- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Intestinal Mesenchymal Cells from Adult Mice
Published: Vol 6, Iss 18, Sep 20, 2016 DOI: 10.21769/BioProtoc.1940 Views: 24861
Reviewed by: Ivan ZanoniMeenal SinhaEmilie Besnard

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Long-term Cultivation of Mouse Alveolar Macrophages
Clara Jana-Lui Busch [...] Michael H. Sieweke
Jul 20, 2019 17015 Views

Isolation and Stimulation of Peritoneal Macrophages with Apoptotic Jurkat Cells to Produce IL-10
Mei Song and Xiaojing Ma
Dec 20, 2019 6116 Views

Isolation and Quantification of Mouse γδT-cells in vitro and in vivo
Isha Rana [...] Colin Jamora
Sep 5, 2021 5006 Views
Abstract
During the last 20 years intestinal mesenchymal cells (IMCs) have emerged as an important cell type that plays a central role in intestinal development and homeostasis, by providing both structural support and growth regulatory elements. IMCs also actively participate in wound healing responses, thus regulating pathologic conditions such as tissue repair, inflammation, fibrosis and carcinogenesis (Powell et al., 2011). We have recently demonstrated that intestinal mesenchymal-specific signals play important in vivo physiological roles in intestinal inflammation and carcinogenesis (Koliaraki et al., 2012; Roulis et al., 2014; Koliaraki et al., 2015). Here we describe the enzymatic method used for the isolation and culture of mesenchymal cells from the adult mouse intestine.
Keywords: Mouse intestineMaterials and Reagents
- Petri dishes (100 mm)
Note: Any Petri dish can be used, according to each researcher’s preference and availability. - Falcon tube
- Transfer pipettes 3.5 ml (SARSTEDT, catalog number: 86.1172.001 )
- 50 ml conical tubes (SARSTEDT, catalog number: 62.547.004 )
- Syringe filters, 0.2 μm pore size (SARSTEDT, catalog number: 83.1826.001 )
- Cell strainers 70 μm (Corning, Falcon®, catalog number: 352350 )
- Cell culture treated flasks (25 cm2) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 136196 )
- Cell culture treated flasks (175 cm2) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 178883 )
- Cell culture treated flasks (80 cm2) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 178905 )
- Mice
- Ethanol
Note: Any ethanol can be used, according to each researcher’s preference and availability. - HBSS (Thermo Fisher Scientific, GibcoTM, catalog number: 14170088 )
- Antibiotic-antimycotic (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15240096 )
- EDTA (Thermo Fisher Scientific, Fisher Scientific, catalog number: 327205000 )
- DTT (DL-Dithiothreitol) (Sigma-Aldrich, catalog number: D9779 )
- DMEM (Thermo Fisher Scientific, GibcoTM, catalog number: 11960044 )
- Fetal bovine serum (FBS) (Biochrom, catalog number: S0115 )
- Penicillin-streptomycin 100x (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- L-glutamine (200 mM) (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-081 )
- MEM non-essential amino acids solution (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 11140-068 )
- Phosphate-buffered saline (PBS) Dulbecco (10x) (Biochrom, catalog number: L1835 )
- Amphotericin B (Sigma-Aldrich, catalog number: A2942 )
- Collagenase from Clostridium histolyticum, type XI (Sigma-Aldrich, catalog number: C7657 )
- Dispase II, neutral protease grade II, from Bacillus polymyxa (Roche Diagnostics, catalog number: 04942078001 )
- Trypsin-EDTA (0.05%) (Thermo Fisher Scientific, GibcoTM, catalog number: 25300054 )
- FACS staining antibodies and material
- Vimentin-A647 (Abcam, catalog number: ab194719 )
- CD45-PE-Cy5 (BD, catalog number: 553082 )
- Intracellular fixation & permeabilization buffer set (eBioscience, catalog number: 88-8824-00 )
- Vimentin-A647 (Abcam, catalog number: ab194719 )
- HBSS/antibiotics (see Recipes)
- HBSS/EDTA/DTT (see Recipes)
- Digestion solution (see Recipes)
- DMEM medium (see Recipes)
Equipment
- Dissection kit
- Two scissors (at least one medium size and one small)
- Two forceps
Note: Any scissors and forceps can be used, according to each researcher’s preference and availability. - A 10 ml syringe equipped with a 21-gauge needle for flushing the intestine (BD, PlastipakTM, catalog number: 308063 )
- Two scissors (at least one medium size and one small)
- Cell culture hood
- Cell culture incubator, 37 °C, 5% CO2
- Shaking water-bath
- Centrifuge compatible with 50 ml conical tubes
- Bright-field inverted microscope for cell culture
- Multicolor FACS analyser (BD, model: BD FACS Canto II )
Software
- FlowJo software (Tree Star Inc.)
Procedure
- At the bench
- Euthanize the mouse by CO2 asphyxiation, following guidelines approved by your Institutional Animal Care and Use Committee.
- Sterilize the skin of the abdomen with 70% ethanol and open it.
- Remove the colon and/or 7-8 cm of the small intestine (from the cecum to the stomach) and place separately in Petri dishes filled with ice-cold HBSS/antibiotics (Figure 1).
Note: It is better to avoid cell isolation from the colon and small intestine together as they need different incubation times in the digestion solution.
Figure 1. Dissection of the mouse intestine. A. The skin is removed and the abdomen is exposed. B. The peritoneum is carefully opened to expose the intestine. C. The intestine is removed from the cavity to expose the stomach and the anus (indicated by arrows). D. The intestine is carefully removed from the anus to the stomach. The colon and small intestine are removed and placed in separate Petri dishes. Intestinal parts are indicated. - Wash the intestinal parts repeatedly by flushing with HBSS/antibiotics using a 10 ml syringe equipped with a 21-gauge needle (Figure 2A). Place the tissue on a pre-wet paper and remove fat and adherent connective tissue (Figure 2B). Cut the intestinal tissue longitudinally and place in 10 ml ice-cold HBSS/antibiotics in a falcon tube per mouse on ice (Figure 2C).
Notes:- Intestinal tissue is sensitive and the duration of this step should be as short as possible. Each mouse tissue should be prepared separately and the tissue should then be placed on ice. If you need to pause the protocol in this step, although not recommended, place all tissues on ice.
- For the small intestine, it is better to also remove Peyer’s patches (Figure 2B).
- Using blunt ended needles in this step might be more convenient for less experienced users.

Figure 2. Representative images of the steps for the preparation of the intestine. A. Flushing of the intestine. B. Removal of fat and connective tissue with the help of forceps. Peyer’s Patches are indicated with arrows. C. Longitudinal opening of the intestinal tissue.
- Intestinal tissue is sensitive and the duration of this step should be as short as possible. Each mouse tissue should be prepared separately and the tissue should then be placed on ice. If you need to pause the protocol in this step, although not recommended, place all tissues on ice.
- Euthanize the mouse by CO2 asphyxiation, following guidelines approved by your Institutional Animal Care and Use Committee.
- In the cell culture hood
- Wash colon or small intestine with ice-cold HBSS/antibiotics (~10 ml) at least 3 times by vigorously shaking the tube, removing the supernatant and adding new HBSS/antibiotics each time.
Notes:- The supernatant should be clear before you move to the next step.
- Removal of the supernatant can be easier if you use the sterile transfer pipettes.
- The supernatant should be clear before you move to the next step.
- Place the tissue in a Petri dish and cut the tissue at 0.5-1 cm pieces with the help of scissors and forceps.
Note: Scissors and forceps can be sterilized before inserting in the cell culture hood. Alternatively, scissors and forceps can be sprayed with 70% ethanol before use. They can be kept in 70% ethanol and emerged in sterile water before manipulation of the intestine, or in-between samples. - Wash the pieces once with ice-cold HBSS/antibiotics (~10 ml) with vigorous shaking and remove the supernatant.
- Add 10 ml pre-warmed HBSS/EDTA/DTT solution and incubate for 20 min, at 37 °C, in a shaking water-bath (~200 rpm) to remove intestinal epithelial cells (both for the colon and small intestine).
- Wash the intestinal pieces at least 3 times with HBSS/antibiotics (it can be at room temperature now) by vigorously shaking the tubes for ~15 sec, removing the supernatant and adding new HBSS/antibiotics each time. After the last wash the supernatant should no longer be cloudy. Decant through a cell strainer (70 μm) to remove all liquid and place in a new Falcon tube.
Note: EDTA can inhibit collagenase, so it has to be removed completely. - Add 10 ml digestion solution and incubate for 1 h (colon) or 30-40 min (small intestine), at 37 °C, in a shaking water-bath (~200 rpm).
Note: You should see the supernatant become cloudy and the tissue more transparent but not completely dissolved (Figure 3).
Figure 3. Intestinal tissue before and after enzymatic digestion. The arrow indicates the presence of some intact tissue after enzymatic digestion. - Shake vigorously and place supernatant in a new Falcon tube.
Optional: You can pass the supernatant through a cell strainer (70 μm). - Centrifuge at 280 x g for 10 min at room temperature.
- Resuspend the cell pellet in 5-6 ml DMEM medium + amphotericin B (2.5 μg/ml) and place in a 25 cm2 flask per mouse.
Important: After 2-3 h you can notice cells adhering to the bottom of the flasks (Figure 4A). Change the medium at this point or the next day by removing the supernatant and adding new medium to reduce chances of contamination. - Watch out daily for signs of bacterial contamination and feed every 2-3 days. Usually, at 4-5 days cells are confluent and can be passaged to a 75 cm2 flask (Figure 4B).
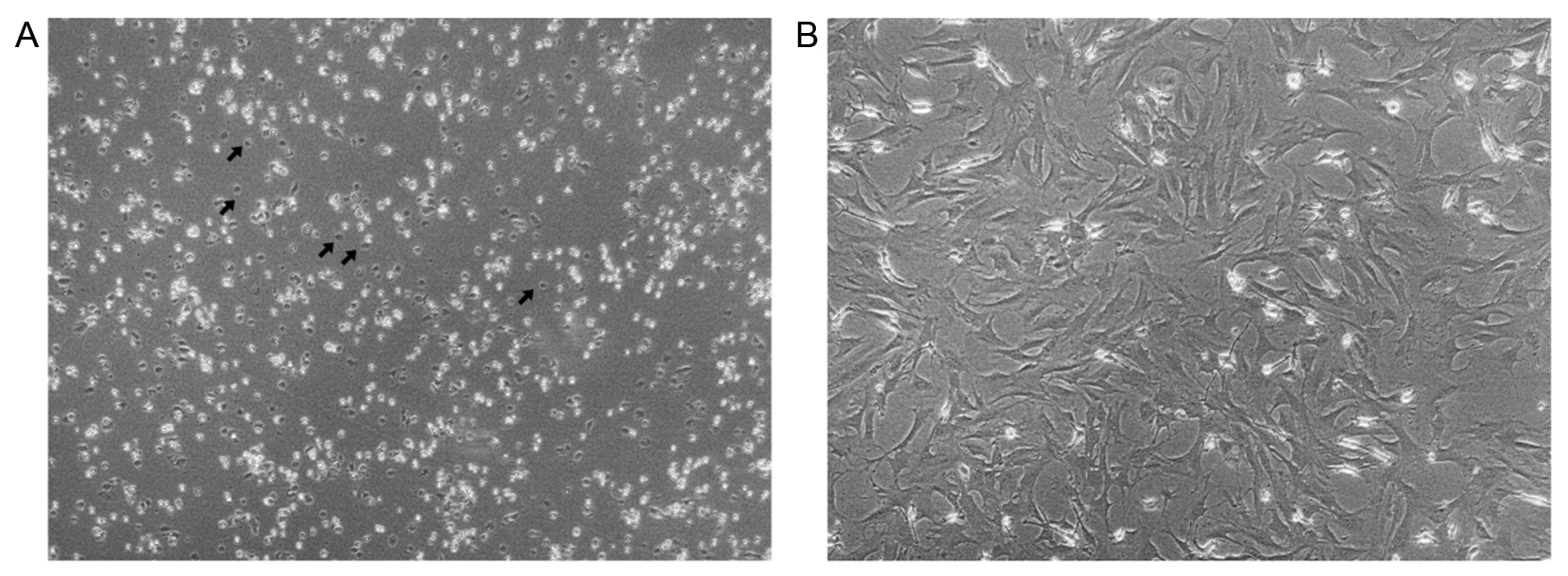
Figure 4. Representative images from IMCs in cell culture after plating (A) and at passage 2 (P2) (B). In (A) selected cells that have attached to the flask are indicated by arrows (Magnification lens 10x). - Passaging of cells can be done by washing the cells twice with 10 ml of 1x PBS (diluted 10x PBS to 1x with H2O) each time and adding trypsin/EDTA (1 ml for a 25 cm2 flask). Incubation with trypsin/EDTA for 5 min, at room temperature should be enough for cells to detach. DMEM medium is then added to inactivate trypsin and cells are split at a 1:2 ratio.
- After the first passage, cells are usually passaged every 3-6 days in a split ratio 1:2 (always split when the cells are almost 100% confluent). Feed every 2-3 days. At passages 1 and 2, amphotericin B is added at 0.25 μg/ml and at passage 3 it is omitted.
- At passage 3, check cells with FACS analysis for their purity. At this stage, cultures are normally CD45 (< 3-4%), therefore negative for hematopoietic cells, and can be used in subsequent experiments. Vimentin, an intracellular cytoskeleton protein, can be used as a positive marker and should stain > 90% of the cells (See example of flow cytometry analysis in Figure 5 and FACS staining details under “Notes” section).
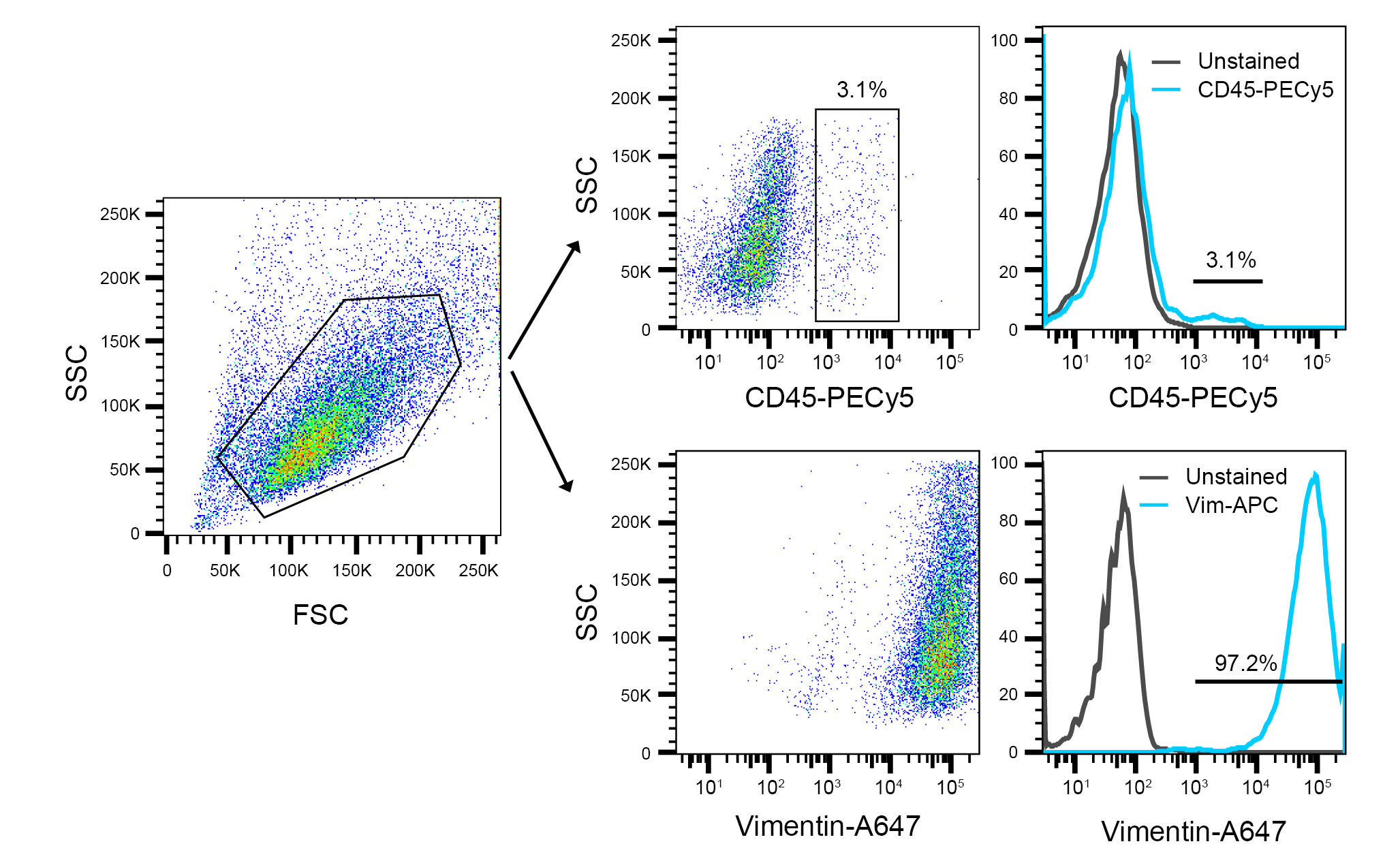
Figure 5. Representative FACS analysis of isolated IMCs at passage 3 (P3) - Try to use isolated IMCs between passages 3-5 and don’t keep them more than one month or if you notice changes in their morphology. One 175 cm2 flask usually yields 3-6 x 106 cells.
- Cells can be used for several functional and biochemical assays. Plating cell concentration to reach ~80% confluence the next day is as follows:
- 96-well plate: 2 x 104 per well
- 24-well plate: 8 x 104 per well
- 6-well plate: 2.5 x 104 per well
- 100 mm plate: 1.6 x 106 per plate
- 96-well plate: 2 x 104 per well
- Wash colon or small intestine with ice-cold HBSS/antibiotics (~10 ml) at least 3 times by vigorously shaking the tube, removing the supernatant and adding new HBSS/antibiotics each time.
Notes
- The above protocol refers to the use of 1 mouse/sample. You can group colon or small intestine from 3 mice and add 15 ml of HBSS/EDTA/DTT and 15 ml of digestion solution. In this case, more washes may be necessary before the supernatant is clear in steps B1 and B5. After the end of the protocol you can plate the isolated cells in a 75 cm2 flask.
- There is increased chance of bacterial contamination after isolation and before the first cell passage. For this reason try to change the medium 2-3 h or the next day after isolation, check cells daily and change the medium every 2-3 days. Make sure to always add amphotericin at the indicated concentration, preferably prepare the amphotericin-supplemented DMEM medium fresh and handle it appropriately, as it is light sensitive.
- FACS staining
- Cell staining for CD45 (0.2 μl per 1-2 x 106 cells) is performed first in 100 μl samples (1-2 x 106 cells) in PBS/5% FBS, for 30 min, at 4 °C, in the dark, followed by two washes with FACS buffer (PBS/2% FBS).
- Cells are then fixed and permeabilized, using the Fixation & Permeabilization kit for 40 min at room temperature, followed by two washes with 1x permeabilization buffer (Perm buffer).
- Cell staining with vimentin (0.2 μl per 1-2 x 106 cells) is then performed in 100 μl 1x Perm buffer, for 1 h, at room temperature, followed by one wash with Perm buffer and one wash with FACS buffer before analysis using a FACS BD Canto II cytometer and the FlowJo software.
- Cell staining for CD45 (0.2 μl per 1-2 x 106 cells) is performed first in 100 μl samples (1-2 x 106 cells) in PBS/5% FBS, for 30 min, at 4 °C, in the dark, followed by two washes with FACS buffer (PBS/2% FBS).
- In case the intestine is inflamed you may notice a higher number of CD45+ cells. In this case and if this is a problem for your subsequent experiments, you may have to add an extra magnetic-bead depletion or cell-sorting step.
- Cryopreservation of primary intestinal mesenchymal cells is not recommended.
Recipes
- HBSS/antibiotics
5 ml antibiotic-antimycotic in 500 ml HBSS
Note: Ice cold before use. - HBSS/EDTA/DTT
5 mM EDTA
1 mM DTT
1x pen/strep
Dissolve in HBSS
Note: Pre-warmed at 37 °C before use. - Digestion solution
300 U/ml collagenase XI
0.08 U/ml dispase II
Dissolve in DMEM medium
Note: The digestion solution should be made fresh. Weigh the appropriate amount of collagenase and dispase according to the number of mice you will use and dilute it in HBSS at 1/10 of the final volume. Sterile filter the solution through a syringe filter with 0.2 μm pore size and then add DMEM medium to the final volume. - DMEM medium
50 ml heat-inactivated FBS
5 ml pen/strep
5 ml L-glutamine
5 ml non-essential amino acids
Dissolve in 500 ml DMEM
Notes:- All mediums apart from the digestion solution can be made in advance and stored at 4 °C.
- Pre-warmed at 37 °C before use.
- All mediums apart from the digestion solution can be made in advance and stored at 4 °C.
Acknowledgments
This protocol was based on a previous protocol used in our lab (Armaka et al., 2008). This work was supported by FP7 Advanced ERC grant MCs-inTEST (Grant Agreement No. 340217) and Innovative Medicines Initiative Joint Undertaking (IMI JU) project BTCure (Grant Agreement No.115142) to GK.
References
- Armaka, M., Apostolaki, M., Jacques, P., Kontoyiannis, D. L., Elewaut, D. and Kollias, G. (2008). Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 205(2): 331-337.
- Koliaraki, V., Roulis, M. and Kollias, G. (2012). Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J Clin Invest 122(11): 4231-4242.
- Koliaraki, V., Pasparakis, M. and Kollias, G. (2015). IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med 212(13): 2235-2251.
- Powell, D. W., Pinchuk, I. V., Saada, J. I., Chen, X. and Mifflin, R. C. (2011). Mesenckhymal cells of the intestinal lamina propria. Annu Rev Physiol 73: 213-237.
- Roulis, M., Nikolaou, C., Kotsaki, E., Kaffe, E., Karagianni, N., Koliaraki, V., Salpea, K., Ragoussis, J., Aidinis, V., Martini, E., Becker, C., Herschman, H. R., Vetrano, S., Danese, S. and Kollias, G. (2014). Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci U S A 111(43): E4658-4667.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Koliaraki, V. and Kollias, G. (2016). Isolation of Intestinal Mesenchymal Cells from Adult Mice. Bio-protocol 6(18): e1940. DOI: 10.21769/BioProtoc.1940.
Category
Immunology > Immune cell isolation > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











