- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sucrose Preference Test to Measure Anhedonic Behaviour in Mice
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1958 Views: 36281
Reviewed by: Soyun KimXi FengAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1295 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1810 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views
Abstract
The sucrose preference test (SPT) is a reward-based test, used as in indicator of anhedonia. Anhedonia, or the decreased ability to experience pleasure, represents one of the core symptoms of depression. Rodents are born with an interest in sweet foods or solutions. Reduced preference for sweet solution in SPT represents anhedonia, while this reduction can be reversed by treatment with antidepressants. SPT is carried out in the animal’s home cage. For the SPT, mice are presented with 2 dual bearing sipper tubes. One tube contains plain drinking water, and the second contains a sucrose solution. Water and sucrose solution intake is measured daily, and the positions of two bottles is switched daily to reduce any confound produced by a side bias. Sucrose preference is calculated as a percentage of the volume of sucrose intake over the total volume of fluid intake and averaged over the testing period. Here, we present our protocol that has been able to detect anhedonia in mice subjected to a chronic depression model.
Keywords: AnhedoniaMaterials and Reagents
- Bearing sipper bottles
- Drinking bottles
- Laboratory-bred mice
Note: Mice housed in groups 3-5 per cage, kept in a room with controlled temperature (~23 °C) and humidity under 12 h light/dark cycle (lights on at 7:00 AM) with ad libitum access to food and water. - Sucrose (EMD Millipore, catalog number: 573113 )
- Plain tap water
Equipment
- Mouse cage without lid, which allows positioning of two bearing sipper water bottles (Figure 1)
- Digital balance

Figure 1. Mouse cage with two bearing sipper bottles
Procedure
- Prior to beginning testing, mice are habituated to the presence of two drinking bottles for at least 3 days in their home cage.
- In order to detect possible side preference in the drinking behaviour, the water intake is measured daily in both bottles.
- After the acclimatization the mice are separated single per cage and presented to two drinking bottles: one containing 1% sucrose and the other water for 3 days in their home cage.
- Water and sucrose solution intake is measured daily by weighing the bottles.
- The positions of two bottles is switched daily to reduce any confound produced by a side bias.
- Sucrose preference is calculated as a percentage of the volume of sucrose intake over the total volume of fluid intake and averaged over the 3 days of testing.
Sucrose preference = V(sucrose solution)/[V(sucrose solution)+V(water)] x 100%
Representative data
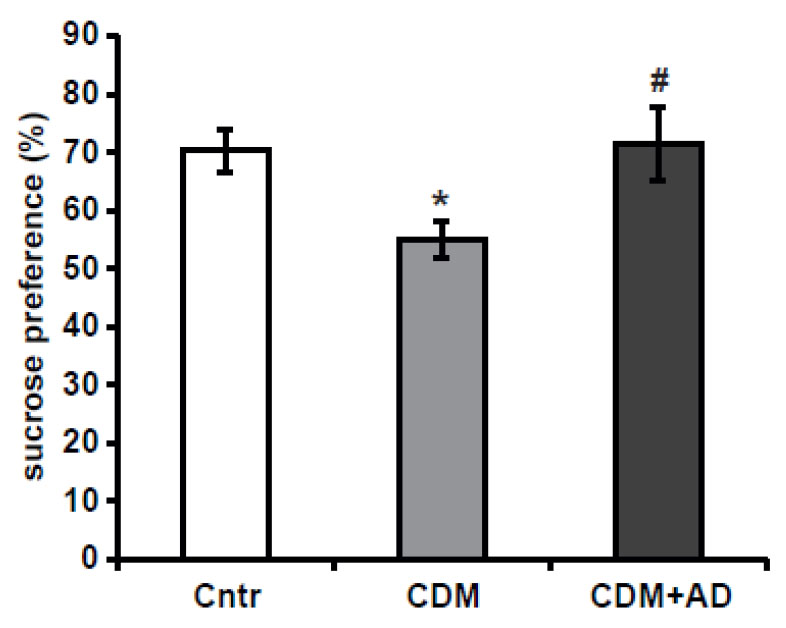
Figure 2. Sample data from sucrose preference test performed on control (Cntr), chronically despaired mice (CDM) and CDM treated for 4 weeks with classical antidepressant (CDM+AD)
Notes
- The SPT is an assay for anhedonia in mice subjected to a chronic depression model (Figure 2). However numerous studies show a remarkable inter-individual variability in animals’ responses to stress (Strekalova et al., 2011).
- Baseline sucrose preference may vary according to mouse strain and age (Pothion et al., 2004).
- Several factors of drinking behavior might essentially interfere with the outcome of the standard free-choice, two-bottle sucrose test paradigm:
- Individual mice from the same group might have a preference for drinking at one or the other corner of the cage. Housing the mice with water on both sides of the cage prior of the experiment should abolish the side preference in drinking behavior in some but not all animals. Switching the position of the bottles containing water or sucrose solution during the test will minimize this artifact (Pothion et al., 2004; Schmidt et al., 2008).
- The individual mice have different values of total liquid intake. The analysis of the drinking behavior should take into account the relative parameter of preference in choice paradigm, rather than of absolute intake values (Pothion et al., 2004; Strekalova and Steinbusch, 2010).
- The major weakness of the sucrose preference test is that mice may initially be neophobic to sucrose solution, a factor which may decrease consumption, and thus make it difficult to distinguish anhedonia from fear of an unfamiliar substance. Neophobia might be abolished by repeated exposure or habituation to sucrose solution (Strekalova and Steinbusch, 2010).
- Pre-exposure of mice to sucrose can increase sucrose intake and preference up to ceiling values, which decreases test’s sensitivity (Strekalova and Steinbusch, 2010). This effect can be counter balanced by application of sucrose solutions of descending concentrations (Strekalova et al., 2006).
- Exposing the sucrose solution to room temperature for longer than 3 days may lead to growth of bacteria and fungus, which will interfere with the final outcome of the test.
- Housing the mice prior to the sucrose preference test in a home cage with a water bottle placed in the middle of the cage could significantly decrease the side preference in the drinking behavior. The mice showing side preference should be excluded from the final evaluation of the results.
Acknowledgments
The protocol described here has been adapted from a previous study (Serchov et al., 2015), which succeeded in assessing anhedonia in mice subjected to model of chronic depression. This work was supported by grants from the German Research Council (DFG) (CA 115/5-4) to D.v.C. and K.B., the European Union FP7 program “MoodInflame” to D.v.C. and German Ministry for Research and Education (DMBF) grant e:bio – Modul I –ReelinSys (Project B: 031 6174A) to K.B.
References
- Pothion, S., Bizot, J. C., Trovero, F. and Belzung, C. (2004). Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155(1): 135-146.
- Serchov, T., Clement, H. W., Schwarz, M. K., Iasevoli, F., Tosh, D. K., Idzko, M., Jacobson, K. A., de Bartolomeis, A., Normann, C., Biber, K. and van Calker, D. (2015). Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 87(3): 549-562.
- Schmidt, M. V., Sterlemann, V. and Muller, M. B. (2008). Chronic stress and individual vulnerability. Ann N Y Acad Sci 1148: 174-183.
- Strekalova, T., Couch, Y., Kholod, N., Boyks, M., Malin, D., Leprince, P. and Steinbusch, H. M. (2011). Update in the methodology of the chronic stress paradigm: internal control matters. Behav Brain Funct 7: 9.
- Strekalova, T., Gorenkova, N., Schunk, E., Dolgov, O. and Bartsch, D. (2006). Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol 17(3): 271-287.
- Strekalova, T. and Steinbusch, H. W. (2010). Measuring behavior in mice with chronic stress depression paradigm. Prog Neuropsychopharmacol Biol Psychiatry 34(2): 348-361.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Serchov, T., van Calker, D. and Biber, K. (2016). Sucrose Preference Test to Measure Anhedonic Behaviour in Mice. Bio-protocol 6(19): e1958. DOI: 10.21769/BioProtoc.1958.
Category
Neuroscience > Behavioral neuroscience > Cognition
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









