, store the slides in a slide storage box in the fridge or proceed directly to microscopic analyses of the slides.
Notes: - Fluorophores may be visualized under a wide-field microscope (DeltaVision Elite; Applied Precision) equipped with a 100x objective lens (U-PLAN S-APO, NA 1.4; Olympus), a cooled EM CCD camera (Evolve 512; Photometrics), and a solid-state illumination source (Insight; Applied Precision) or similar fluorescence microscope.
- Images can be acquired using softWoRx (Applied Precision) software or similar.
- Add colcemid to 0.1 µg/ml final concentration to the 5 ml cell culture in each of the flasks and incubate for 150 min in the CO2 incubator.
Data analysis
Images can be processed and analyzed with Volocity software (PerkinElmer) or ImageJ. Macrochromosomes suitable for analysis are selected based on the following criteria; they should be in focus, they should not overlie other chromosomes and they should be longer than 2 μm. Selected macrochromosomes is then analyzed for gaps or breaks and TopBP1 foci localization (Figure 3). Gaps and breaks are defined as areas on chromosomes with no DAPI staining. Many macrochromosomes do not hold TopBP1 foci. When TopBP1 foci are present they can be classified into three categories: I: at chromosome ends, II: at gaps/breaks or III: at internal sites (which corresponds to the remaining foci found on the chromosomes).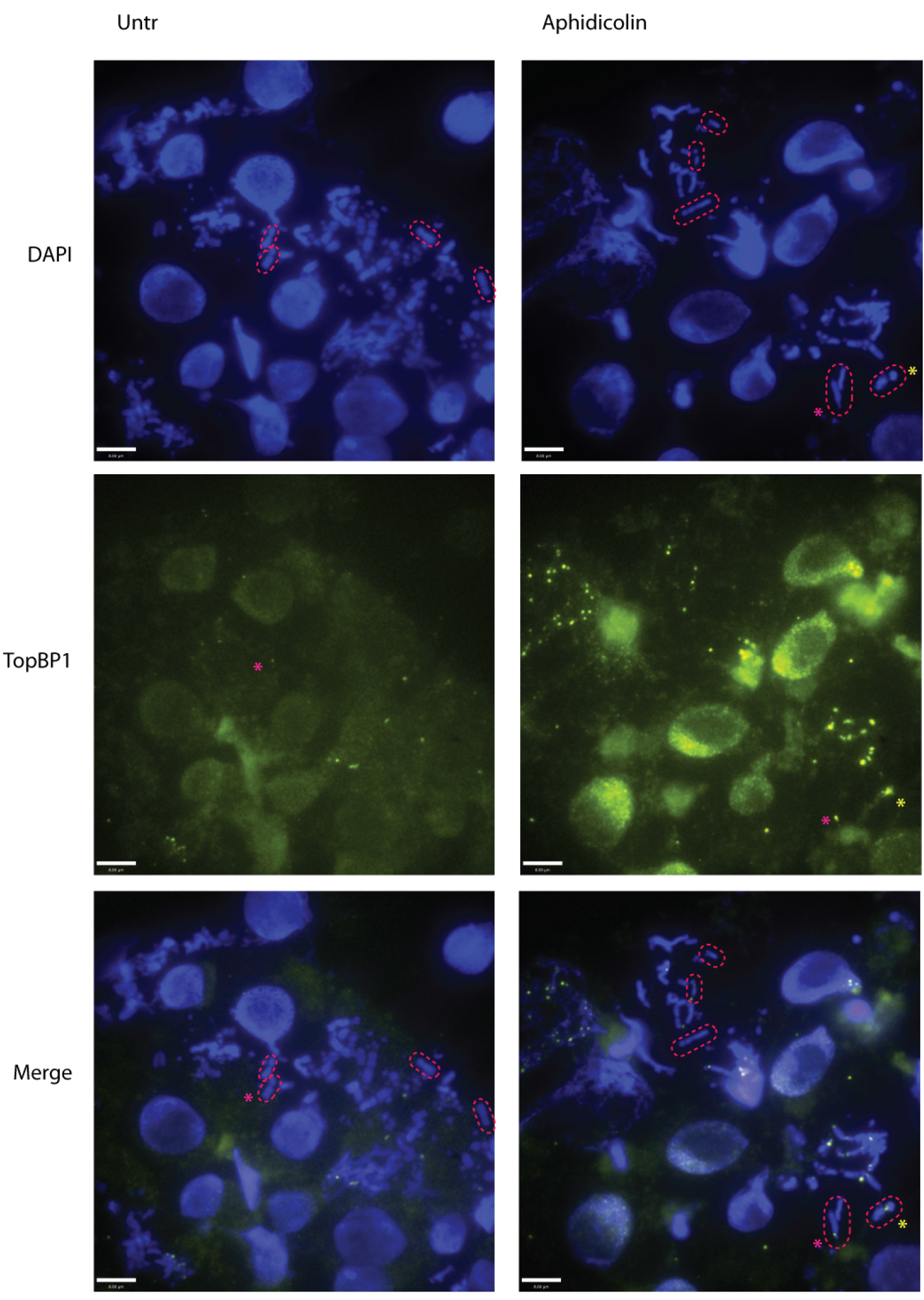
Figure 3. Representative images of slides prepared according to the protocol. Images showing interphase nuclei and metaphase chromosome spreads. Macrochromosomes suitable for analysis are encircled by read dashed line. Left panel: metaphase spreads from untr. cells. Top, DAPI staining. Middle, Alexa-488 staining of YFP-tagged TopBP1. Bottom, merge. Right panel: metaphase spreads from aphidicolin-treated cells. Left, DAPI staining. Middle, Alexa-488 staining of YFP-tagged TopBP1. Bottom, merge. Chromosomes with TopBP1 at the end are marked by red asterisks. Chromosome with TopBP1 at a break is marked by a yellow asterisk. Scale bars = 8 μm.
The data can be quantified by analyzing 250-300 macrochromosomes from both untreated and aphidicolin-treated cells from three independent experiments (80-100 from each condition of each experiment). Data can be presented in a column diagram showing for each condition: a. the percentage of macrochromosomes with TopBP1 at the end, b. the percentage of macrochromosomes with TopBP1 at gaps and breaks, and c. the percentage of macrochromosomes with TopBP1 at an internal site. It is recommended that data be analyzed blinded.
Notes
- An empty tip box with water in the reservoir can be used as a humidified chamber.
- Experiments should be analyzed blinded to avoid bias in quantification.
- Optimization of incubation time and temperature (step B6, day 2: incubation in hypotonic buffer) was crucial to obtain good quality metaphase spreads.
Recipes
- DT40 cells medium
RPMI 1640 medium GlutaMAX supplemented with:
2% chicken serum
8% FBS
55 µM β-mercaptoethanol
50 U/ml penicillin and 50 µg/ml streptomycin - Aphidicolin solution
Dissolved in DMSO to a stock concentration of 4 mM and aliquots are stored at -20 °C for maximum 6 months. The aphidicolin solution does not need to be sterilized - Hypotonic buffer
Prepared fresh by mixing 1 volume FBS, 1 volume 75 mM KCl with 3 volumes of (Milli-Q) H2O - 3% paraformaldehyde in PBS
Dissolve 0.3 g paraformaldehyde in 7 ml (Milli-Q) H2O, 1 ml 10x PBS, 200 μl NaOH (2 N) by warming the solution to 65 °C
When the paraformaldehyde is dissolved, the solution is adjusted to pH 7.4 with HCl. Finally the volume is adjusted to 10 ml with (Milli-Q) H2O
After preparation aliquots of 0.5 ml are stored at -20 °C for maximum 12 months
Note: Aliquots should not be refrozen or reused after the day of thawing. - KCM buffer
120 mM KCl
20 mM NaCl
10 mM Tris/HCl pH 8.0
0.5 mM EDTA
0.1% Triton X-100
Note: KCM buffer can be stored at room temperature for 3 months. - Phosphate buffered saline (PBS), pH 7.4
37 mM NaCl
10 mM phosphate
2.7mM KCl - PBS-T
Prepared by adding 0.1% Tween 20 to PBS
PBS-T can be kept for one month at 4 °C - Blocking solution
Prepared by supplementing PBS-T with 3% BSA
The solution can be stored for 5 days at 4 °C - Alexa 488-secondary antibody working solution
Alexa Fluor 488-conjugated anti-mouse IgG is diluted 1:1,000 in blocking solution
Note: The solution should be stored on ice, protected from light and used the same day. - Anti-GFP antibody working solution
Mouse anti-GFP antibody is diluted 1:1,000 in blocking solution, stored on ice and used the same day - DAPI-mounting buffer
Contains 85% glycerol and 2.5% n-propyl-gallate with 1.5 µg/ml DAPI
Stored at -20 °C, protected from light, DAPI-mounting buffer can be stored for 6 months
Acknowledgments
This work was supported by the Villum Foundation. This protocol is adapted from (Jeppesen, 2000).
References
- Baba, T. W. and Humphries, E. H. (1985). Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci U S A 82(1): 213-216.
- Durkin, S. G. and Glover, T. W. (2007). Chromosome fragile sites. Annu Rev Genet 41: 169-192.
- Gallina, I., Christiansen, S. K., Pedersen, R. T., Lisby, M. and Oestergaard, V. H. (2016). TopBP1-mediated DNA processing during mitosis. Cell Cycle 15(2): 176-183.
- Germann, S. M., Schramke, V., Pedersen, R. T., Gallina, I., Eckert-Boulet, N., Oestergaard, V. H. and Lisby, M. (2014). TopBP1/Dpb11 binds DNA anaphase bridges to prevent genome instability. J Cell Biol 204(1): 45-59.
- Hungerford, D. A. (1965). Leukocytes cultured from small inocula of whole blood and the preparation of metaphase chromosomes by treatment with hypotonic KCl. Stain Technol 40(6): 333-338.
- Jeppesen, P. (2000). Immunofluorescence in cytogenetic analysis: method and applications. Genet Mol Biol 23:1003-1014.
- Oestergaard, V. H. and Lisby, M. (2016). TopBP1 makes the final call for repair on the verge of cell division. Mol Cell Oncol 3(2): e1093066.
- Pedersen, R. T., Kruse, T., Nilsson, J., Oestergaard, V. H. and Lisby, M. (2015). TopBP1 is required at mitosis to reduce transmission of DNA damage to G1 daughter cells. J Cell Biol 210(4): 565-582.
- Ronne, M., Andersen, O. and Erlandsen, M. (1979). Effect of colcemid exposure and methanol acetic acid fixation on human metaphase chromosome structure. Hereditas 90(2): 195-201.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Oestergaard, V. H. (2017). Immunostaining of Formaldehyde-fixed Metaphase Chromosome from Untreated and Aphidicolin-treated DT40 Cells. Bio-protocol 7(9): e2259. DOI: 10.21769/BioProtoc.2259.
-
Pedersen, R. T., Kruse, T., Nilsson, J., Oestergaard, V. H. and Lisby, M. (2015). TopBP1 is required at mitosis to reduce transmission of DNA damage to G1 daughter cells. J Cell Biol 210(4): 565-582.
Category
Cancer Biology > General technique > Cell biology assays > Cell cycle
Cell Biology > Cell staining > Nucleic acid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link